Abstract
Honey samples from apiaries located in the province of Catamarca, Argentina, were analysed. Most of the territory is arid and covered by mountains (80%). Beekeeping is situated in the arid Chaco and Yunga regions. A total of 86 pollen types, belonging to 39 families, were identified from the honey samples and 68 (79%) were native flora. Ten types were classified as predominant pollen types and were present in the monofloral honeys (n = 18), Prosopis spp., Cercidium praecox, Larrea divaricata and Tournefortia lilloi are those with the highest frequency. Pollen from Fabaceae and Asteraceae has the greatest representation in honey, with 14 and 11 types, respectively. Most of the honey samples correspond to groups II (normal pollen grain content, 39%) and III (rich in pollen grain content, 30%). The honeys analysed had a typical pollen array of arid Chaco and southern Andean Yungas flora. The present research has contributed to describe new types of monofloral honeys from native plants. This finding points to the potential commercial worth of ethnobotanical studies in the honey market and may help future investigations, which need to establish conservation categories for endemic species.
Pollination ecology and melissopalynology, the study of pollen content in honey, share a link through the fertilisation of plants. Quality characterisation of honey includes pollen identification and quantification that are regularly studied to ensure the nectar source. In fact, the level of anthropogenic influence and the existence of wild flora could be evaluated since honey may be used as a bio-indicator of environment conservation (Kevan, Citation1999; Lambert et al., Citation2012). Thus, data from melissopalynological analyses of honey from Catamarca could be useful to evaluate environmental health.
Honey from Catamarca offers a unique opportunity to evaluate the conservation status of the native flora from the different regions since honey bee colonies utilise native flora, where a diversity of pollen is provided. Some of the richest sources of pollen and nectar are Prosopis spp., Larrea spp., Geoffraea decorticans (Gillies ex Hook. et Arn.) Burkart and Cercidium praecox (Ruiz et Pav. ex Hook.) Harms ssp. praecox (Salgado & Pire, Citation1998; PROSAP, Citation2007).
Beekeeping is situated in regions that can be ascribed to a variety of eco-regions with a distinctive flora each. Dense forested areas of the southern Andean Yungas flora [between 300–500 and 900 m above sea level (a.s.l.)] have recently been characterised (Hilgert & Gil, Citation2006; Sánchez & Lupo, Citation2009). The main species are Anadenanthera colubrina (Vell.) Brenan var. cebil (Griseb.) Altschul, Schinopsis lorentzii (Griseb.) Engl.; less frequently found are Enterolobium contortisiliquum (Vell.) Morong, Juglans australis Griseb. and Sapium haematospermun Müll. Arg., Tabebuia nodosa (Griseb.) Griseb. and Pentapanax angelicifolius Griseb. Also, shrub flora like Acacia tucumanensis Griseb., Tournefortia lilloi I.M.Johnst. and Duranta serratifolia (Griseb.) Kuntze are present (Palmieri et al., Citation2010). Chaco arid and semi-arid regions are characterised by tree species such as Schinopsis marginata Engl., Acacia visco Lorentz ex Griseb. as main species and Celtis ehrenbergiana (Klotzsch) Liebm., Lithraea molleoides (Vell.) Engl., Geoffraea decorticans, Ziziphus mistol Griseb., Prosopis alba Griseb. var. alba, Prosopis nigra (Griseb.) Hieron. var. nigra, Prosopis kuntzei Harms and Zanthoxylum coco Gillies ex Hook.f. et Arn., but also shrub species such as Mimosa detinens Benth., Condalia microphylla Cav., Maytenus viscifolia Griseb., Schinus spp., Capparis atamisquea Kuntze, Acacia spp. and Mimosa ephedroides (Gillies ex Hook. et Arn.) Benth. (PROSAP, Citation2007; Palmieri et al., Citation2010).
The Chaco district flora is loosely related with the Yunga flora, which has denser forest vegetation. Some species are present in both regions, while others are distinctive of only one of them, i.e., Prosopis spp. (Algarrobo), Acacia spp. (Espinillo, Garabato), Condalia microphylla (Piquillín) and Schinopsis marginata (Orco Quebracho) are present in the former, while Tournefortia lilloi (Alcanflor), Anadenanthera colubrina var. cebil (Cebil) and Schinopsis lorentzii (Quebracho Colorado) in the latter (Palmieri et al., Citation2010).
Melissopalynologycal study from different phytogeographic regions, i.e. the Pampa region (Tellería, Citation1988, Citation1993), the Espinal region (Costa et al., Citation1995; Andrada & Tellería, Citation2005), the Monte region (Tellería & Forcone, Citation2000), the Subantarctic region (Forcone et al., Citation2005) and the east district of the Chaco region (Salgado & Pire, Citation1998) have been reported. Data on the botanical origin of honey produced in Catamarca are scarce and unpublished in international or national scientific journals.
In this study, 33 honey samples from apiaries located in different phytogeographic regions were analysed and constitute the first contribution of pollen analyses in the honeys from Catamarca, Argentina.
Material and methods
Description of the study sites
Catamarca is a province of Argentina, located in the northwest of the country, that spans 25º–30° S and among 69º–65° W (). Eighty per cent of the territory is characterised by mountains, and it is an arid and semi-arid zone. Four different ecosystems can be distinguished: the Pampean sierras, the Narváez-Cerro Negro-Famatina system, the Cordilleran–Catamarca area of transition and the Puna system, an elevated portion in the northwest (Morláns, Citation1995; Gutiérrez et al., Citation2010).
Figure 1. Depiction of the study sites. A. Map showing location of the study area. B. A representative site of the Chaco region C. A representative site of the Yunga region.
The sites of honey collection were situated in eco-regions of the Yunga (southern Andean Yungas, NT0165) and Chaco (Chaco Occidental, NT0210; Arid Chaco, NT0701; Chaco Serrano, NT0706). The eco-regions denominations and codes used are those of the World Wildlife Fund, consisting of two letters and two sets of two numbers (realm code + biome code + specific eco-region code, thus NT means neotropic) (Olson, Citation2001).
Samples and honey analyses
Thirty-three artisanal honey samples from Apis mellifera L. were collected by beekeepers that are registered with the RENAPA (Argentinean National Registry of Beekeepers), in the southeast of Catamarca during the summer of 2008 to 2009. Honeys were collected from eight different departments: Andalgalá (n = 3), Capayán (n = 14), Fray Mamerto Esquiú (n = 1), La Paz (n = 3), Paclín (n = 5), Santa Rosa (n = 4), Tinogasta (n = 1) and Valle Viejo (n = 2). Sampling occurred from the end of 2008 to the beginning of 2009. The honey was obtained by centrifugation and stored at room temperature until analysis.
Melissopalynological qualitative studies were done as previously reported (Louveaux et al., Citation1978). A sample of 10 g honey was dissolved in 50 ml distilled water, centrifuged at 1500 g for 10 min, washed once with distilled water and acetolysed (Erdtman, Citation1960). Pollen sediment was mounted in glycerine jelly and sealed with paraffin. To determine frequency classes, 500 pollen grains were counted per sample (Louveaux et al., Citation1970). The four following categories were used for frequency classes: predominant pollen (D, more than 45% of pollen grains counted), secondary pollen (S, 16–45%), important minor pollen (M, 3–15%) and minor pollen (T, 1–3%).
Pollen types were identified by comparing with a reference collection that was made from the plants in the area surrounding the beehives. A pollen atlas by Markgraf and d’Antoni (Citation1978) was also consulted. The reference collection is housed in the Palynotheca of the Facultad de Ciencias Exactas, Físicas y Naturales of the Universidad Nacional de Córdoba. The herbarium specimens recollected were deposited in the Cátedra de Palinología of the faculty. Mimosa ephedroides pollen was classified along with the following identification characteristics: ovoid dissymmetric bitetrads, diameter 18 μm; pyramidal monads 3-porate, distinctly aspidate; thin exine (Caccavari, Citation1985, Citation2002). The pollen types were identified at species level whenever possible and otherwise to genus or family ranks.
When one pollen type was represented ≥ 45% of the total number of pollen grains, the sample was classified as a monofloral honey (Maurizio & Louveaux, Citation1961).The frequency occurrence of pollen is expressed as a percentage. It was calculated by totalling the number of samples, in which a taxon occurs and dividing the result by the total number of samples.
Quantitative analysis was done following the methodology previously described (Moar, Citation1985) spiking with tablets of Lycopodium clavatum L. spores. A subsample of 10 g honey was dissolved in distilled water, and two L. clavatum tablets (dissolved in 5 ml 5% hydrochloric acid) were added, each containing 12 000 ± 200 spores. The sediment was concentrated by repeated centrifugation at 1500 g for 10 min, mounted in glycerine jelly, and sealed with paraffin. In order to obtain the percentage and frequency classes of spores and pollen taxa, up to 500 pollen grains were counted. Pollen concentration was derived from the ratio of total pollen × Lycopodium spores added to the number of Lycopodium spores counted. Honeydew elements were not counted.
Honey was classified as follows: Group I (< 20 000 grains), Group II (20 000–100 000 grains), Group III (100 000–500 000 grains), Group IV (500 000–1 000 000 grains), Group V (> 1 000 000 grains) (Maurizio, Citation1975).
Microphotography of pollen and honey samples was done with an Olympus FV 300 spectral confocal microscope equipped with a 40× (NA: 0.75) and a 60× (NA: 1.42) Uplan SApo oil-immersion objectives. Fluorescence images were acquired using a 488 nm Argon or 543 nm Helium-Neon laser excitation line. Emission was collected between 510–540 nm and 565–610 nm, respectively. In each independent experiment, the PTM voltage, gain and offset were calibrated in accordance with the auto-fluorescence and secondary controls, with similar parameters for all experiments. The confocal aperture (CA) was set at 100 μm. Z-stacks were acquired and retinal image slices were used for making two-dimensional (2D) projections with the BioImage software using the maximum intensity projection method.
Results
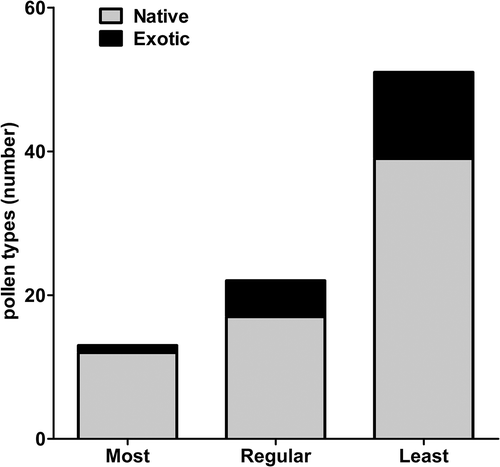
A total of 86 pollen types belonging to 39 families were identified in the honey samples, 68 (79% from the total) of which were from native flora (). An analysis to discriminate the most frequent pollen species (species found in ten or more samples) revealed only one exotic pollen type (Eucalyptus sp., 8%). In contrast, an analysis of the least frequent species (found in four or less samples) revealed 12 exotic plant species (24%) ().
Figure 2. Level of native and exotic plants in honey samples grouped by frequency of appearance: Most (found in ten or more honey samples), Regular (between nine and four) and Least (less than four).
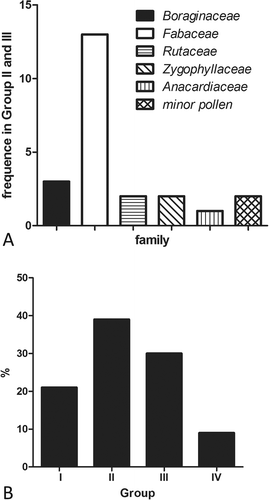
Ten taxa, classified as predominant pollen types, were present in the monofloral honeys (n = 18). Three of these were from the most frequent species (Prosopis spp., Cercidium praecox and Larrea divaricata Cav.; , ). When species were grouped by life habit (trees, shrub, herbs and bindweeds), it was observed that pollen types mainly represented trees and shrubs (60%). Bindweeds such as Tripodanthus sp., Clematis sp. or Cardiospermum spp., were less represented in honey.
Figure 3. Predominant (D) pollen types found in honey that was classified as monofloral honeys. Horizontal bars represent the percentage of a taxon’s pollen in the monofloral honeys. The last bar represents the percentage of multifloral honeys in the samples examined.
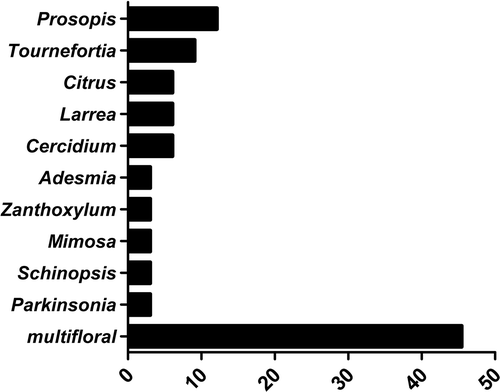
Pollen from the families Fabaceae and Asteraceae were most common in the honeys, with 14 and 11 types, respectively (). Lamiaceae and Anacardiaceae were represented with four pollen types each (). Native species were present in most of the samples, with Prosopis sp. occurring in 88% of the samples, Larrea in 82% of the samples, Mimosa in 76% of the samples, Cercidium in 70% of the samples and Acacia and Baccharis in 52% of the samples (, ). The diversity of pollen types ranged from six to 23 different types in the whole population analysed ().
Honey samples from the Yunga region represented 24% of the samples (eight out of 33 samples). These honeys had a distinctive pollen pattern due to the content of Tournefortia lilloi in the samples from Alijilán, being the predominant pollen in 75% of them and present in 62% of the Yunga honey samples (). Other pollen types found in the honeys of this region were Myrcianthes mato (Griseb.) McVaugh and Hyptis mutabilis (Rich.) Briq. (). In two samples from Palo Labrado (881 m a.s.l.), Zanthoxylum coco pollen was found.
The pollen types more frequently found in honey samples from the Chaco region were Prosopis spp. and Mimosa spp. (92%; ), Cercidium praecox (68%) and Larrea divaricata (60%) of the samples from the region (). Pollen from Gomphrena pulchella Mart., Geoffraea decorticans, Mimosa spp. and Senna aphylla (Cav.) H.S.Irwin et Barneby, were present in about 30% of these honey samples and were much less frequent in Yunga honey samples (less than 13%). Geoffraea decorticans was not present in honeys from the Yunga region.
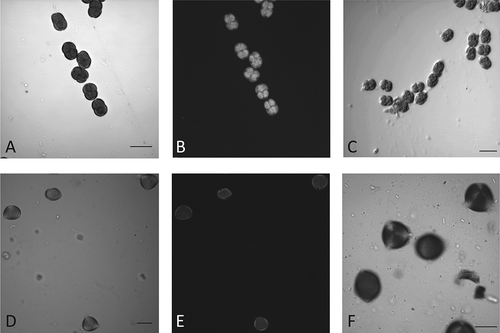
Figure 4. Pollen content of monofloral honeys of Mimosa sp. (A–C) and Prosopis sp. (D–F). Microphotgraph of honey samples (A, B, D, E) are compared against reference standards of Mimosa sp. (C) or Prosopis sp. (F). A, C, D, F are 2D projections of a Z-stack taken with a 40× objective. B, E are auto-fluorescence views from de equatorial section of A and D, respectively. Scale bars – 20 μm.
Quantitative analysis demonstrates that most honey samples correspond to groups II (39%) and III (30%), mainly containing predominant or secondary pollen from the Fabaceae family, while samples classified as groups IV and I were the least frequent (9% and 21%, respectively; ).
Table I. Pollen types identified in the 33 honeys analysed, their frequency classes and pollen diversity
Discussion
This report is the first melissopalynological study about honey from Catamarca. Here, nectar sources come from native species mostly indicating that the territory origin of the samples had little anthropogenic influence. Our data support the relationship between the presence of wild flora pollen in honey and environment conservation (Kevan, Citation1999; Lambert et al., Citation2012).
One important finding was the high percentage (79% from the total) of native flora pollen identified in the honey samples; native nectariferous taxa were the predominant pollen in 48.5% of the samples and represented 89% in the monofloral honeys.
The presence of Tournefortia lilloi in monofloral honey samples was unexpected, since this species is characteristic of the Yunga region and has not previously been described in Catamarca. This finding is of interest since T. lilloi has medical importance (Hilgert & Gil, Citation2006). The relationship between secondary metabolites of the nectariferous flora and honey is a subject now under research (Gil et al., Citation1995; Iurlina et al., Citation2009; Alvarez-Suarez et al., Citation2012; Jasicka-Misiak et al., Citation2012).
Asteraceae along with Fabaceae had the highest pollen diversity in the honey samples analysed. This result is in line with the high pollen diversity of Asteraceae in honeys from the Yungas montane forest (Jujuy, Argentina; Sánchez & Lupo, Citation2009).
Pollen content of honeys from Catamarca differed from that of the Pampa phytogeographic province because of the high percentage of the native tree and shrub species present. In contrast, honey from the central and south Argentinean Pampa have mainly pollen from introduced herbs (Tellería, Citation1988; Forcone, Citation2008).
The analysed honey samples had some pollen types from genera like Larrea, Prosopis and Schinus that also were found in honeys from the Espinal phytogeographic region (Costa, Citation1982; Costa et al., Citation1995; Fagúndez & Caccavari, Citation2006). Furthermore, high frequency of pollen types from taxa like Schinus spp., Baccharis spp., Prosopis spp., Mimosa spp., Acacia spp., Larrea divaricata and Aloysia gratissima (Gillies et Hook. ex Hook.) Tronc. were found in honeys from other districts of the Chaco phytogeographic region (Salgado & Pire, Citation1998; Pistone & Costa, Citation2007).
Our results are in line with those of Aizen and Feinsinger (Citation1994) studying native insect pollinators, since they found Prosopis and Cercidium dominated the entomophilous flora of the sites in subtropical dry forest (Chaco serrano) of the Tucumán province, north-western Argentina. However, several differences could be found when the comparison was done with other Chaco districts that have high frequency of taxa like Eryngium spp., Acicarpha tribuloides Juss., Sapium haematospermum Müll. Arg., Tabebuia type, Arecaceae and Polygonum spp. (Fagúndez & Caccavari, Citation2006). These taxa have also been found in Brazilian honeys (Barth, Citation2004).
The presence of pollen from Mimosa ephedroides (Mimosoideae) seems to be a distinctive feature of honeys from Catamarca, since it is not found in honeys from other regions of Argentina (Aizen & Feinsinger, Citation1994; Costa et al., Citation1995). This characteristic must be evaluated in the future to ascertain if it can be used as an indicator of geographical origin because although Mimosa ephedroides is an endemic plant in Catamarca (Burkart, Citation1948), it was absent in honeys from Alijilán (Yunga region).
In México and Brazil, Mimosa species have been reported as a nectar source (Barth, Citation2004; Ramírez-Arriaga et al., Citation2011). Moreover, in the south-eastern region of Brazil, the presence of several species of Mimosa pollen in honey samples have been established (Barth, Citation2004). Recently, it has been suggested that Mimosa is not solely a polleniferous resource as has been previously thought (Villanueva-Gutiérrez et al., Citation2009; Ramírez-Arriaga et al., Citation2011).
Asteraceae and Myrtaceae monofloral honeys were reported in the south of Brazil (Barth, Citation2004). Contrary to those results, Asteraceae and Myrtaceae honeys were poorly represented in this study, since they were recorded only as minor or trace components in the samples analysed.
Melissopalynological research could aid to identify nectariferous plants characteristic of arid phytogeographic regions that are important to the ecosystem and might help beekeepers to preserve and recover some affected vegetation types. This may be accomplished by reforesting with relevant melliferous native plants.
Conclusion
Honey from Catamarca can be distinguished from those of other areas of Argentina by melissopalynologycal analysis. Pollen associations from native plants, including Prosopis and Mimosa, could be markers of honey from this Argentinean province; our group is extending the research to other areas of Catamarca to ascertain this hypothesis.
Honey from the Yunga region of Catamarca is distinguished by the frequent occurrence of pollen types from species distinctive of this region such as Tournefortia lilloi, Schinus areira and Myrcianthes mato. In contrast, honey from the arid and semi-arid Chaco contains pollen types such as Mimosoideae, Larrea spp., Cercidium praecox and Astereae. Finally, it is concluded that the pollen spectrum of the honey indicates that honeybees (Apis mellifera) foraged pollen and nectar mainly from the native flora of each region. The present study may contribute to extend the production of monofloral honeys from wild flora, and points to the potential commercial worth of ethnobotanical studies on the honey market and may help future investigations, which need to establish conservation categories for endemic species.
Acknowledgements
Estela Pistone and María José Loyola from Área de Proyectos Especiales are thanked for their technical support. V.A.V.R. received a postgraduate scholarship of the Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Catamarca. Proyecto Federal de Innovación Productiva-PFIP: Desarrollo y fortalecimiento de laboratorio para la determinación del origen botánico, caracterización física, química y microbiológica de miel producida en Catamarca. Help and support of the beekeepers of the Catamarca province and of Alejandro Álvarez of the Instituto Nacional de Tecnología Agropecuaria-INTA, are greatly acknowledged.
References
- Aizen, M. A. & Feinsinger, P. (1994). Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine ‘Chaco Serrano’. Ecological Applications, 4, 378–392.
- Alvarez-Suarez, J. M., Giampieri, F., González-Paramás, A. M., Damiani, E., Astolfi, P., Martinez-Sanchez, G., Bompadre, S., Quiles, J. L., Santos-Buelga, C. & Battino, M. (2012). Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food and Chemical Toxicology, 50, 1508–1516.
- Andrada, A. & Tellería, M. C. (2005). Pollen collected by honey bees (Apis mellifera L.) from south of Caldén distrito (Argentina): Botanical origin and protein content. Grana, 44, 1–8.
- Barth, O. M. (2004). Melissopalynology in Brazil: A review of pollen analysis of honeys, propolis and pollen loads of bees. Scientia Agricola, 61, 342–350.
- Burkart, A. (1948). Las especies de Mimosa de la Flora Argentina. Darwiniana, 8, 9–231.
- Caccavari, M. A. (1985). Granos de polen de leguminosas de la Argentina. IV. Genero Mimosa. Boletín de la Sociedad Argentina de Botánica, 24, 151–167.
- Caccavari, M. A. (2002). Pollen morphology and structure of Tropical and Subtropical American genera of the Piptadenia-group (Leguminosae: Mimosoideae). Grana, 41, 130–141.
- Costa, M. C. (1982). Contribución al conocimiento de la flora melífera de la provincia de Córdoba. I. Departamento Río Segundo. Boletín de la Sociedad Argentina de Botánica, 21, 247–258.
- Costa, M. C., Decolati, N. & Godoy, E. (1995). Análisis polínico en mieles del norte de la Provincia de San Luis (Argentina). Kurtziana, 24, 133–144.
- Erdtman, G. (1960). The acetolysis method. A revised description. Svensk Botanisk Tidskrift, 54, 561–564.
- Fagúndez, G. A. & Caccavari, M. A. (2006). Pollen analysis of honeys from the central zone of the Argentine province of Entre Ríos. Grana, 45, 305–320.
- Forcone, A. (2008). Pollen analysis of honey from Chubut (Argentinean Patagonia). Grana, 47, 147–158.
- Forcone, A., Ayestarán, G., Kutschker, A. & Garcia, J. (2005). Palynological characterisation of honeys from the Andean Patagonia (Chubut- Argentina) Grana, 44, 202–208.
- Gil, M. I., Ferreres, F., Ortiz, A., Subra, E. & Tomas-Barberan, F. A. (1995). Plant phenolic metabolites and floral origin of rosemary honey. Journal of Agricultural and Food Chemistry, 43, 2833–2838.
- Gutiérrez, P. R., Zavattieri, A. M., Ezpeleta, M. & Astini, R. (2010). Palynology of the La Veteada Formation (Permian) in the Sierra de Narváez, Catamarca Province, Argentina. Ameghiniana, 48, 154–176.
- Hilgert, N. & Gil, G. (2006). Medicinal plants of the Argentine Yungas plants of the Las Yungas biosphere reserve, northwest of Argentina, used in health care. Biodiversity and Conservation, 15, 2565–2594.
- Iurlina, M. O., Saiz, A. I., Fritz, R. & Manrique, G. D. (2009). Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chemistry, 115, 1141–1149.
- Jasicka-Misiak, I., Poliwoda, A., Dereń, M. & Kafarski, P. (2012). Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chemistry, 131, 1149–1156.
- Kevan, P. G. (1999). Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agriculture, Ecosystems & Environment, 74, 373–393.
- Lambert, O., Veyrand, B., Durand, S., Marchand, P., Bizec, B. L., Piroux, M., Puyo, S., Thorin, C., Delbac, F. & Pouliquen, H. (2012). Polycyclic aromatic hydrocarbons: Bees, honey and pollen as sentinels for environmental chemical contaminants. Chemosphere, 86, 98–104.
- Louveaux, J., Maurizio, A. & Vorwhol, G. (1970). Methods of melissopalynology by International Commission for Bee Botany of IUBS. Bee World, 51, 125–138.
- Louveaux, J., Maurizio, A. & Vorwohl, G. (1978). Methods of melissopalynology by International Commission for Bee Botany of IUBS. Bee World, 59, 139–157.
- Markgraf, V. & d’Antoni, H. (1978). Pollen flora of Argentina. Tucson, AZ: University of Arizona Press.
- Maurizio, A. (1975). Microscopy of honey. In E. Crane (Ed.), Honey: A comprehensive survey (pp. 240–257). London: Heinemann.
- Maurizio, A. & Louveaux, J. (1961). Pollens de plantes mellifères d’Europe. Pollen et Spores II, 3, 219–246.
- Moar, N. T. (1985). Pollen analysis of New Zealand honey. New Zealand Journal of Agricultural Research, 28, 39–70.
- Morláns, M. C. (1995). Regiones Naturales de Catamarca, Provincias Geológicas y Provincias Fitogeográficas. Revista de Ciencia y Técnica- Área Ecología, II, 1–42.
- Olson, D. M. (2001). Terrestrial ecoregions of the world: A new map of life on earth. BioScience, 51, 933–938.
- Palmieri C. N., Carma, M. I. & Quiroga A. (2010). Las ecorregiones presentes en Catamarca. Atlas Catamarca. San Fernando del Valle de Catamarca: Gobierno de la Provinci a de Catamarca. ETISIG Catamarca. http://www.atlas.catamarca.gov.ar
- Pistone, E. & Costa, M. C. (2007). Vinculación entre el contenido polínico de mieles y la flora de la provincia fitogeografica chaqueña. In Universidad Tecnológica Nacional (Ed.), Terceras Jornadas de Economía Ecológica (pp. 56–56). Tucumán: Facultad Regional Tucumán.
- Programa de Servicios Agrícolas Provinciales (PROSAP). (2007). Tipos de mieles. Secretaría de Agricultura, Ganadería, Pesca y Alimentación. Buenos Aires: Ministerio de Economia Argentina.
- Ramírez-Arriaga, E., Navarro-Calvo, L. A. & Díaz-Carbajal, E. (2011). Botanical characterisation of Mexican honeys from a subtropical region (Oaxaca) based on pollen analysis. Grana, 50, 40–54.
- Salgado, C. R. & Pire, S. M. (1998). Análisis polínico de mieles del Noroeste de la provincia de Corrientes (Argentina). Darwiniana, 36, 87–93.
- Sánchez, A. C. & Lupo, L. C. (2009). Asteraceae de interés en la Melisopalinogía: Bosque montano de las yungas (Jujuy, Argentina). Boletín de la Sociedad Argentina de Botánica, 44, 57–64.
- Tellería, M. C. (1988). Analyse pollinique des miels du nord-ouest de la Province de Buenos Aires (République Argentine). Apidologie, 19, 275–290.
- Tellería, M. C. (1993). Floraison et récolte du pollen par les abeilles domestiques (Apis mellifera L. var. ligustica) dans la pampa argentine. Apidologie, 24, 109–120.
- Tellería, M. C. & Forcone, A. (2000). El polen de las mieles del Valle de Río Negro, Provincia Fitogeográfica del Monte (Argentina). Darwiniana, 38, 273–277.
- Villanueva-Gutiérrez, R., Moguel-Ordóñez, Y. B., Echazarreta-González, C. M. & Arana-López, G. (2009). Monofloral honeys in the Yucatán Peninsula, Mexico. Grana, 48,214–223.