Abstract
Twenty-five samples of autumnal honeys from the western Mediterranean (Mallorca and Eivissa, Balearic Islands) were examined for pollen content (qualitative and quantitative melissopalynological analysis), moisture, electrical conductivity, colour, sensorial qualities and volatile components. Quantitative analysis showed that the honey contained Maurizio’s Class II: 64%, Class III: 28%, Class IV: 4% and Class V: 4%. Fifty-four pollen types, with an average number of 16.68 per sample, were identified, belonging to 29 botanical families. Only two taxa (Ceratonia siliqua and Erica multiflora) were found in all samples. Seventeen samples were unifloral (68%) – ten (40%) of C. siliqua, six (24%) of E. multiflora and one (4%) of Hedera helix. All honeys have a low honeydew index (<?0.09%), while the values for electrical conductivity and water content were high. The major honey volatile components are: cis- and trans-linalool oxides (64.2%) and hotrienol (10.4%) for the carob (C. siliqua) and trans-linalool oxide (13.4%), p-menthane-1,8-diol (11.1%), safranal (9.7%), limonene (5,4%), α-pinene (3.7%) and oxoisophorone (3.4%) for the winter heather (E. multiflora).
In a Mediterranean context, the human colonisation of the Balearic archipelago took place quite late. Archaeological data relating to the first stable settlement suggest human presence in the area between 4400 and 3600 bp (Calvo et al., Citation2002). This region has a long history of beekeeping extending back at least 2500 years (Bonet & Mata, Citation1997) and includes structures such as the stone beehives of the Punic, Greek and Roman civilisations on the island of Eivissa.
In the abundant production of autumnal honeys, traditionally, beekeepers recognised that Apis mellifera L. visits flowers of Ceratonia siliqua L., Erica multiflora L., Diplotaxis erucoides (L.) DC., Dittrichia viscosa (L.) Greuter, Hedera helix L. and Rosmarinus officinalis L. These are the predominant autumn-flowering species, but there are also a several others (e.g. Herrera, Citation1986, Citation1988; Guardia et al., Citation1998; Pons, Citation2002).
One aspect of honey production is the intensive cultivation of carob trees (Ceratonia siliqua). Ceratonia siliqua honey is limited to warmer Mediterranean territories (Balearic Islands, Sicily, Sardinia, Greece, Turkey, northern Africa and France), where it has been mostly studied. Sáenz Lain and Gómez Ferreras (Citation2000) reported that in Spain, this honey type is only present in the Balearic Islands, but there is no reference to any physico-chemical parameters. However, Ceratonia pollen appears in some honeys from Israel, Morocco, Algeria and the southern Iberian Peninsula (Damblon, Citation1988; Ricciardelli d’Albore, Citation1998; Persano Oddo et al., Citation2000; Ricciardelli d’Albore & Intoppa, Citation2000; Terrab et al., Citation2000, Citation2001, Citation2003a).
Erica multiflora is a common species in dry and calcareous coastal shrubland regions, and its unifloral honey is common in the eastern Iberian Peninsula, Malta and Croatia; it is found occasionally in Italy and Tunisia (Persano Oddo & Piro, Citation2004). Erica manipuliflora Salisb., a similar species, found in the phryganas or garrigue of the central and eastern Mediterranean region, can also be unifloral. Other unifloral Erica honeys have been studied in northern Spain and Portugal, but belong to other species such as E. umbellata L., E. australis L. and E. arborea L. (Sà-Otero et al., Citation1991, Citation2006; Seijo et al., Citation1992; Sáenz Lain & Gómez Ferreras, Citation2000) or Calluna sp. (Andrade et al., Citation1999). Hedera helix honey is rarely found in Spain, Italy, France or Croatia (Persano Oddo & Piro, Citation2004).
One of the main features of the Mediterranean climate is the dry summers (Rivas-Martínez, Citation1995), with concentrated rainfall during winter or spring. Some territories in the western Mediterranean, however, have their maximum rainfall during the autumn (Llasat et al., Citation1996). This pattern of autumnal rain is located in the thermo-Mediterranean belt (Rivas-Martínez, Citation1983), and it favours an abundance of autumnal-flowering plants. The Balearic Islands, located in the centre of the western Mediterranean region, represent an area that has an autumn locally known as ‘winter spring’.
Honey aroma has been studied for years in order to develop analytical methods for its determination based on the volatile organic fraction. The composition of the honey’s volatile fractions derives from its floral origin and from the foraging habits and physiology of the bees. For this reason, the chemical volatile composition is of great importance in characterising the floral and/or geographical origin of honeys. Volatile components have been determined as specific markers for unifloral honeys. The use of dynamic heat space techniques (Bouseta et al., Citation1992; Radovic et al., Citation2001) or solid-phase micro extraction could prove to be useful for testing the authenticity of the botanical origin. Marker components of unifloral honeys can probably be detected among the less volatile components (e.g. Guidotti & Vitali, Citation1998; Pérez et al., Citation2002; Wolski et al., Citation2006). In different honeys, a range of natural organic substances of the plant source have been identified as phytochemicals (e.g. de Maria & Moreira, Citation2003; Castro-Vázquez et al., Citation2009) and several markers are identified for the determination of the floral origin of the respective honeys (e.g. Radovic et al., Citation2001; Piasenzotto et al., Citation2003; Cajka et al., Citation2009; Melliou & Chinou, Citation2011).
The main objectives of this study of autumn/winter honeys from the western Mediterranean region are: (a) melissopalynological characterisation; (b) evaluation of the physicochemical parameters (colour, humidity, conductivity) and organoleptic characteristics; (c) characterisation of the major volatile components of the more specific honeys; (d) the establishment of markers that can help to distinguish these honeys from those with a different geographical origin.
Material and methods
Study area
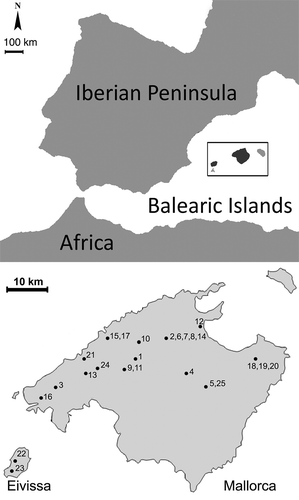
This study analysed 25 honey samples collected in autumn/winter from Mallorca (2004/2005) and Eivissa (2006/2007) (). The climate of the sampling localities corresponds to the Mediterranean maritime type (de Bolòs, Citation1985) of mild winters, with a maximum rainfall in autumn and at the end of winter or beginning of spring (de Bolòs & Vigo, Citation1984–2001). According to the bioclimatic classification of Rivas-Martínez (Citation1995, Citation1997), the study area has a Mediterranean macrobioclimate, a Mediterranean pluviseasonal-oceanic or xeric-oceanic bioclimate and a thermo-Mediterranean thermoclimate. Certain zones in the study area have a diverse ombroclimate. In general, the semi-arid, dry and sub-humid ombrotypes are common, whereas in southern Mallorca and in Eivissa the dry type prevails. Vegetation around the beehives consists of a mix of carob culture (different varieties, mainly Bugadera, De la mel, Vera, Costella and Duraió in Mallorca, and Panesca, Boval and Uraiona in Eivissa), scrub communities and, in some localities, rupicolous vegetation.
Figure 1. Map of Balearic Islands (upper part) and map with distribution of the apiaries investigated (lower part). Collection sites: 1, 9, 11 – Binissalem; 2, 6, 7, 8, 14 – Campanet; 3 – Puigpunyent; 4 – Llubí; 5, 25 – Petra; 10 – Biniamar; 12 – Pollença; 13 – Esporles; 15, 17 – Soller; 16 – Andratx; 18, 19, 20 – Artá; 21 – Estellencs; 22, 23 – Eivissa; 24 – Orient.
Beekeeping
For beekeeping, the standard Langstroth beehive is generally used. The extracted honey is produced by centrifugation. The honeybee species Apis mellifera is identified as the Western-European race (de la Rua et al., Citation2002).
Melissopalynological analysis
In order not to damage the pollen or to destroy fungal spores, the qualitative analysis of the samples was prepared without acetolysis (Louveaux et al., Citation1978; Terrab et al., Citation2004; von der Ohe et al., Citation2004). Qualitative analysis of melissopalynological characters were carried out by a standardised method of Louveaux et al. (Citation1978). While it has been demonstrated that water dilution results in lower yields that can be biased against certain taxa (Jones & Bryant, Citation2004) due to the crystallised nature of some samples, we used warm water dilution to aid dissolution of the crystallised honey. The sample of 10?g of honey was dissolved in 20?ml of distilled water at 30?°C for 10?min with continuous stirring. Two 10?ml sub-samples were centrifuged two times for 10?min at 2200g (at 3500?rpm; cf. Pendleton, Citation2006). The sediment was centrifuged and dehydrated with different ethanol concentrations (50%, 70% and 96%). The pure sediment was stained with glycerine-fuchsine, mounted on microscope slides and examined under an Olympus BX41 microscope.
Two samples were analysed and >?700 pollen grains were identified and counted with an Olympus BX41 microscope at 400× or 1000× magnification. According to the qualitative analysis, the pollen types were identified on the lowest possible taxonomic level (family, genus, species or type). For each pollen type, in relation to the total number of pollen grains recognised, the frequency and percentage were calculated. The pollen types identified and counts were classified into five different categories (Louveaux et al., Citation1978) according to percentage of occurrence: dominant pollen (D, >?45% of the total pollen grains counted); secondary pollen (S, 16–45%); important minor pollen (I, 3–15%); minor (trace) pollen (m, <?3%); and sporadic pollen (p, <?1%).
For the quantitative analysis, natural honey (Vorwohl, Citation1967) was used following the methodology proposed by La Serna et al. (Citation1999) and La Serna and Gómez (Citation2006). The pollen grains were counted from a homogenised suspension of 10?g honey in 20?ml of distilled water, using a haemocytometer (Fuchs–Rosenthal counting chamber) and an optical microscope at 40× magnification. The result is expressed as the number of pollen grains per gram of honey and the samples have been assigned Maurizio’s classes (Maurizio, Citation1939, 1979): I, <?2000 grains; II, 2000–10?000; III, 10?000–50?000; IV, 50?000–100?000; V, >?100?000.
According to the criteria of Louveaux et al. (Citation1978) and Maurizio (Citation1979), a honey is considered unifloral if the pollen frequency of one plant is larger than 45% with exceptions to this due to ‘under’ and ‘over’ representation. The honeydew index (HDE/P) was calculated from the ratio of honeydew elements and pollen grains of nectariferous plants (Louveaux et al., Citation1978).
Table I. Pollen types and frequency of each type found in the samples as dominant (D, >45%), secondary (S, 16–44%), important pollen (I, 3–15%), minor pollen (m, 1–3%) and pollen present (pp, <?1%)
Physicochemical parameters
Determination of the physicochemical parameters such as colour, electrical conductivity and water content may help with the identification of certain honey types (Bogdanov & Martin, Citation2002). Colour was measured in mm Pfund. Values in the range 84–114 mm were considered as amber, over 114 mm as dark or very dark. Determinations were assessed with a digital Hanna C-221 colorimeter (Hanna Instruments Ltd, Leighton Buzzard, UK). The electrical conductivity of a 20% honey solution in distilled water was measured at 20?°C with a Hanna Dist3 conductimeter (Hanna Instruments Ltd) and the values were expressed as μS/cm. Water content (moisture) was determined with an Atago HHR-2N refractometer (Atago Co. Ltd, Tokyo, Japan) reading at 20?°C, using the Wedmore table (AOAC, Citation1990).
This evaluation was important in order to verify the authenticity of unifloral honey, since it can reveal the presence of botanical components not detected by melissopalynological and physico-chemical analysis. For the sensorial analysis of honey (visual, olfactory and gustative sensations), the method of Piana et al. (Citation2004) was used.
Chemical analysis
The samples of the more characteristic unifloral honeys of Ceratonia siliqua and Erica multiflora were analysed for volatile organics. Extraction of the volatile organic fraction followed the methodology of Verzera et al. (Citation2001). Solid phase microextraction (SPME) was used to extract the organic volatile components of the honey without adding solvents. The SPME fibre used was covered with polydimethylsiloxane/divinylbenzene (PDMS/DVB) with a 65?μm film thickness. A honey sample of 16?g was dissolved with 2?g sodium chloride (NaCl) in 7?ml water in a 40?ml glass headspace vial. The vial was gently heated to 30?°C and stirred for 30?min. Subsequently, the SPME fibre was exposed to the vapour phase with the sample for 25?min with stirring. Each sampling of the different varieties of honey was made in triplicate.
Samples were then analysed using a gas chromatograph-mass spectrometer (GC-MS). The SPME fibre was introduced onto the GC injector in splitless mode and held for 3?min to permit the complete thermic (250?°C) secretion of the compounds from the fibre onto the column. The extracted volatiles enter into the system of gas chromatography-mass coupled to spectrometry-mass (GC-EM) for detection and identification. A Clarus 600 GC-MS (Perkin–Elmer, Inc., Wellesley, MA, USA) with a capillary column ZB-5MS (30?m length?×?0.25?mm internal diameter?×?0.25?mm film thickness; Phenomenex, Inc., Torrance, CA, USA) was used to analyse the samples. The injector temperature was 220?°C. The temperature program followed this schedule: 60?°C for 5?min, increasing to 180?°C at rate of 3?°C/min, thereafter by 20?°C/min until 280?°C, holding this temperature for 10?min.
The carrier gas was helium at a constant flux of 1?ml/min. Detection used an electron impact (EI) with energy (ionisation potential) of 70?eV, while the ionisation source up to 180Â?°C. Mass spectra were obtained in scan mode in a mass range of 45–300?uma. The chromatograms and spectra of the samples were processed using the GC-EM software, Turbomass version 5.1 (Perkin-Elmer, Inc.).
A mixture of n-alkanes (C8–C40) dissolved in n-hexane was supplied by Supelco (Bellefonte, PA, USA) to retain index determinations. The control was done between n-octane and n-eicosane. For components with retention indices (RI) less than 800, extrapolation using n-octane and n-nonane was employed. Main volatile honey components were identified taking the RI (determined with reference to a homologous series of normal alkanes) into account, and by comparing their mass spectral fragmentation patterns (Adams, Citation2007) and stored on the MS library [NIST MS Search Program (version 2.0)]. For the quantitative component, the peak areas were considered according to the concentrations from GC.
Results
Melissopalynological analysis
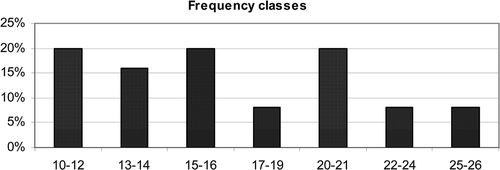
Qualitative analysis yielded a total of 54 pollen types (–) belonging to 29 botanical families. Most of the samples were low in pollen types with a mean value of 16.7 ± 5.21 (). Only two taxa (Ceratonia siliqua and Erica multiflora) were present in all samples ( ), five taxa (Hedera helix, Phoenix sp., Dittrichia viscosa, Diplotaxis erucoides and Cistus sp.) were found in more than 75% of samples and three taxa [Eryobotria japonica (Thunb.) Lindl., Asparagus acutifolius L. and Myrtaceae] were present in 50–75% of the samples.
Figure 2. Distribution of the samples in frequency classes according to their richness in pollen types; X: number of the pollen, Y: percentage of samples.
According the Maurizio’s classification of the quantitative analysis, 64% of the samples belonged to Class II, 28% to Class III and 8% to Class IV and V, respectively. The overall pollen content can be considered as medium (pollen density 4438–135?500?grains/g, average 18?484?grains/g; ). The nectariferous taxa represent 78% of the taxa, while polliniferous taxa are represented by 3%. The honeydew index is very low in all samples (below 0.09), thus can be regarded as practically nil (Loveaux et al., 1978) and is here ignored.
Physicochemical parameters
The dominant colour in 22 samples was dark, amber colour was found in three samples only. Most of the samples have high or very high values for electrical conductivity (410–1780 μS/cm). The water content varied between 16.1% and 22% ( ).
Honey types
Seventeen honeys were unifloral (ten samples of Ceratonia siliqua, six of Erica multiflora and one of Hedera helix), and eight were multifloral (, ).
Table II. Melissopalynological analysis and physicochemical parameters and frequency-class distribution.
In the Ceratonia siliqua honey, between 11 and 22 types of pollen were found (average 18), with only one sample revealing an absence of Erica multiflora pollen (the second most common nectariferous species). Quantitative analysis revealed a medium-high pollen content (80% in Class II, 10% in Class IV and 10% in Class V; ). The pollen density ranged between 5292 and 135?500 grains/g (average 28?957). The physical state of this honey is liquid. The rate of crystallisation depends on the water content, but it is always very low (>1 year); however, eventually, fine, powdery crystals are produced. When C. siliqua pollen predominates no or very low crystallisation occurs. The crystallisation process is linked to the presence of Erica nectar, which, when present, causes the formation of fine crystals with a powdery appearance. When the honey is liquid, its colour is dark or amber, but when it is crystallised, it is lighter. The odour is intense and has smells of flowers, liquorice, wood, mushrooms and forest. The taste is sweet, a little salty with a touch of bitterness, caramel, liquorice, humus, mushrooms and forest. A retronasal aroma that is somewhat floral, wood, forest, liquorice, which clears the nose.
In the Erica multiflora honey, between 11 and 16 pollen types occur (average 15.58), with 40% of the samples being characterised by the presence of up to 6% Arbutus unedo L. pollen (an under-represented type that adds a very singular flavour). Only 20% of the samples contained Ceratonia siliqua pollen. Quantitative analysis ( ) showed a medium pollen content (50% Class II, 50% Class III). The pollen density ranges between 4438 and 23?547 grains/g (average 12?030). The organoleptic analysis shows the physical state can transition from liquid to crystal rapidly (crystals may appear in one month). The crystals are fine, similar to powder or grit, and sometimes, there may appear separated phases. The colour when liquid is dark (toffee or dark brown) with an orange tonality, but when it is crystallised, there is a remarkable orange colour variation. The taste is sweet, somewhat salty, acidic, humus, mushrooms and forest with a final bitter taste. The odour suggests caramelised sugar and is sometimes strong, intense, fresh and flowery. A retronasal aroma is of forest and syrup. Due to the harvest period, these honey types may have high moisture content. This fact and their rapid rate of crystallisation create problems for its preservation.
Table III. Results of the qualitative analysis, represented as percentages
The only sample analysed of Hedera helix honey had a pollen density of 7828?grains/g (Class II) and 18 pollen types (predominantly from nectariferous species; ). The organoleptic analysis reveals that the physical state is extremely smooth. When crystallisation occurs, it is very fine, which is the reason for the honey’s smooth texture. These characteristics will remain for about two years. The colour is dark (brown leather, toffee dark brown) and with an odour of liquorice and forest that is intense. The taste is acidic, bitter, medicines, roasted and coffee. A retronasal aroma of liquorice expectorant was noted.
Multifloral honey contains between ten and 25 types of pollen per sample (average 18.5). This type of honey contains different proportions of Ceratonia siliqua and Erica multiflora (Classes II or III) and the pollen density range from 5786 to 16?797?grains/g (average 11?564; ). The organoleptic analysis reveals that the physical state has fine crystals. The colour varies from amber to dark. The odour is intense with smells of liquorice, forest, wood, caramelised sugar and flowery. The taste is sweet, salty, caramel and liquorice, humus, mushrooms and forest. A retronasal floral aroma with wood, forest, liquorice and syrup can be detected. This type of honey has different fractions of C. siliqua and E. multiflora, but the flavour and other characteristics of the C. siliqua predominate.
Chemical volatile analysis
The main volatile components of Ceratonia siliqua and Erica multiflora samples showed significant differences depending on the floral source of the honey. For example, the C. siliqua honey has a high content of linalool derivate, such as trans-linalool oxide (57.7%), cis-linalool oxide (6.6%) and hotrienol (10.4%). However, in Erica multiflora honey, components such as cis-linalool oxide, linalool and hotrienol are absent and trans-linalool oxide is present in lower levels. This type of honey is characterised by the presence of p-menthane-1,8-diol (11.1%), safranal (9.7%), limonene (5.4%) and oxoisophorone (3.4%) and significant amounts of α-pinene and camphene ( ).
Discussion
Melissopalynology is recognised as the most applicable method in order to determine the botanical and, in many cases, geographical origin of a honey sample (Anklam, Citation1998). The pollen spectrum of the honey usually reflects the vegetation of the area, where the beehives are located (La Serna & Gómez, Citation2006). In some places of the south-western Mediterranean region, autumn, the rainiest season, represents a period characterised by intense flowering of a limited number of species (Kummerov, Citation1983; Arroyo, Citation1988; Thompson, Citation2005).
The identified pollen reflects the current natural vegetation of the two islands under study. The common flowering species at this time of the year are Ceratonia siliqua, Diplotaxis erucoides (from disturbed areas), Dittrichia viscosa, Phoenix sp. (cultivated), Hedera helix (wild and cultivated), Erica multiflora and Rosmarinus officinalis (abundant in the scrublands), which could be of a more general interest. Species such as Arbutus unedo and Micromeria inodora (Desf.) Benth., with a more limited wild distribution, could have a more specific ecological or biogeographical importance (de Bolòs & Molinier, Citation1984; Llorens et al., Citation2007).
Palynological investigation reveals that the autumnal honeys of Mallorca and Eivissa have no remarkable differences in their pollen spectra and shows a medium presence of pollen types, with clear dominance of a few species, such as Ceratonia siliqua, Erica multiflora and, locally, Rosmarinus officinalis. The presence of Micromeria inodora in the honeys of Eivissa is remarkable (sample 22: unifloral E. multiflora and sample 23: multifloral; ). This species can be considered a territorial trace guide species and a strong geographical marker for honeys produced in the Pityusic Islands (Eivissa and Formentera). Besides the importance of C. siliqua and E. multiflora, it is also evident that 72% of these autumnal honeys are unifloral.
Most honeys have a high value for electrical conductivity, which is related to their mineral content according to their botanical origin (Vorwohl, Citation1964). In the autumnal honeys, this fact seems to be determined by the presence of Ceratonia siliqua honeydew (as fruit exudates). However, quantitative analysis shows that most honeys do not contain HDE and, according to Louveaux et al. (Citation1978), this character indicates that nectar is the main source of the autumnal honeys.
The water content of honey reflects the relation between the climatic conditions and the season of production (Conti et al., Citation2007; Mohammed & Babiker, Citation2009) and consequently, the autumnal honeys may have a high water content value. Moreover, these values are similar to the results measured in other autumnal honeys (c. 21%) such as Arbutus unedo (Persano Oddo et al., Citation2004) or various Ericaceae (Crane, Citation1975, Citation1990). According to the ranges established by the European Community Directive, this value can be considered as high (The Council of the European Union, Citation2002) and finally this does not reduce the quality of the honey.
Unifloral honeys
Since ancient times, the carob tree grew in most countries of the Mediterranean basin; it is a characteristic species of the subtropical Mediterranean vegetation. Its value was recognised by the ancient Greeks, who brought it from Middle East to Greece and Italy and by the Romans and Arabs, who disseminated it along the North African coast and northwards into Spain and Portugal (Batlle & Tous, Citation1997; Ramón-Laca & Mabberley, Citation2004). Nowadays, the main carob populations – spontaneous (feral) and cultivated – are concentrated predominantly in Spain and in the south of Italy (mainly in Sicily), Morocco, Greece, Cyprus, Turkey and Algeria.
Table IV. The main volatile phenolic components in Ceratonia siliqua and Erica multiflora honeys (in %)
Ceratonia siliqua has an autumnal flowering (Retana et al., Citation1994; Bosch et al., Citation1996) and it is recognised as an important nectariferous and polliniferous species (Santas & Bikos, Citation1979; Ortiz et al., Citation1996). At the same time, it can provide honeydew (Damblon, Citation1988). In different honeys of the meridional territories of the Mediterranean such as Sicily, Turkey, the Iberian Peninsula and North Africa, the contents of Ceratonia pollen appears in varying percentages, many times over-represented, due to the juxtaposition of the stamen and the nectariferous disc (Clemente & Salas, Citation1996; Terrab et al., Citation2001, Citation2002). Despite this, only a few honeys are known to be derived from the carob tree: Turkey (Ricciardelli d’Albore & Vorwohl, Citation1979), Monti Iblei in Sicily (Ricciardelli d’Albore, Citation1998), Morocco (Terrab et al., Citation2003a), and the Balearic Islands (Sáenz Laín & Gómez Ferreras, Citation2000).
Ricciardelli d’Albore and Intoppa (Citation2000) declare that the unifloral Ceratonia siliqua honeys are very rare and have a delicate smell, yellow colour and bitter taste. These characteristics are not comparable to those from the honeys in our study, which have a medium-high total pollen content, with a high percentage of C. siliqua pollen (57%), with amber or dark colour and infrequent bitter taste (probably due to the lack or rare presence of Arbutus unedo).
The presence of Ericaceae in Ceratonia siliqua honey determines the reduction in electrical conductivity values (Guyot et al., Citation1999; Martins et al., Citation2008); this trend also appears in the studied samples of C. siliqua honey. The high electrical conductivity values in C. siliqua honeys analysed and the absence or almost absence of honeydew characteristics (HDE index <?0.09%), which reveal the circumstantial value of C. siliqua as a source of honeydew, have been verified by some other authors (Damblon, Citation1988; Terrab et al., Citation2003b)
The other unifloral important honey is the Erica multiflora honey, an autochthon abundant plant in Mallorca and Eivissa, which flowers generously in autumn. It is recognised as an important nectariferous and polliniferous species predominant in the unifloral honey having an average of pollen of 50%, with a toffee or brown tonality rarely accompanied by Arbutus unedo.
The electrical conductivity values in the Erica multiflora honey are variable; higher levels correspond to those honeys with more content of Ceratonia pollen, as it occurs with other Erica species honeys (400–700 μS/cm; Persano Oddo & Piro, Citation2004; Martins et al., Citation2008).
Hedera helix honey, which can be found in Mallorca, is an obviously special unifloral honey from the Tramuntana Mountains. Hedera helix is cultivated in gardens or grown in feral form in humid and rocky mountain sites. Hedera helix honey has high electrical conductivity values, although less than the Ceratonia siliqua or Erica multiflora honeys. In H. helix honey, the significant presence of pollen of C. siliqua and E.a multiflora is continuous. The sample of H. helix honey detected a sensory characteristic different from the ones studied in Piana et al. (Citation1989).
Differences were discovered within the various amounts of volatile compounds observed depending upon the floral source of honey. Ceratonia siliqua honey volatile composition is characterised by a predominance of linalool and especially, its derivatives cis-linalool oxide, trans-linalool oxide and hotrienol, with a total amount of 75.3%. These values coincide with those of analytical data concerning nectar volatile composition of flowers analysed in several C. siliqua varieties studied (Custódio et al., Citation2006). However, this predominance is also characteristic in different honeys from tropical lands, as the ones from tahonal (Viguiera dentata Blake), tzitzilché (Gymnopodium floribundum Rolfen) in Yucatán (Cuevas-Glory et al., 2012) or from other climatic areas, such as the ones from corontillo [Escallonia pulverulenta (Ruiz et Pav.) Pers.] in Chile (Montenegro et al., Citation2009). In the Mediterranean context, these fruity aromatic compounds can be considered as typical markers of this unifloral honey.
Erica multiflora honey shows a more diverse and balanced chemical volatile composition with six major represented compounds (46.6% of the total): α-pinene, limonene, trans-linalool oxide, p-menthane-1,8-diol, oxoisophorone, and safranal. Safranal and oxoisophorone can be considered as volatile markers of this honey. Isophorone, a 3,5,5-trimethyl-cyclohex-2-ene-family derivative, was considered a reliable contributor in order to authenticate other honeys such as Calluna (Guyot et al., Citation1999) or Salix (Jerković & Marijanović, Citation2010). The presence of trans-linalool oxide could be related to the influence of Ceratonia siliqua.
Conclusions
Three unifloral honeys are recognised: carob (Ceratonia siliqua), winter heather (Erica multiflora) and ivy (Hedera helix). These species, which provide both nectar and pollen, are essential for bee nutrition during autumn (Lavie, Citation1975; Ortiz et al., Citation1996; Persano Oddo et al., Citation2004). The pollen of nectariferous species is dominant (78%). Ceratonia siliqua is the most important species of the autumnal honeys in the Balearic Islands and its presence is widespread in all the samples.
Erica multiflora is a wild, natural, important and abundant autumnal plant. In general, autumnal honeys have a medium pollen component (pollen density between 4438 and 135?500?grains/g, average 18?484). However, they have a narrow spectrum of pollen types with a limited number of species that bloom at this time of the year. High pollen levels of of Ceratonia siliqua and E. multiflora, in association with a few types such as Diplotaxis erucoides, Dittrichia viscosa, Phoenix sp. and Rosmarinus officinalis, distinguish the honeys from the Balearic Islands, characterised by other taxa typical in the Mediterranean region. These species differ to other honeys in the Mediterranean region like Morocco or Italy. The pollen can differentiate the honeys with origin in the Balearic Islands from those harvested in other latitudes. However, the presence of Micromeria inodora in the honeys of Eivissa is remarkable. This species can be considered as a guide species for the honeys obtained in the Pityusic Islands. It flowers in June–August and is very important for the survival of the bees.
The volatile components are useful to distinguish different honeys. The Ceratonia siliqua honey has as a main feature a high presence of linalool and its derivatives, while Erica multiflora honey is distinguished by the abundance of p-menthane-1,8-diol and safranal. Phenolic composition obviously shows an exclusive presence of high values of trans-linalool oxide in the C. siliqua honey and exclusive presence of hotrienol and cis-linalool oxide, whereas in Erica honey the particular compounds are p-menthane-1,8-diol, safranal, limonene and α-pinene.
Acknowledgements
The authors would like to extend their gratitude to the Mallorca Rural ‘Leader plus’ programme and the beekeepers of Mallorca and Eivissa for their support and friendly collaboration. The authors also thank an anonymous reviewer for useful comments and suggestions on an earlier version of the manuscript.
References
- Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream: Allured Publishing Corporation.
- Andrade, P. B., Amaral, M. T., Isabel, P., Carvalho, J., Seabra, R. M. & Proença da Cunha, A. (1999). Physical attributes and pollen spectrum of Portuguese heather honeys. Food Chemistry, 66, 503–510.
- Anklam, E. (1998). A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chemistry, 63, 549–562.
- Association of Official Analytical Chemists (AOAC). (1990). Official methods of analysis, association of official analytical chemists. Gaithersburg: AOAC Press.
- Arroyo, J. (1988). Atributos florales y fenología de la floración en matorrales del sur de España. Lagascalia, 1, 43–78.
- Batlle, I. & Tous, J. (1997). Carob tree. Ceratonia siliqua L. Promoting the conservation and use of underutilized and neglected crops. Gatersleben: Institute of Plant Genetics and Crop Plant Research/Rome: International Plant Genetic Resources Institute.
- Bogdanov, S. & Martin, P. (2002). Honey authenticity. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und -hygiene, 93, 232–254.
- Bonet, H. & Mata, C. (1997). The archaeology of beekeeping in pre-roman Iberia. Journal of Mediterranean Archaeology, 10, 33–47.
- Bosch, J., García del Pino, F., Ramoneda, J. & Retana, J. (1996). Fruiting phenology and fruit set of carob, Ceratonia siliqua L. (Caesalpiniaceae). Israel Journal of Plant Sciences, 44, 359–368.
- Bouseta, A., Collins, S. & Dufour, J. P. (1992). Characteristic aroma profiles of unifloral honey obtained with a dynamic headspace GC-MS System. Journal of Apicultural Research, 31, 96–109.
- Cajka, T., Hajslova, J., Pudil, F. & Riddellova, K. (2009). Traceability of honey origin based on volatiles pattern processing by artificial neural networks. Journal of Chromatography A, 1216, 1458–1462.
- Calvo, M., Guerrero, V. M. & Salvà, B. (2002). Los orígenes del poblamiento balear. Una discusión no acabada. Complutum, 13, 159–191.
- Castro-Vázquez, L., Díaz-Maroto, M. C., González-Viñas, M. A. & Pérez-Coello, M. S. (2009). Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chemistry, 112, 1022–1030.
- Clemente, B. P. & Salas, Z. J. (1996). Análisis polínico de mieles monoflorales comercializadas en España. In B. Ruiz Zapata (Ed.), Estudios palinológicos: XI Simposio Palinología. (A.P.L.E.) (pp. 133–135). Madrid: Universidad de Alcalá de Henares.
- Conti, M. E., Stripeikis, J., Campanella L., Cucina, D. & Tudino, M. B. (2007). Characterization of Italian honeys (Marche Region) on the basis of their mineral content and some typical quality parameters. Chemistry Central Journal, 1, 14.
- Crane, E. (1975). Honey. A comprehensive survey. London: Heinemann, International Bee Research Association.
- Crane, E. (1990). Bee and beekeeping: Science, practice and world resources. New York: Cornell University Press, International Bee Research Association.
- Custódio, L., Serra, H., Nogueira, J. M., Gonçalves, S. & Romano, A. (2006). Analysis of the volatiles emitted by whole flowers and isolated flower organs of the carob tree using HS-SPME-GC/MS. Journal of Chemical Ecology, 32, 929–942.
- Cuevas-Glory, L., Ortiz-Vázquez, E., Pino, A. & Sauri-Duch, E. (2012). Floral classification of Yucatan Peninsula honeys by PCA & HS-SPME/GC-MS of volatile compounds. International Journal of Food Science & Technology, 47, 1378–1383.
- Damblon, F. (1988). Caractérisation botanique, écologique et géographique des miels du Maroc. Institute Francaise de Pondichery, Travaux Section Sciences Technologie, 25, 309–329.
- de Bolòs, O. (1985). Chorology of the flora of Catalan countries. Introductory volume. Barcelona: ORCA, Institut d’Estudis Catalans.
- de Bolòs, O. & Molinier, R. (1984). Vegetation of the Pityusic Islands. In H. Kuhbier, J. A. Alcover & G. Arellano (Eds), Biogeography and ecology of the Pityusic Islands (pp. 185–221). The Hague: W. Junk.
- de Bolòs, O. & Vigo, J. (1984–2001). Flora dels països Catalans (vol. 1–4). Barcelona: Barcino.
- de la Rua, P., Galián, J. & Serrano, J. (2002). Caracterización molecular de las abejas Baleares. Vida Apícola, 112, 45–50.
- de Maria, C. A. B. & Moreira, R. F. A. (2003). Volatile compounds in floral honeys. Química Nova, 26, 90–96.
- Guardia, R., Casas, C. & Ninot, J. M. (1998). Phenological patterns in Mediterranean pastures and scrubs of Catalonia. Acta Botanica Barcinonencia, 45, 557–576.
- Guidotti, M. & Vitali, M. (1998) Identificazione di composti orgnici volatili in miele mediante SPME e GC/MS. Industrie Alimentari, 37, 351–356.
- Guyot, C., Scheirman, V. & Collin, S. (1999). Floral origin markers of heather honeys: Erica arborea and Calluna vulgaris. Food Chemistry, 64(1), 3–11.
- Herrera, J. (1986). Flowering and fruiting phenology in the coastal shrublands of Doñana, south Spain. Vegetatio, 68, 91–98.
- Herrera, J. (1988). Pollination relationships in southern Spanish Mediterranean shrublands. Journal of Ecology, 78, 274–287.
- Jerković, I. & Marijanović, K. (2010). Volatile composition screening of Salix spp. nectar honey: Benzenecarboxylic acids, norisoprenoids, terpenes, and others. Chemistry & Biodiversity, 7, 2309–2325.
- Jones, G. & Bryant, V. M. (2004). The use of EtOH for the dilution of honey. Grana, 43, 174–182.
- Kummerov, J. (1983). Comparative phenology of Mediterranean-type plant communities. In F. J. Kruger, D. T. Mitchell & J. U. M. Jarvis (Eds), Ecological studies 43: Mediterranean-type ecosystems (pp. 300–317). New York: Springer.
- La Serna, I. & Gómez, C. (2006). Pollen and sensorial characterization of different honeys from El Hierro (Canary Island). Grana, 45, 146–159.
- La Serna, I. R., Méndez, B. P. & Gómez, C. F. (1999). Aplicación de nuevas tecnologías en mieles de Canarias para su tipificación y control de calidad. Editorial Confederación Española de Cajas de Ahorro. Madrid: Registro de Empresa Editorial Número 936 del Ministerio de Cultura.
- Lavie, P. (1975). Reparto y valor melífero de las Ericaceas en la cuenca mediterránea. Actas de XXV Congreso Internacional de Apicultura, Grenoble, France, 8–14 September 1975, 423–424.
- Llasat, M. C., Ramis, C. & Barrantes. J. (1996). The meteorology of high-intensity rainfall events over the west Mediterranean region. Remote Sensing Reviews, 14, 51–90.
- Llorens, L., Gil, L. & Tébar, F. J. (2007). La vegetació de l’illa de Mallorca. Bases per a la interpretació d’hàbitats. Palma de Mallorca: Associació Jardí Botànic de Palma.
- Louveaux, J., Maurizio, A. & Vorwohl, G. (1978). Methods of melissopalynology. Bee World, 59, 139–157.
- Martins, R. C., Lopes, V. V., Valento, P., Carvalho, J. C. M. F., Isabel, P., Amaral, M. T., Batista, M. T., Andrade, P. B. & Silva, B. M. (2008). Relevant principal component analysis applied to the characterisation of Portuguese heather honey. Natural Product Research, 22, 1560–1582.
- Maurizio, A. 1939. Untersuchungen zur quantitativen Pollenanalyse des Honigs. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und -hygiene, 30, 27–69.
- Maurizio, A. 1979. Microscopy of honey. In E. Crane (Ed.), Honey: A comprehensive survey (pp. 240–257). London: Heinemann.
- Melliou, E. & Chinou, I. (2011). Chemical constituents of selected unifloral Greek bee-honeys with antimicrobial activity. Food Chemistry, 129, 284–290.
- Mohammed, S. A. & Babiker, E. E. (2009). Protein structure, physicochemical properties and mineral composition of Apis mellifera honey samples of different floral origin. Australian Journal of Basic and Applied Sciences, 3, 2477–2483.
- Montenegro, G., Gómez, M., Casaubon, G., Belancic, A., Mujica, A. M. & Pena, R. C. (2009). Analysis of volatile compounds in three unifloral native Chilean honeys. Phyton, 78, 61–65.
- Ortiz, P. L., Arista, M. & Talavera, S. (1996). Producción de néctar y frecuencia de polinizadores en Ceratonia siliqua L. (Caesalpinaceae). Anales del Jardín Botánico de Madrid, 54, 540–546.
- Pendleton, M. (2006). Descriptions of melissopalynological methods involving centrifugation should include data for calculating Relative Centrifugal Force (RCF) or should express data in units of RCF or gravities (g). Grana, 45, 71–72.
- Pérez, R. A., Sánchez-Brunete, C., Calvo, R. M. & Tadeo, J. L. (2002). Analysis of volatiles from Spanish honeys by solid-phase microextraction and gas chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry, 50, 2633–2637.
- Persano Oddo, L., Piana, L., Bogdanov, S., Bentabol, A., Gotsiou, P., Kerlvliet, J., Martín P., Morlot, M., Ortiz, A., Ruoff, K. & von der Ohe, K. (2004). Botanical species giving unifloral honey in Europe. Apidologie, 35, S82–S93.
- Persano Oddo, L. & Piro, R. (2004). Main European unifloral honeys: Descriptive sheets. Apidologie, 35, S38–S81.
- Persano Oddo, L., Sabatini, A. G., Accorti, M., Colombo, R., Marcazzan, G. L., Piana, M. L. & Pulcini, P. (2000). I mieli uniflorali italiani. Nuove schede di caratterizzazione. Rome: Istituto Sperimentale Zoologia Agraria, Ministerio delle Politiche Agricole e Forestali.
- Piana, M. L., Persano Oddo, L., Bentabol, A., Bruneau, E., Bogdanov, S. & Guyot, C. (2004). Sensory analysis applied to honey: State of the art. Apidologie, 35, S26–S37.
- Piana, M. L., Ricciardelli d’Albore, G. & Isola, A. (1989). La miel. Madrid: Mundi- Prensa.
- Piasenzotto, L., Gracco, L. & Conte, L. (2003). Solid phase microextraction (SPME) applied to honey quality control. Journal of the Science of Food and Agriculture, 83, 1037–1044.
- Pons, M. (2002). Estudis sobre la biologia de la reproducció de les brolles i de les timonedes (Rosmarinus officinalis) de les Pitiuses. Palma: Universitat de les Illes Balears, PhD Diss.
- Radovic, B. S., Careri, M., Mangia, A., Musci, M., Gerboles, M. & Anklam, E. (2001). Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chemistry, 72(4), 511–520.
- Ramón-Laca, L. & Mabberley, D. J. (2004). The ecological status of the carobtree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Botanical Journal of the Linnean Society, 144, 431–436.
- Retana, J., Ramoneda, J., García del Pino, F. & Bosh, J. (1994). Flowering phenology of carob, Ceratonia siliqua L. (Caesalpinaceae). Journal of Horticultural Science, 69, 97–103.
- Ricciardelli d’Albore, G. (1998). Mediterranean melissopalynology. Perugia: Institute of Agricultural Entomology, University of Perugia.
- Ricciardelli d’Albore, G. & Intoppa, F. (2000). Fiori e api. La flora visitada dalle api e dagli altri Apoidei in Europa. Bologna: Calderini Edagricole.
- Ricciardelli d’Albore, G. & Vorwohl, G. (1979). Mieles monoflorales en el Mediterráneo documentado con ayuda del análisis microscópico de mieles. Actas de XXVII Congreso Internacional de Apicultura, Athens, Greece, 14–20 September 1979, 201–208.
- Rivas-Martínez, S. (1983). Pisos bioclimáticos de España. Lazaroa, 5, 33–43.
- Rivas-Martínez, S. (1995). Clasificación bioclimática de la tierra. Folia Botanica Matritensis, 16, 1–26.
- Rivas-Martínez, S. (1997). Syntaxonomical synopsis of the North American natural potential vegetation communities, I. Itinera Geobotanica, 10, 5–148.
- Sà-Otero, M. P., Ramil Rego, P. & Aira, M. J. (1991). Análisis polínico de las mieles procedentes de las provincias de Lugo y Ourense (NW España). Nova Acta Científica Compostelana, 2, 57–63.
- Sà-Otero, M. P., Armesto-Batzan, S. & Díaz-Losada, E. (2006). A study of variation in the pollen spectra of honeys sampled from the Baixa Limia-Serra do Xurés Nature Reserve in north-west Spain. Grana, 45, 137–145.
- Sáenz Lain, C. & Gómez Ferreras, C. (2000). Mieles españolas. Características e identificación mediante el análisis del polen. Madrid: Mundi-Prensa.
- Santas, L. A. & Bikos, A. A. (1979). The apicultural flora of Greece. Apiacta, 14, 115–123.
- Seijo, M. C., Aira, M. J., Iglesias, I. & Jato, V. (1992). Palynological characterisation of honey from La Coruña province (NW Spain). Journal of Apicultural Research, 36, 133–139.
- Terrab, A., Díez, M. J. & Heredia, F. J. (2003a). Palynological, physico-chemical and colour characterization of Moroccan Honeys: III. Other unifloral honeys type. International Journal of Food Science and Technology, 38, 395–402.
- Terrab, A., Díez, M. J. & Valdés, B. (2000). Análisis polínico de mieles de la zona noroccidental de Marruecos: Región del Rif Occidental. Lagascalia, 21, 323–334.
- Terrab, A., Pontes, A., Heredia, F. J. & Díez, M. J. (2004). A preliminary palynological characterization of Spanish thyme honeys. Botanical Journal of the Linnean Society, 146, 323–330.
- Terrab, A., Valdés, B. & Díez, M. J. (2001). Análisis polínico de mieles de la zona noroccidental de Marruecos: región de Targuist. Lazaroa, 22, 51–58.
- Terrab, A., Valdés, B. & Díez, M. J. (2002). Análisis polínico de mieles en la región del Alto Ouerrha (noroeste de Marruecos). Lagascalia, 22, 21–33.
- Terrab, A., Valdés, B. & Díez, M. J. (2003b). Pollen analysis of honeys from the Mamora forest region (NW Morocco). Grana, 42, 47–54.
- The Council of the European Union. (2002). Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities, L 10, 47–52.
- Thompson, J. D. (2005). Plant evolution in the Mediterranean. Oxford: Oxford University Press.
- Verzera, A., Campisi, S., Zappalà, M. & Bonaccorsi, I. (2001). SPME-GC-MS analysis of honey volatile components for the characterization of different floral origin. American Laboratory, 33, 18–21.
- von der Ohe, W., Persano Oddo, L., Piana, M. L., Morlot, M. & Martín, P. (2004). Harmonized methods of melissopalynology. Apidologie, 35, S18–S25.
- Vorwohl, G. (1964) Die Beziehungen zwischen der elektrischen Leitfähigkeit der Honige und ihrer trachtmässigen Herkunft. Annales de l’Abeille, 7, 301–309.
- Vorwohl, G. (1967). The microscopic analysis of honey, a comparison of its methods with those of the other branches of palynology. Review of Palaeobotany and Palynology, 3, 287–290.
- Wolski, T., Tambor, K., Rybak-Chmielewska, H. & Kêdzia, B. (2006). Identification of honey volatile components by solid phase microextraction (SPME) and gas chromatography/mass spectrometry (GC/MS). Journal of Apicultural Science, 50, 115–125.