Abstract
An ongoing investigation of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin continues to show that it contains an extremely rich assemblage of angiosperm taxa. The Fagales to Rosales pollen record documented here contains 34 different taxa belonging to the Betulaceae (Alnus, Betula, Carpinus, Corylus, Ostrya), Fagaceae (Castanea, Fagus, Quercus Groups Cerris, Ilex, Cyclobalanopsis, Quercus/Lobatae), Juglandaceae (Engelhardioideae, Carya, Juglans, Pterocarya), Myricaceae (Morrella vel Myrica), Cannabaceae (Celtis), Elaeagnaceae (Elaeagnus), Rhamnaceae, Rosaceae (Prunus) and Ulmaceae (Cedrelospermum, Ulmus, Zelkova). Two of the pollen types represent extinct genera, Trigonobalanopsis and Cedrelospermum, and are also reported for the first time from the Lavanttal Basin along with pollen of Rhamnaceae and Prunus. The different types of Quercus pollen are now affiliated with Groups Cerris, Cyclobalanopsis, Ilex and Quercus/Lobatae based on sculpturing elements observed using scanning electron microscopy (SEM). Köppen signatures of potential modern analogues of the fossil Fagales and Rosales suggest a subtropical (Cfa, Cwa) climate at lower elevation and subsequent subtropical to temperate climate with altitudinal succession (Cfa → Cfb/Dfa→ Dfb; Cwa → Cwb → Dwb) in the Lavanttal area during accumulation of the palynoflora. Most of the fossil taxa have potential modern analogues that can be grouped as nemoral and/or merido-nemoral vegetation elements, and the diversity of Fagales indicates a varying landscape with a high variety of niches.
This contribution is the fourth part of a series of papers (Grímsson & Zetter Citation2011; Grímsson et al. Citation2011, Citation2015a) on the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria. It completes the record of an important subclade of the fabid (rosid I) clade within the rosids: the nitrogen-fixing clade. The nitrogen-fixing clade includes four angiosperm orders (APG Citation2009): Fabales, Rosales, Cucurbitales and Fagales. The Fabales have already been covered in the third part (Grímsson et al. Citation2015a). No member of Cucurbitales has been found in the Lavanttal assemblage. Hence, this paper will cover the remaining members of the nitrogen-fixing clade, palynomorphs that can be assigned to either the Fagales or Rosales. In total, eight families, 16 extant and two extinct genera, and a total of 34 pollen taxa are covered in the current study. Members of the Fagales can be particularly informative for palaeoclimatic and ecological conditions. Hence, we used the recently proposed ‘Köppen signatures’ (Denk et al. Citation2013), which aim at characterising the general climatic niche of an extant or extinct taxon. The ‘Köppen signature’ of a taxon describes in which general climate zones (using the Köppen-system) the taxon can be found today (e.g. Cfa-Cfb; warm temperate, fully humid climates with warm to hot summers), or thrived in the past. By summarising the ‘Köppen signatures’ of all elements in a palaeoflora, general climatic trends can be identified (e.g. locally delayed Miocene cooling in the northern North Atlantic; see Denk et al. Citation2013). In most cases, this involves choosing modern analogues to represent fossil taxa. However, it is (theoretically) possible to score a fossil taxon decoupled from modern analogues based on available secondary evidence. Using ‘Köppen signatures’ already established for a number of modern species, which are potential modern analogues of the Lavanttal taxa and including a substantial number of species so far not covered, we draw preliminary conclusions about the climate and ecology of the Lavanttal flora (a final, comprehensive assessment will be done in the final series contribution).
Material and methods
For a detailed account on the geographical position, geology and age of the Lavanttal Basin and its surroundings, sedimentology, palaeoenvironment, preservation of organic matter, sediment sampling and methods used for studying the palynomorphs, see Grímsson et al. (Citation2011). For descriptions of ginkgophytes and gnetophytes and their ecological implication as well as fossil records, see Grímsson et al. (Citation2011). For corresponding information on the conifer pollen from the Lavanttal Basin, see Grímsson and Zetter (Citation2011), and for information on pollen of the Magnoliales to Fabales, see Grímsson et al. (Citation2015a).
Nomenclatural conventions for climate and vegetation zones
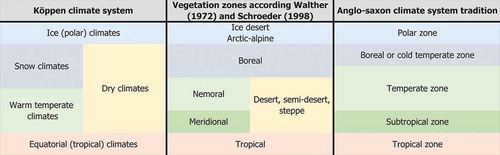
We apply three nomenclatural conventions/systems that serve different aspects of climate and vegetation (): (a) the Köppen climate system, which distinguishes climate zones by certain abiotic parameters or combinations thereof (see Kottek et al. Citation2006) and represents them in a three letter code referring to the general climate types (first letter), the seasonal distribution of precipitation (second letter) and the seasonal distribution or general level of warmth (third letter; ). (b) The general categorisation of vegetation zones according to Walter (Citation1973) and Schroeder (Citation1998), distinguishing five major latitudinal-altitudinal vegetation belts on the Northern Hemisphere: the tropical zone, the meridional zone, the nemoral zone, the boreal zone and the artic-alpine zone, and the commonly used terminology for latitudinal climate belts in Anglo-saxon literature, which recognises a subtropical climate zone in addition to the tropical, temperate and polar zones. shows (roughly) the correlation of the different concepts.
Table I. Explanation of the three letter code used in the Köppen climate classification.
Figure 1. Generalised climate and vegetation systems for the Northern Hemisphere used in this study. Shown is how the Köppen climate system and its Anglo-Saxon modification correlates with the vegetation zones used by Walther (1973) and Schroeder (Citation1998), in order to establish synonymy of terminologies.
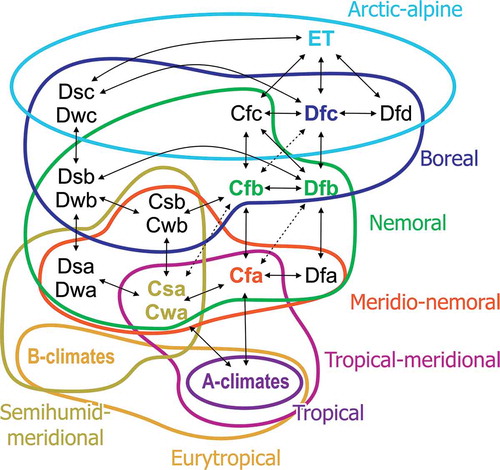
Climatic and ecological categorisation of modern species used as modern analogues for determined fossil pollen taxa
We used the recently proposed ‘Köppen signatures’ (Denk et al. Citation2013) to summarise the climatic niche occupied by potential modern analogues (species groups, genera) of the determined pollen taxa as provided in the ClimGrim database and updated for not yet covered species. We further categorised the modern species, the group of potential modern analogues, as vegetation elements (‘generalists’, ‘meridio-nemoral’, ‘nemoral’, ‘boreal’, ‘arctic-alpine’; ; see Denk et al. Citation2013) and include three new categories (based on Schroeder Citation1998; see Velitzelos et al. Citation2014, table 23) to accommodate species with hygric and thermic preferences (availability of water and warmth) not covered by the data set used by Denk et al. (Citation2013): (i) ‘tropical’ – species restricted to tropical A-climates; (ii) ‘tropical-meridional’ – species occurring in tropical A-climates and Cw-(Cfa-)climates; (iii) ‘semihumid-meridional’ – species occurring in winter- and/or summer-dry warm temperate climates mostly (Cw-, Cs-climates) but not tropical A-climates or fully humid warm temperate Cf-climates; (iv) ‘eurytropical’ – species restricted to dry climates (B-climates). shows the updated concept (modified after Denk et al. Citation2013). Extracted data from the updated ClimGrim database v. 0.2.1 (http://www.palaeogrimm.org/data) is provided in File S1 in the electronic supplement.
Figure 2. Circumscription of vegetation elements by Köppen climate types (‘Köppen signatures’), introducing four new categories (scheme modified after Denk et al. Citation2013).
Systematic palaeontology
All descriptions of angiosperm pollen presented here include the most diagnostic features observed both in light microscopy (LM) and scaning electron microscopy (SEM). The pollen terminology follows mostly Punt et al. (Citation2007) and Hesse et al. (Citation2009). The classification and author names of orders and families follow APG III (Citation2009). Families and genera are arranged in alphabetical order. When present, incertae sedis taxa are listed at the end of each larger taxonomic group.
Fabids
Order Fagales Engl.
Family Betulaceae Gray
Subfamily Betuloideae Arn.
Genus Alnus Mill.
Alnus sp. 1 (Subgenus Alnus vel Clethropsis)
(–)
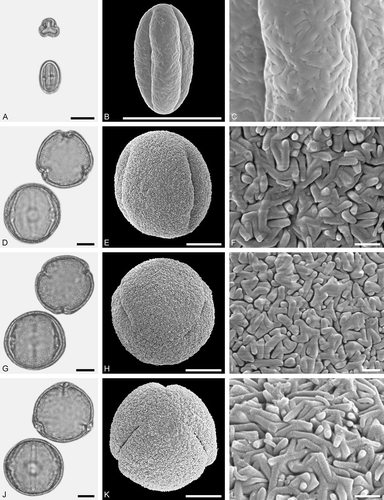
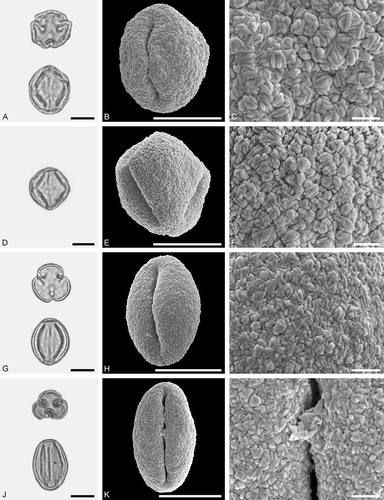
Figure 3. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Betulaceae pollen. A–C. Alnus sp. 1, close-up of polar area. D–F. Alnus sp. 2 with five pori, close-up of polar area. G–I. Alnus sp. 2, grain with four pori, close-up of polar area. J–L. Betula sp. 1, close-up of polar area. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
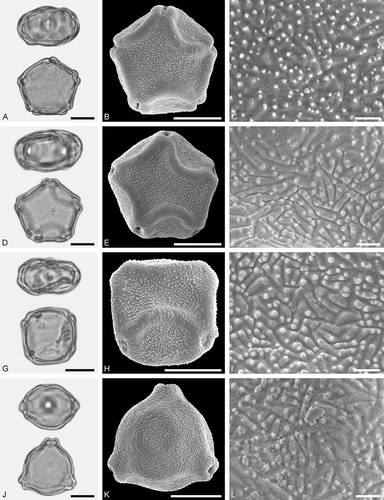
Description
Pollen, monad, oblate, outline pentangular to quadrangular in polar view, elliptic in equatorial view; polar axis 16–19 µm long in LM, equatorial diameter 20–27 µm wide in LM, 18–24 µm wide in SEM; stephanoporate (4–5), pori verstibulate, annulate, meridionally elongated, arci distinct, connecting apertures; exine 0.9–1.2 µm thick, nexine thinner or as thick as sexine, sexine thickened around pori; tectate; sculpturing psilate to scabrate in LM, rugulate to microrugulate in SEM, rugulae with a microechinate suprasculpture, rugulae narrow and of low relief, sculpture elements less conspicuous on arci (SEM).
Remarks
Pollen morphology (LM, SEM) and partly the ultrastructure (SEM) of Betulaceae has been described in detail by Chen (Citation1991) and Blackmore et al. (Citation2003). The pollen morphology (LM and SEM) of all subgenera of Alnus, including pollen from most of the extant Alnus species has been documented by Huang (Citation1972), Lieux (Citation1980), Chen (Citation1991), Jones et al. (Citation1995), Wang et al. (Citation1995), Fritz and Allesch (Citation1999), Perveen and Qaiser (Citation1999), Blackmore et al. (Citation2003), Beug (Citation2004), Fujiki and Ozawa (Citation2007), Li et al. (Citation2011a), May (Citation2011), Miyoshi et al. (Citation2011), Leopold et al. (Citation2012), May and Lacourse (Citation2012) and Liu et al. (Citation2014). Fossil pollen showing LM-based morphological affinities to pollen of modern Alnus have commonly been assigned to the pollen form-genera Alnipollenites (e.g. Potonié Citation1931; Stuchlik et al. Citation2009; Zetter et al. Citation2011) and Polyvestibulopollenites (e.g. Thomson & Pflug Citation1953; Stuchlik et al. Citation2009). Assignment of individual fossils to one of the (two or) three modern subgenera Alnus, Clethropsis, Alnobetula (Furlow Citation1979; Leopold et al. Citation2012) is, however, difficult. Only the subgenus Alnobetula is somewhat distinct by the frequency of polar arci (common in the two Asian species A. sieboldiana Matsum. and A. firma Siebold et Zucc.) and by having a dominant pore number of ≥ 5. Species of subgenera Alnus and Chletropsis are dominantly four- or five-pored. Distinctness in pollen is corroborated by other characteristics of Alnus species. Extant species of subgenus Alnobetula differ from those of the other two subgenera in a number of vegetative characteristics (Murai Citation1964; Furlow Citation1979; Chen & Li Citation2004) and nuclear ITS data (Navarro et al. Citation2003; Chen & Li Citation2004), whereas the sister subgenera Alnus and Chlethropsis show large morphological overlap despite their genetic distinctness.
Fossil record
The oldest fossil records of Alnus are of Paleocene to (early) Eocene age from North America, the Russian Far East, China and Japan (e.g. Budantsev Citation1982; Zetter et al. Citation2011; Liu et al. Citation2014). The earliest megafossils in western North America are A. parviflora (Berry) Wolfe et Wehr from the early Eocene McAbee flora (c. 52 Ma) of British Columbia, Canada (Dillhoff et al. Citation2005), and about co-eval localities such as Republic, Washington (state), USA (Wolfe & Wehr Citation1987), where they are represented by very common foliage and the typical pistillate (female) ‘cones’ (woody catkins) associated with Alnus pollen and various Alnus pollen catkins (‘cones’). The early middle Eocene A. clarnoensis X.Y.Liu, Manchester et J.H.Jin (Liu et al. Citation2014), considered by the authors to represent subgenus Alnus, is based on associated leaves, staminate inflorescences with in situ pollen, and woody, fruiting infructescences found in the Clarno Formation (central Oregon, USA). From the Eocene onwards isolated organs of Alnus (leaves, catkins with or without in situ pollen, dispersed pollen) have been commonly reported in palaeofloras of North America (e.g. Wolfe Citation1966; Meyer & Manchester Citation1997; Liu et al. Citation2014), Europe (from mid-Oligocene onwards; e.g. Mai Citation1987; Mai & Walther Citation1991; Dašková Citation2008; Denk et al. Citation2011) and across Asia (e.g. Chung & Huang Citation1972; Budantsev Citation1982; Liu Citation1996). Only by the Pleistocene has Alnus pollen been reported in the (northern) Andes of South America, which still host a species of Alnus, A. acuminata Kunth. Due to common convergence in preservable traits, individual fossils (isolated organs) are difficult to assign to distinct intrageneric lineages within Alnus (Liu et al. Citation2014). A recent molecular dating suggests that the modern species of genus Alnus did not diverge prior to the mid-Miocene (Grimm & Renner Citation2013). Based on this study, all fossil species assigned to Alnus older than the Miocene may represent ancestral or extinct lineages within the genus. Earlier dating studies (e.g. Forest et al. Citation2005) produced even younger crown ages. Liu et al. (Citation2014) described A. clarnoensis on a combination of leaves, infructescences (woody catkins) and staminate inflorescences with in situ pollen, and considered it as a member of subgenus Alnus. This led them to the conclusion that the subgenera were already established in the Eocene. In fact, due to data availability, the five included species in the final dating run by Grimm and Renner (Citation2013) only included members of a clade corresponding to subgenus Alnus. The data set did include A. nepalensis D.Don, a species originally assigned to the subgenus Clethropsis but nesting within the subgenus Alnus clade according to molecular data (e.g. Chen & Li Citation2004; Grimm & Renner Citation2013), a position further supported by overall pollen features (Leopold et al. Citation2012). Hence, the molecular estimates only give an estimate for crown group radiation within this subgenus, but not the genus itself, which would resolve the potential conflict between the finding of Alnus clarnoensis (Liu et al. Citation2014) and the c. 15 Ma younger crown group divergence estimates (Grimm & Renner Citation2013). In addition, all highlighted diagnostic characters are also found in at least one of the three species of subgenus Chletropsis sensu stricto (Leopold et al. Citation2012; Liu et al. Citation2014, table 2), which is resolved as the sister clade of subgenus Alnus in available molecular phylogenies (Navarro et al. Citation2003; Chen & Li Citation2004) and morphologically overlapping with the latter.
Ecological implications
Up to 35 Alnus species can be grouped into three subgenera, which, based on ITS data, largely group according to the morphological-systematic concepts of Murai (Citation1964) and Furlow (Citation1979): the subgenera Alnus and Clethropsis are resolved as sister clades, and the subgenus Alnobetula as the first diverging lineage. Alnus occurs usually as an azonal element accompanying water bodies, alongside streams, on riverbanks, typically in forested areas (e.g. Li & Skvortsov Citation1999; Denk et al. Citation2001). With about 25 species, the subgenus Alnus is the most diverse lineage and the most widespread. Alnus acuminata extends into the mid- to high-altitudinal forest belts (1400–3100 m above sea level [a.s.l.] of the Andes in South America (until south Argentina), whereas Alnus hirsuta (Spach) Rupr. is found in the northern parts of eastern Eurasia (Siberia to northeast China, Korea, Russian Far East and Japan) at elevations of 700 to 1500 m (Li & Skvortsov Citation1999; see Grimm & Renner Citation2013, file S4, for a compilation of geographic, altitudinal and climate distribution of Betulaceae genera and commonly accepted species). With up to ten species, the subgenus Alnobetula is less diverse than the subgenus Alnus, and, except for A. alnobetula (Erh.) K.Koch (incl. Alnus viridis [Chaix.] DC.), which has adapted to the subarctic climate conditions, is exclusively found in the temperate zone of northeast Asia. The two Asian species of the subgenus Clethropsis occur in montane forests of the foothills of the Himalayas and on Taiwan between sea-level and 2900 m a.s.l. (Li & Skvortsov Citation1999), whereas their North American relative has a scattered distribution in mild temperate lowlands of eastern United States (south-central Oklahoma, southern Delaware and south-eastern Maine).
The genus is widespread in the extra-tropical part of the Northern Hemisphere (per-humid or seasonally dry climates; BS-, C-, D-climates) showing no clear preference for a certain climate (Grimm & Renner Citation2013, file S4) or vegetation zone (Schroeder Citation1998). Individual species of Alnus can be categorised as generalists, semihumid-meridional, meridio-nemoral, nemoral, boreal, or arctic-alpine elements (File S1). Based on the modern distribution of the subgenera Alnus and Clethropsis, the trees producing the fossil Alnus pollen from the Lavanttal Basin could have been elements of various habitats such as swamp and riparian forests (levee and back-swamp forests), but also as accessory element in the hinterland montane forests.
Alnus sp. 2 (subgenus Alnus vel Clethropsis)
(–)
Description
Pollen, monad, oblate, outline pentangular to quadrangular in polar view, elliptic in equatorial view; polar axis 12–19 µm long in LM, equatorial diameter 21–29 µm wide in LM, 19–24 µm in SEM; stephanoporate (4–5), pori verstibulate, annulate, meridionally elongated, arci distinct, connecting apertures; exine 0.7–1.0 µm thick (LM), nexine thinner or as thick as sexine, sexine thickened around pori; tectate; sculpturing psilate to scabrate in LM, rugulate to microrugulate in SEM, rugulae with a microechinate suprasculpture, rugulae wide and of high relief, sculpture elements less conspicuous on arci (SEM).
Remarks
Rugulae are broader, more conspicuous and of higher relief and the microechini are also much larger than in Alnus sp. 1.
Genus Betula L.
Betula sp. 1
(–, –)
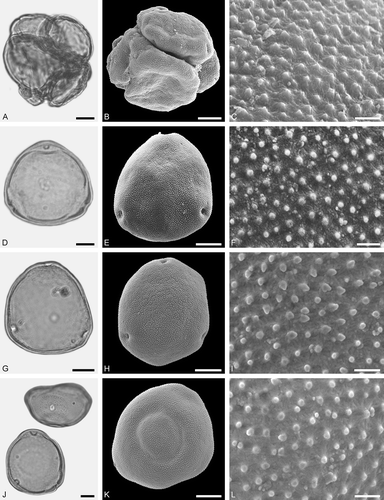
Figure 4. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Betulaceae pollen. A–C. Betula sp. 1, close-up of polar area. D–F. Betula sp. 2, close-up of polar area. G–I. Carpinus sp. 1, close-up of polar area. J–L. Carpinus sp. 2, close-up of polar area. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
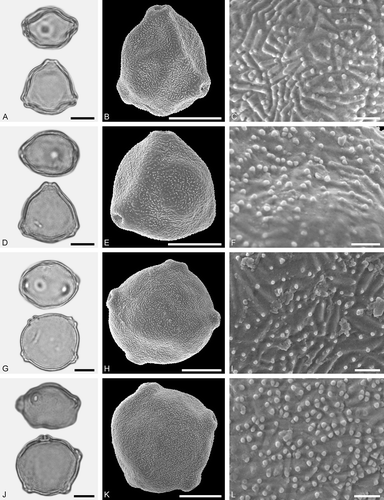
Description
Pollen, monad, oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 18–20 µm long in LM, equatorial diameter 23–26 µm wide in LM, 21–23 µm wide in SEM; triporate, pori verstibulate, annulate; exine 0.8–1.1 µm thick (LM), nexine thinner than sexine, circular thickening of nexine in polar areas; tectate; sculpturing psilate to scabrate in LM, rugulate to microrugulate in SEM, rugulae with a microechinate suprasculpture, rugulae wide and of high relief (SEM).
Remarks
The pollen morphology (LM and SEM) of many extant Betula species has been presented by Birks (Citation1968), Lieux (Citation1980), Zavada and Dilcher (Citation1986), Chen (Citation1991), Jones et al. (Citation1995), Wang et al. (Citation1995), Mäkelä (Citation1996), Fritz and Allesch (Citation1999), Blackmore et al. (Citation2003), Wei (Citation2003), Beug (Citation2004), Clegg et al. (Citation2005), Karlsdóttir et al. (Citation2007, Citation2008), Li et al. (Citation2011a), Miyoshi et al. (Citation2011) and Lin et al. (Citation2013). The pollen ultrastructure (SEM, transmission electron microscopy [TEM]) of Betula has been described and figured by Zavada and Dilcher (Citation1986), Pehlivan (Citation1987) and Blackmore et al. (Citation2003). Fossil pollen showing LM-based morphological affinities to pollen of modern Betula have commonly been assigned to the pollen form-genus Betulapollenites (e.g. Potonié Citation1960; Stuchlik et al. Citation2009).
Fossil record
The fossil record of Betula parallels the one of its putative sister genus Alnus, both regarding its spatial and temporal distribution (e.g. Mai Citation1995). As with Alnus, the oldest fossil with unambiguous affinities to Betula, B. leopoldae P.R.Crane et Stockey (foliage, aments and associated Betula pollen), has been reported from the Eocene McAbee flora of western North America (Dillhoff et al. Citation2005) predating earliest unambiguous records in western Eurasia and East Asia (mostly pollen, but also nuts and bracts, B. longisquamosa Mädler, and infructescences, e.g. Zastawniak and Walther Citation1998, late Miocene, Poland). Betula macrofossils and microfossils are common elements in numerous post-Eocene temperate fossil floras. Alnus and Betula are commonly found in the same assemblages; this is why Mai (Citation1981b) placed them in the same group (‘group 1’) of arcto-tertiary elements.
Ecological implications
The Kew Checklist for Betulaceae lists 90 accepted species of Betula (see Grimm & Renner Citation2013, file S4). In both altitudinal and latitudinal distribution, Betula exceeds other genera of Fagales (Li & Skvortsov Citation1999; Thompson et al. Citation1999b, Citation2006; Fang et al. Citation2009; Grimm & Renner Citation2013). In contrast to other genera of the Betulaceae, a large number of Betula species have been described from the cold-temperate to arctic regions of the Northern Hemisphere such as northern North America (10 spp.) and Siberia (13 spp.) and the mountainous, central part of Asia (about 25 spp.). In comparison, 19 species are known from China and 14 from Korea and Japan. Six species occur in Europe, four in south-western Asia, and ten in North America outside the subarctic region. Some species thrive at sea-level, while others are pure montane taxa (e.g. Furlow Citation1997; Li & Skvortsov Citation1999). Other species prefer temperate to subarctic swamps and bogs (e.g. North American B. pumila L. and the East Asian B. fructicosa Pall). The most temperature tolerant species are B. humilis Schrank, B. nana L. (arctic and high mountains of Eurasia; Meusel et al. Citation1965; IOPI Citation1996–2007) and B. neoalaskana Sarg. (Russian Far East to northern North America; Furlow Citation1997; Thompson et al. Citation2006).
Species of Betula are (co-)dominant elements of boreal northern hemispheric forests thriving in warm temperate or snow climates with relatively to very short (cold) summers (Cfc-, Dfb-, Dfc-, Dfd- climates), but are frequently found also in nemoral habitats such as temperate broad-leaved forests, open woods and thickets (Furlow Citation1997; Li & Skvortsov Citation1999) and associated climate zones (snow to warm temperate climates, fully humid or winter-dry with warm to hot summers: Cwa, Cwb, Cfa, Cfb, Dfb, Dwa, Dwb). A few species (B. alnoides Buch.-Ham. ex D.Don, B. cylindrostachya Lindl. ex Wall., B. jinpingensis P.C.Li, B. luminifera H.J.P.Winkel) can be found in distinctly subtropical settings (Cwa-, Cfa-climates) in the Himalayan foothills (mostly below 2000 m a.s.l.) and south-western China (Li & Skvortsov Citation1999; Shaw et al. Citation2014a). Most species of Betula can be categorised as nemoral or boreal elements, but a few represent semihumid-meridional, meridio-nemoral, and arctic-alpine elements (File S1). Thus, the occurrence of Betula can be taken as an indication of forested habitats of various temperature regimes, but excludes tropical (A-climates) and desert/steppe climates (B-climates) and habitats.
Betula sp. 2
(–)
Description
Pollen, monad, oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 20–22 µm long in LM, equatorial diameter 25–27 µm wide in LM, 20–22 µm wide in SEM; triporate, pori verstibulate, annulate; exine 1.0–1.2 µm thick (LM), nexine thinner than sexine, circular thickening of nexine in polar areas; tectate; sculpturing psilate to scabrate in LM, rugulate to microrugulate in SEM, rugulae with a microechinate suprasculpture, rugulae wide and of low relief (SEM).
Remarks
The sculpture elements (rugulae) are much less conspicuous in Betula sp. 2 than in Betula sp. 1, and they are also wider apart and shorter.
Subfamily Coryloideae Hook.f.
Genus Carpinus L.
Carpinus sp. 1
(–)
Description
Pollen, monad, oblate, outline convex quadrangular in polar view, elliptic in equatorial view; polar axis 23–25 µm long in LM, equatorial diameter 30–32 µm wide in LM, 28–30 µm wide in SEM; stephanoporate (4), pori annulate; exine 0.8–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate to scabrate in LM, rugulate in SEM, rugulae with a microechinate suprasculpture, rugulae long and conspicuous, widely spaced (SEM).
Remarks
The pollen morphology (LM and SEM) of Carpinus has been documented by Huang (Citation1972), Lieux (Citation1980), Chen (Citation1991), Jones et al. (Citation1995), Wang et al. (Citation1995), Fritz and Allesch (Citation1999), Blackmore et al. (Citation2003), Beug (Citation2004), Akhondnezhad et al. (Citation2011), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The pollen ultrastructure (TEM) of Carpinus has been described and figured by Pehlivan (Citation1987). Fossil pollen showing LM-based morphological affinities to pollen of modern Carpinus have commonly been assigned to the pollen form-genera Carpinuspollenites (e.g. Thiergart Citation1937; Stuchlik et al. Citation2009) and Carpinipites (e.g. Srivastava Citation1966; Stuchlik et al. Citation2009). Of the four extant genera in the Coryloideae, three (Ostrya, Ostryopsis and Corylus) have predominantly three-porate pollen in contrast to Carpinus, which are usually four-porate. According to plastid or combined plastid-nuclear phylogenies, Carpinus is nested in Ostrya (e.g. Yoo & Wen Citation2002, Citation2007; Grimm & Renner Citation2013); hence, the four-porate pollen can be seen as an apomorphy of Carpinus within the coryloid subclade of Betulaceae.
Fossil record
The earliest fossils with affinities to Coryloideae (including the modern genera Carpinus, Corylus, Ostrya and Ostryopsis) are Palaeocarpinus from the Paleocene of north-western China and North Dakota (Manchester & Guo Citation1996; Manchester et al. Citation2004). Earliest fossils that can be reliably assigned to the modern Carpinus (and Corylus, see later) are fruits that have been described from the Republic flora of the latest early Eocene of Washington (state), north-western North America (C. perryae Pigg, Manchester et Wehr; Pigg et al. Citation2003). In Eurasia, the genus can be traced back to the late Eocene of Japan (Tanai Citation1972; Uemura & Tanai Citation1993), and the latest Eocene/earliest Oligocene of Europe based on macrofossils and pollen (Berger Citation1953; Mai Citation1995; Stuchlik et al. Citation2009). Carpinus has a continuous fruit record in the Neogene of Poland (middle Miocene onwards), studied in detail and compared to extant species of North America, western Eurasia and East Asia (morphology and anatomy) by Jentys-Szaferowa (Citation1975). Apparently, species with similarity to modern North American (fruits, leaves), western Eurasian and East Asian morphotypes co-existed in the Miocene and Pliocene of Europe (Jentys-Szaferowa Citation1975; Mai Citation1995).
Ecological implications
Carpinus is a widespread, northern hemispheric genus with c. 39 species. Two species, C. caroliniana Walter and C. tropicalis (Donn.Sm.) Lundell, occur in eastern North America and Mesoamerica, respectively, two in western Eurasia. The genus is most diverse in East Asia (c. 34 species). In China, species of Carpinus grow at altitudes between 200 and 2900 m, in subtropical to temperate forests of hilly/mountainous regions, on mountain slopes and in valleys. Most of the species diversity is concentrated in the subtropical broad-leaved forests of south-western China (Sichuan, Yunnan, Guangxi and Guizhou provinces); six species have ranges including or limited to subtropical and temperate regions of Korea and Japan. Carpinus londoniana H.J.P.Winkl. extends into north Vietnam (Ohwi Citation1965; Li & Skvortsov Citation1999); C. faginea Lindl. has a distribution restricted to western and central part of the Himalayas. The most widespread species is C. viminea Wall. ex Lindl. (Himalayas to Korea and Vietnam). In the Himalayas, Carpinus is found at moderately high altitudes of up to 2800 m (Nepal) and at 2700–3300 m (Meusel et al. Citation1965). The western Eurasian species C. betulus L. extends from France (up to 1000 m a.s.l. in the Alps) and southernmost Sweden (Scania) to Iran. In the south-eastern part of its range, it co-occurs with the second western Eurasian species, C. orientalis Mill. (Meusel et al. Citation1965). The North American C. caroliniana is limited to the nemoral zone (see Walter Citation1973; Schroeder Citation1998) of the eastern part of the United States (excluding southern Louisiana to Georgia) into southernmost Canada, where it grows as an understory tree in moist habitats lowland deciduous forest and at altitudes below 300 m (Furlow Citation1997). Its Mesoamerican counterpart C. tropicalis, occurring from northern Mexico to Costa Rica, can be the dominant tree in the montane cloud forests along streams in canyons (Stritch Citation2014).
In general, Carpinus prefers warm temperate climates with sufficient rain during growing season (Cf-, Cw-climates), with only one species, C. orientalis extending locally (Albania, western Greece; Adana, Osmaniye and Hatay provinces, south-eastern Turkey) into summer-dry climates of the Mediterranean (Browics & Zieliński Citation1982). Together with Corylus, Carpinus is an indicator for well-drained, mixed mesophytic broad-leaved forests of the humid warm temperate zone (nemoral zone; Walter Citation1973; Schroeder Citation1998). Species of Carpinus can be categorised as semihumid-meridional, meridio-nemoral and/or nemoral elements (File S1). Since all modern species prefer moist habitats, it is likely that the Carpinus pollen from the Lavanttal basin originated from trees growing either in lowland levee or riparian forests or along streams and rivulets in mountain valleys and adjacent slopes.
Carpinus sp. 2
(–, –)
Figure 5. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Betulaceae pollen. A–C. Carpinus sp. 2, close-up of polar area. D–F. Corylus sp., close-up of polar area. G–L. Ostrya sp., close-ups of polar area. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
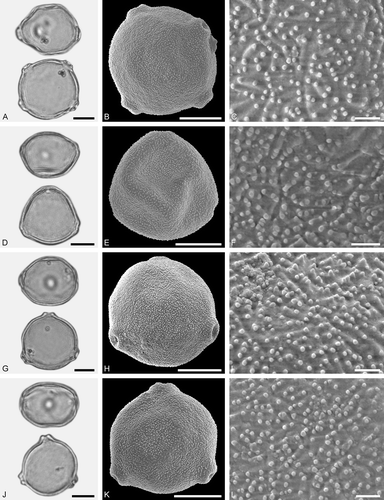
Description
Pollen, monad, oblate, outline convex quadrangular in polar view, elliptic in equatorial view; polar axis 22–25 µm long in LM, equatorial diameter 31–33 µm wide in LM, 28–30 µm wide in SEM; stephanoporate (4), pori annulate; exine 0.9–1.1 µm thick, nexine thinner than sexine; tectate; sculpturing psilate to scabrate in LM, rugulate in SEM, rugulae with a microechinate suprasculpture, rugulae short and inconspicuous, closely packed (SEM).
Remarks
Rugulae are much shorter, less conspicuous, more closely packed, and with a much higher number of microechini than in Carpinus sp. 1.
Genus Corylus L.
Corylus sp.
(–)
Description
Pollen, monad, oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 19–21 µm long in LM, equatorial diameter 24–26 µm wide in LM, 23–25 µm wide in SEM; triporate, pori small, circular; exine 0.8–0.9 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate to scabrate in LM, microrugulate in SEM, rugulae with a microechinate suprasculpture, high density of microechinate per rugulae (SEM).
Remarks
The pollen morphology (LM and SEM) of Corylus has been documented by Chen (Citation1991), Fritz and Allesch (Citation1999), Blackmore et al. (Citation2003), Wei (Citation2003), Beug (Citation2004), Wang et al. (Citation1995), Li et al. (Citation2011a), Miyoshi et al. (Citation2011) and Nikolaieva et al. (Citation2014). The pollen ultrastructure (SEM, TEM) of Corylus has been described and figured by Pehlivan (Citation1987) and Blackmore et al. (Citation2003). Fossil pollen showing LM-based morphological affinities to pollen of modern Corylus have commonly been assigned to the pollen form-species Triporopollenites coryloides H.Pflug (e.g. Thomson & Pflug Citation1953; Stuchlik et al. Citation2009).
Fossil record
The early macrofossil record of the Coryleae has been summarised by Crane (Citation1989) and Pigg et al. (Citation2003). The earliest macrofossils that can be reliably assigned to Corylus are infructescences with nuts, C. johnsonii Pigg, Manchester et Wehr, from the middle Eocene of Republic, Washington. These fossil fruits are considered most similar to several modern Chinese species (Pigg et al. Citation2003). Older coryloid records from the Paleocene of western North America (Montana), Greenland and England are of uncertain generic affinity (Pigg et al. Citation2003). Corylus apparently did not reach Europe before the late Oligocene after the closure of the Turgai (Mai Citation1995). In his overview of arcto-tertiary elements, Mai (Citation1995) noted that the genus was also present in the Paleocene of East Asia, but this has yet to be confirmed. Late Cretaceous and Paleogene pollen that has occasionally been assigned to Corylus (e.g. Muller Citation1981) may in fact belong to extinct, ancestral lineages of Coryleae (especially Palaeocarpinus), the clade comprising Corylus, Carpinus, Ostrya and Ostryopsis (Pigg et al. Citation2003; Zetter et al. Citation2011). Unambiguous Corylus pollen can only be determined by a combination of LM and SEM, and so far, is unknown from Central European sediments older than the Miocene (R. Zetter, pers. observation through c. 40 years of work on fossil pollen material form all over Europe).
Ecological implications
Corylus consists of c. 16 species with a disjunct distribution in the Northern Hemisphere (Li & Skvortsov Citation1999; Whitcher & Wen Citation2001). Ten species occur in East Asia, four are found in western Eurasia, and two in North America. Widespread species are common pioneer trees; Corylus species are common accessory and shrubby elements of broad-leaved dry to mesic forests (Coladonato Citation1993, Fryer Citation2007). The East Asian species are found in temperate broad-leaved forest, one species, however, C. heterophylla Fisch. ex Trautv., extends into eastern Mongolia, south-eastern Siberia, and the southern Russian Far East. In China, the species occur at mid- to high-altitudes (400–3000 m) in montane areas, preferring forested valleys and mountain slopes (Li & Skvortsov Citation1999). Three of the four western Eurasian species are restricted to south-eastern Europe and the Euxinian, Colchic and Hyrcanian regions south of the Black Sea, in the Caucasus and adjacent areas of northern Iran (Browics & Zieliński Citation1982). The most widespread western Eurasian species, C. avellana L., the common hazelnut, is widely cultivated but also naturally found throughout temperate zone of western Eurasia with a range extending from the Iberian Peninsula to southern Scotland, southern Scandinavia (up to 600 m a.s.l. in southern Norway) and into the European part of Russia, the Black Sea region, the Caucasus and south-eastern Turkey and adjacent Iran/Iraq. In the Alps, it reaches up to the subalpine zone (Meusel et al. Citation1965). The two North American species, C. americana Walter and C. cornuta Marshall, are found in eastern North America in moist to dry, open woods and thickets, including disturbed areas, up to 750 m a.s.l. Both species can be considered as weeds, sometimes as pests in managed forests (Furlow Citation1997). In the Pacific states (California to British Columbia), Corylus cornuta prefers rocky slopes.
Corylus is typical for the nemoral zone (Schroeder Citation1998), with a climate preference for warm temperate, fully humid climates with warm summers (Cfb; see Grimm & Renner Citation2013, file S4). Most species have a range covering also Cwb- or Dfb-climates (warm temperate to snow climates with sufficient precipitation during growing season and warm summers). Species of Corylus can hence be categorised as nemoral elements (File S1). Based on the characteristic occurrence of the genus in its disjunct areas in North America, western Eurasia and East Asia, we figure that Corylus has grown in the understory of mixed broad-leaved deciduous forests or at margins of beech-dominated forests surrounding the wetland basin.
Genus Ostrya Scop.
Ostrya sp.
(–)
Description
Pollen, monad, oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 21–25 µm long in LM, equatorial diameter 27–30 µm wide in LM, 24–27 µm wide in SEM; triporate, pori annulate; exine 1.0–1.2 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate to scabrate in LM, microrugulate to rugulate in SEM, rugulae with a microechinate suprasculpture, rugulae of low relief, microechinii conspicuous and of high density (SEM).
Remarks
Pollen of extant Ostrya has been described and figured using LM and SEM by Lieux (Citation1980), Accorsi et al. (Citation1991), Chen (Citation1991), Jones et al. (Citation1995), Wang et al. (Citation1995), Fritz and Allesch (Citation1999), Blackmore et al. (Citation2003), Beug (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The ultrastructure (TEM) of Ostrya pollen has been documented by Pehlivan (Citation1987). Fossil pollen showing LM-based morphological affinities to pollen of modern Ostrya have commonly been assigned to the pollen form-genus Ostryoipollenites (e.g. Potonié Citation1960; Stuchlik et al. Citation2009). Whereas pollen of the closely related Carpinus is usually four-porate, pollen of species of Ostrya are usually three-porate as the pollen in their more distant relatives Ostryopsis and Corylus (e.g. Chen Citation1991). According to plastid or combined plastid-nuclear phylogenies, Ostrya is paraphyletic to Carpinus (e.g. Yoo & Wen Citation2002, Citation2007; Grimm & Renner Citation2013). Hence, the three-porate situation may be plesiomorphic within the Carpinus-Ostrya(-Ostryopsis) lineage, and the pollen could possibly also represent an extinct or ancestral taxon predating the formation of Carpinus as a distinct lineage.
Fossil record
Reports of fossil Ostrya are scarcer than of other extant Coryloideae. The earliest unequivocal fossil record of Ostrya in North America is O. oregoniana Chaney fruits and leaves from the earliest Oligocene Bridge Creek flora of John Day Formation, Oregon, western North America (c. 33 Ma; Meyer & Manchester Citation1997), where it occurred until the middle Miocene, and has a continuous record in eastern North America until present times (e.g. Graham Citation1999; Manchester Citation1999). In Europe, Ostrya is known from the lower Oligocene (diagnostic involucres surrounding the nutlets; Mai Citation1995; Kvaček & Walther Citation1998) and Pliocene (fruits; Jentys-Szaferowa Citation1975). The earliest East Asian records (involucres, fruits, wood) are from the Miocene of Japan (e.g. Tanai Citation1961, Citation1972) and China (WGCPC Citation1978).
Ecological implications
Ostrya is composed of c. nine tree species with a northern hemispheric distribution. Five are growing in East Asia and three in North America. The only western Eurasian species, O. carpinifolia Scop., ranges from south-central and south-eastern Europe via Turkey (Taurus Mountains) to the Caucasus and into Lebanon (e.g. Browics & Zieliński Citation1982). It prefers shrubby, sunny hillsides, is often found on dry, rocky slopes (on limestone), and is an important element of deciduous forests and scrubs and open coniferous woodland (Shaw et al. Citation2014b). The subtropical Chinese species, found in mixed forests at altitudes of 200–1300 m (Li & Skvortsov Citation1999) have fairly restricted distributions (Fang et al. Citation2009). The two other East Asian species have a distinctly temperate distribution. Ostrya japonica Sarg. is found in temperate forests, ranging from north-central China via Korea to Japan (Ohwi Citation1965; Li & Skvortsov Citation1999). Ostrya yunnanensis W.K.Hu has only been reported from the Luquan Xian Mountain in Yunnan, where it grows in moist forest at an elevation of 2600 m a.s.l. Ostrya virginiana (Mill.) K.Koch is the most widespread among the North American species, with a continuous distribution area in eastern North America and numerous disjunct occurrences throughout Mesoamerica (O. virginiana guatemalensis (H.Winkl.) E.Murray; IOPI Citation1996–2007). In the United States and Canada, it occurs throughout the lowlands (0–300 m a.s.l.) on moist to dry slopes and ridges, and occasionally on moist, well-drained flood plains. The other two species, O. knowtonii Sarg. and O. chisosensis Correll have a restricted, scattered distribution in Utah, Arizona, New Mexico and the Texan-Mexican borderlands (along the Rio Grande) along rivulets and rocky slopes in moist canyons at 1200–2300 m a.s.l. (Furlow Citation1997).
Generally restricted to the subtropical belt of the Northern Hemisphere (Browics & Zieliński Citation1982; Furlow Citation1997; Li & Skvortsov Citation1999), a few species of Ostrya appear to be adapted to climates and habitats with substantial draught during growing season (Cs- and BS-climates) such as O. carpinifolia in the Mediterranean and O. chisosensis growing in south-west Texas along the Rio Grande (Mexican-Texan border). All others prefer climates with sufficient or excess precipitation in summer (Cf-climates in North America and western Eurasia; Cf- and Cw-climates in East Asia). All species of Ostrya except for the East Asian O. trichocarpa D.Fang et Y.S.Wang (semihumid-meridional element) and the North American O. chisosensis (eurytropical element) can be categorised as meridio-nemoral or nemoral elements (File S1). With respect to the habitat ranges of extant Ostrya, the fossil pollen may have either produced by trees thriving in mixed deciduous broad-leaved and coniferous hinterland forests (dry or sun-exposed hillsides, mountains; in analogy to the habitats of the Eurasian species) or in lowland riparian and floodplain forests (in analogy to the O. virginiana-habitat).
Family Fagaceae Dumort.
Genus Castanea Mill.
Castanea sp.
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 13–15 µm long in LM, 11–13 µm long in SEM, equatorial diameter 8–10 µm wide in LM, 6–8 µm wide in SEM; tricolporate, pori lalongate, colpi long; exine 0.8–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, rugulate, fossulate, perforate in SEM.
Remarks
The pollen morphology (LM and SEM) and ultrastructure (SEM) of Castaneoideae pollen has been thoroughly documented by Crepet and Daghlian (Citation1980), Miyoshi (Citation1982), Praglowski (Citation1984), Wang and Pu (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The pollen of Castanea, Castanopsis, Chrysolepis, Lithocarpus and Notholithocarpus are very similar in size and shape. Using LM only, they are indistinguishable at the generic level. Pollen of Castanopsis and Lithocarpus/Notholithocarpus commonly overlap in size and shape and arrangement of sculpturing elements seen under SEM, and cannot be distinguished. Castanea-type pollen appears to be generally smaller and narrower than pollen of the other genera. Under the SEM the rugulate sculpturing is flattened and smoother (fused) in appearance (e.g. Praglowski Citation1984). Fossil pollen showing LM-based morphological affinities to pollen of modern Castanea has commonly been assigned to the pollen form-genus Cupuliferoipollenites (e.g. Potonié Citation1960; Stuchlik et al. Citation2014).
Fossil record
According to the summary of Mai (Citation1995), Castanea has its earliest occurrence in the Paleocene of North America and Eocene of East Asia. In Europe and West Asia, it is recorded from the Oligocene onwards. To date, the earliest reliable Castaneoideae fossils are staminate catkins (inflorescences), Castaneoidea puryearensis Crepet et Daghlian, with in situ Castaneoideae-type pollen from the middle Eocene of Tennessee, south-eastern United States (Crepet & Daghlian Citation1980). The early Cainozoic macrofossil record of Castanea or Castanea-like leaf fossils is not convincing because of the overlapping features in leaf morphology within several genera of Fagaceae. This has only been partly resolved using epidermal anatomy from both North American (Jones & Dilcher Citation1988) and European (Kvaček & Walther Citation2010) leaf fossils. Based on cuticle features, Jones and Dilcher (Citation1988) described Castaneophyllum tenesseense (Berry) Jones et Dilcher from the Eocene of Tennessee (south-eastern United States), and Kvaček and Walther (Citation2010) described two different European Castaneophyllum leaf types affiliated with Castanea from the late Eocene of Czech Republic (Castaneophyllum venosum [Rossm.] Erw.Knobloch et Kvaček), and from the early Oligocene of Slovenia, Germany and Czech Republic (Castaneophyllum lonchitiforme Kvaček et H.Walther). Unequivocal records of Castanea leaves with cuticle features and fruits are known mostly from the Miocene in Europe (e.g. Mai Citation1995). The fossil pollen records of Castaneoideae-type pollen based on LM studies date back to the Upper Cretaceous (e.g. Muller Citation1981). Since pollen of the different genera within the subfamily Castaneoideae cannot be differentiated using LM only, and in most cases not even using SEM, it unclear when the first real Castanea-type pollen occur. Dispersed fossil Castanea-type pollen has been described using combined LM and SEM by Bouchal et al. (Citation2014) from the late Eocene of Colorado, USA.
Ecological implications
Castanea is a small genus with c. eight extant species with a wide (disjunct) distribution in the temperate zone of the Northern Hemisphere, occurring in North America, eastern and western Asia, and southern Europe. Four species are native to East Asia, C. crenata Siebold et Zucc., C. henryi (Skan) Rehder et Wilson, C. mollissima Blume and C. seguinii Dode. The American chestnuts, C. dentata (Marshall) Borkh., C. ozarkensis Ashe and C. pumila Mill. are all native to south-eastern North America. The European chestnut, C. sativa Mill. is native to southern Europe and western Asia (e.g. Nixon Citation1997; Huang et al. Citation1999; Dane et al. Citation2003). The Asian Castanea species in China (Korea, Japan) are known to thrive in mixed mesophytic forests, especially on mountain slopes, ranging from sea level to an altitude of 2800 m (Huang et al. Citation1999). The American C. dentata and C. ozarkensis are growing in rich deciduous forests and mixed forests, occurring at an altitude between 0 and 1200 m. Castanea pumila occurs in forests and open woods, forest understory, dry sandy and wet sandy barrens, at an altitude of 0 to 1000 m (Nixon Citation1997).
Six of the about eight species of Castanea can be categorised as nemoral elements following the definition of Denk et al. (Citation2013), and two (C. ozarkensis, a North American endemic with very restricted distribution; C. pumila) as meridio-nemoral (File S1). Kvaček and Walther (Citation2010) noted that the early Eocene and Oligocene Castaneophyllum species (affiliated with Castanea) were deciduous trees adapted to warm temperate conditions that occurred within subtropical evergreen forests and mixed mesophytic forests.
Genus Fagus L.
Subgenus Fagus
Fagus sp.
(–)
Description
Pollen, monad, spheroidal, outline circular in polar and equatorial views; polar axis 33–35 µm long in LM, 30–33 µm long in LM, equatorial diameter 31–36 µm wide in LM, 28–31 µm wide in SEM; tricolporate, colpi narrow, nexine thickened around pori; exine 1.0–1.3 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, rugulate, fossulate in SEM, rugulae often diverging and protruding (SEM).
Remarks
The pollen morphology (LM and SEM) of Fagus has been described and figured in detail by Crepet and Daghlian (Citation1980), Miyoshi (Citation1982), Saito (Citation1992), Denk (Citation2003), Wang and Pu (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The ultrastructure (TEM) of Fagus pollen has been studied by Crepet and Daghlian (Citation1980), Praglowski (Citation1982) and Wang and Pu (Citation2004). The pollen morphology of extant Fagus pollen is very similar to that observed in fossil grains. Pollen from species of the subgenus Engleriana according to Shen (Citation1992; F. engleriana Seemen, F. japonica Maxim., F. okamotoi C.F.Shen) are slightly different to those of the subgenus Fagus (all other species), they usually have a narrower colpi that reaches to the poles, and also the pollen grains are generally smaller than those of subgenus Fagus (Praglowski Citation1982; Denk Citation2003). Fagus pollen grains commonly occur in the Lavanttal samples and show a remarkable high variability in size, form and sculpturing under SEM. The same phenomenon has been documented for other palaeo-palynofloras and material from extant Fagus flowers (e.g. Grímsson et al. Citation2015b). The size of the fossil Fagus pollen grains from the Lavanttal Basin and the arrangement of their colpi suggest that they belong to the subgenus Fagus. Fossil pollen showing LM-based morphological affinities to pollen of modern Fagus have commonly been assigned to the pollen form-genus Faguspollenites (e.g. Raatz Citation1937; Stuchlik et al. Citation2014).
Fossil record
Fagus has an extremely well documented Cainozoic fossil record extending back to the Eocene (e.g. Tralau Citation1962; Zetter Citation1984: Kvaček & Walther Citation1991; Denk & Meller Citation2001; Denk Citation2004; Manchester & Dillhoff Citation2004; Grímsson & Denk Citation2005). The fossil record and biogeographic history of Fagus was recently summarised by Denk and Grimm (Citation2009a). The earliest Fagus records (cupules, fruits, foliage, pollen) to date are from the late early Eocene of British Columbia, western Canada (F. langevinii Manchester et Dillhoff; Manchester & Dillhoff Citation2004; for age determination see Denk & Dillhoff Citation2005), and Washington, north-western United States (Republic locality; S.R. Manchester, pers. communication, 2015). Various additional Fagus macrofossils and pollen have been documented from the middle Eocene of Vancouver Island (western Canada), Axel Heiberg Island (north-eastern Canada) and western Greenland (e.g. McIntyre Citation1991; Richter & LePage Citation2005; Mindell et al. Citation2009; Grímsson et al. Citation2015b). The oldest Asian Fagus fossils to date were found in the Russian Far East and north-eastern China and are of middle to late Eocene age (e.g. Denk & Grimm Citation2009a). The fossil record of Fagus further indicates that this genus dispersed from East Asia into Europe during the early Oligocene following the closure of the Turgai Seaway. In the Miocene, Fagus is found throughout the Northern Hemisphere (Denk & Grimm Citation2009a).
Dispersed fossil Fagus pollen studied using combined LM and SEM has been reported from the late early Eocene of British Columbia (Manchester & Dillhoff Citation2004), the middle Eocene of West Greenland (Grímsson et al. Citation2015b), the early Oligocene of Germany (Denk et al. Citation2012) and the late Miocene of Japan (Saito Citation1992).
Ecological implications
Fagus is a small tree genus consisting of two distinct putative subgenera, subgenus Fagus (seven species) and subgenus Engleriana (three species) with a northern hemispheric distribution (Shen Citation1992; Denk Citation2003; Denk et al. Citation2005). Fagus is an important component of mixed broad-leaved evergreen-deciduous forest in North America and East Asia, and it is the most prominent broad-leaved forest tree in Europe and western Asia (Zhou & Li Citation1994; Peters Citation1997).
Fagus is one of the most ecologically and climatically indicative tree genera of the temperate zones of the Northern Hemisphere (Köppen Citation1936; Peters Citation1997; Grimm & Denk Citation2012); all three widespread and common species or species-complexes, F. grandifolia Ehrh. in eastern North America (northern Mexico to southern Canada), F. sylvatica L. (s.l.) in western Eurasia (northern Spain to northern Iran) and F. crenata Blume in Japan (Ryushu to southern islands, Hokkaido) can be (single-)dominant elements of mesic forests (e.g. Maycock Citation1994) within the warm temperate to snow climates with warm summers and ample precipitation throughout the year (Cfb-, Dfb-climates). In the warmer, subtropical Cfa-climates, Fagus becomes an accessory element (Maycock Citation1994). The genus Fagus shows also a strong correlation between altitudinal and latitudinal distribution (Maycock Citation1994; Cao Citation1995; Grimm & Denk Citation2012), and appears to be entirely absent from the summer-rain, winter-dry monsoon climates of East Asia in contrast to other Fagaceae genera such as Quercus, Castanopsis (sister genus of Castanea) and Lithocarpus. All c. nine species of Fagus can be categorised as nemoral elements (File S1; see Grimm & Denk Citation2012).
Genus Quercus L.
Quercus sp. 1 (Quercus Group Cerris)
(–)
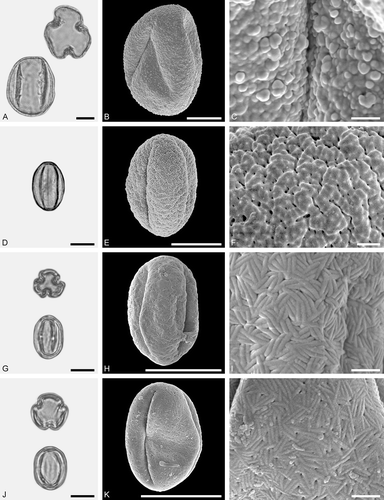
Figure 7. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Fagaceae pollen. A–I. Quercus sp. 1 (Quercus Group Cerris), close-ups of central mesocolpium. J–L. Quercus sp. 2 (Quercus Group Ilex), close-up of mesocolpium. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
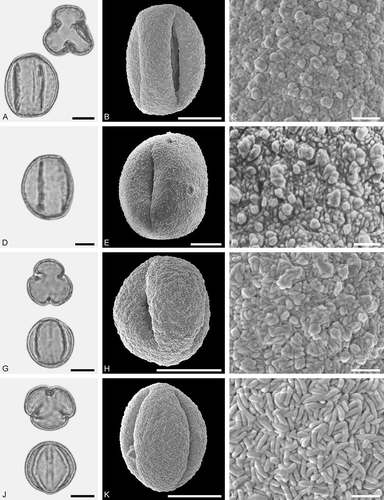
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 27–34 µm long in LM, 25–33 µm long in SEM, equatorial diameter 23–27 µm wide in LM, 20–26 µm wide in SEM; tricolporoidate; exine 0.9–1.2 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microrugulate, perforate in SEM, rugulae forming irregularly distributed and protruding agglomerates (SEM).
Remarks
The pollen morphology (LM and SEM) of Quercus Group Cerris has been presented by Miyoshi (Citation1982), Wei (Citation2003), Wang and Pu (Citation2004), Denk and Grimm (Citation2009b), Makino et al. (Citation2009), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The ultrastructure of this group has been documented by Wang and Pu (Citation2004) and Denk and Tekleva (Citation2014). Fossil pollen showing LM-based morphological affinities to pollen of modern Quercus have commonly been assigned to the pollen form-genus Quercoidites (e.g. Potonié Citation1960; Stuchlik et al. Citation2009).
Fossil record
The rich fossil record of Quercus is in dire need of revision, partly because the systematic concepts that have been used are to some degree outdated, partly because of the many morphological convergences observed in the genus (Denk & Grimm Citation2010). The most diagnostic feature to distinguish major oak lineages in the fossil record is the sculpturing of pollen seen under SEM (Denk & Grimm Citation2009b). In this respect, the oldest unambiguous fossil record of Quercus Group Cerris (according to Denk & Grimm Citation2010) is pollen from the Oligocene–Miocene boundary of central Europe (Kmenta Citation2011). Further well-documented pollen records are from the early and middle Miocene and Pliocene of Central Europe (Ferguson et al. Citation1998; Van der Burgh & Zetter Citation1998; Hofmann et al. Citation2002) and the Miocene of eastern China (Liu et al. Citation2007) until the Holocene of the Sea of Japan (Tekleva et al. Citation2014). Macrofossils that have traditionally been linked to the section/subgenus Cerris may be of different systematic affinities. Modern types of Group Cerris (very similar or identical to extant species) start to occur in the Pliocene of the Eastern Mediterranean at regional scales (Velitzelos et al. Citation2014).
Ecological implications
Group Cerris is one of the least-diverse lineages of oaks; nevertheless, its about ten species are ecologically relatively diverse. In contrast to their evergreen sister lineages Group Ilex and Group Cyclobalanopsis, most species are essentially deciduous, however they are relatively tolerant against summer draught except for the East Asian species. Species like Quercus suber L., the cork oak, and Q. ithaburensis Decne. (incl. Q. macrolepis Kotschy, the Valonia oak) are particularly adapted to the summer dry climates of the Mediterranean (e.g. Ne’Eman Citation1993; Petroselli et al. Citation2013), and widely cultivated. Other species such as Q. cerris L. can be found in dry and mesic forests of the Mediterranean region and have been successfully introduced e.g. to the British Islands, where they naturalised (Stace Citation1997). Quercus libani Oliv. and Q. brantii Lindl. are typical elements across the entire range of the Taurus and Zagros Mountains, where they thrive in cultivated lands and montane forests alike (south-eastern Turkey, northern Iraq, south-western Iran; Browics & Zieliński Citation1982). In contrast to their western Eurasian counterparts, the East Asian species prefer fully humid, mild climate conditions and can be found in mixed mesophytic, subtropical to temperate forests of China and Japan (Huang et al. Citation1999).
The three East Asian and two of the western Eurasian species of Quercus Group Cerris can be categorised as meridio-nemoral and nemoral elements; the remaining five western Eurasian species with a combined range from the eastern Mediterranean to the Levant and eastern Iran as semihumid-meridional (File S1).
Quercus sp. 2 (Quercus Group Ilex)
(–)
Description
Pollen, monad, prolate to spheroidal, outline lobate in polar view, elliptic in equatorial view; polar axis 22–24 µm long in LM, 19–21 µm long in SEM, equatorial diameter 20–22 µm wide in LM, 16–18 µm wide in SEM; tricolporate; exine 0.9–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, rugulate to microrugulate, perforate in SEM, rugulae partly interwoven (SEM).
Remarks
The pollen morphology (LM and SEM) of Quercus Group Ilex has been presented by Miyoshi (Citation1982), Wei (Citation2003), Wang and Pu (Citation2004), Denk and Grimm (Citation2009b), Fujiki and Ozawa (Citation2007), Makino et al. (Citation2009), Li et al. (Citation2011a), Miyoshi et al. (Citation2011) and Denk and Tekleva (Citation2014). The pollen ultrastructure of this group has been documented by Wang and Pu (Citation2004) and Denk and Tekleva (Citation2014).
Fossil record
Because of its generally plesiomorphic type (Denk & Grimm Citation2009b), pollen of Quercus Group Ilex cannot be as straightforwardly identified as pollen of the other intrageneric groups of oaks. Nevertheless, due to a recent study covering all species of Group Ilex and focussing in particular on intragroup/intraspecies variation (Denk & Tekleva Citation2014), it is possible to identify types that are indistinguishable from pollen of modern species of Group Ilex. The oldest records of Group Ilex are from the early Oligocene of Central Europe (Denk et al. Citation2012), whereas similar but older pollen from western Greenland represents an extinct lineage of Quercus/Fagaceae (Grímsson et al. Citation2015b). In western Eurasia, Group Ilex becomes most diverse in the Miocene of the eastern Mediterranean, where macrofossil remains with affinity to either the western Eurasian Q. ilex L. as well as several East Asian species of Group Ilex are abundant and collectively addressed as Q. drymeja Unger and Q. mediterranea Unger (electronic supplement to Denk et al. Citation2014; Velitzelos et al. Citation2014). Pollen of Group Ilex has also been documented for the Miocene of eastern China (Liu et al. Citation2007), the Pleistocene and Holocene of Japan (Nakagawa et al. Citation1996; Tekleva et al. Citation2014) and from the Miocene onwards in Central Europe (Ferguson et al. Citation1998; Van der Burgh & Zetter Citation1998; Hofmann et al. Citation2002)
Ecological implications
Traditionally, members of this group have been considered to indicate seasonal, subtropical conditions with pronounced phases of draught (‘subxerophytic’; e.g. Kovar-Eder et al. Citation2004) probably because of the distribution of Quercus ilex and Q. coccifera L. in the Mediterranean region and the fact that all species are evergreen (e.g. Axelrod Citation1983). However, only three (Q. aucherii Jaub. et Spach, endemic to southwest Turkey; Q. alnifolia Poech, endemic to Cyprus; Q. coccifera, widespread in the Mediterranean) of the four western Eurasian species are limited to summery-dry, Mediterranean climates (Csa, Csb). The most widespread species, Q. ilex, extends its range into fully humid, mostly subtropical habitats (e.g. Petroselli et al. Citation2013), profiting from global warming trends (Delzon et al. Citation2013) and is a common element in various habitats of the Mediterranean region including dry and mesic forests.
The same holds for the western Asian and East Asian members of the group which have a distribution spanning from the southern flanks of the Himalayas, where they can locally occur until nearly 4000 m a.s.l. (Bisht et al. Citation2013), via the southern half of China (Huang et al. Citation1999), into low- to mid-altitudes of southern and central Japan (southern islands, Hokkaido; Ohwi Citation1965) where they grow usually in montane, mixed mesophytic and subalpine forests under climates with moderate to high summer precipitation (Cfa-, Cfb-, Cwa-, Cwb-, Dwa-, Dwb-climates). As exemplarily illustrated in the Miocene of western Turkey, the fossil members of this group thrived in a similar environment (Denk et al. Citation2014). Overall, most species of Group Ilex can be categorised as nemoral elements, but also include meridio-nemoral (Q. ilex) and semihumid-meridional taxa (File S1).
Quercus sp. 3 (Quercus Group Ilex)
(–)
Figure 8. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Fagaceae pollen. A–F. Quercus sp. 3 (Quercus Group Ilex), close-ups of central mesocolpium. G–L. Quercus sp. 4 (Quercus Group Ilex), close-ups of mesocolpium (I) and area around colpus (L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
Description
Pollen, monad, prolate to spheroidal, outline circular to lobate in polar view, elliptic in equatorial view; polar axis 18–21 µm long in LM, 16–18 µm long in SEM, equatorial diameter 17–19 µm wide in LM, 13–15 µm wide in SEM; tricolporate; exine 0.8–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microrugulate in SEM, microrugulae clustered, clusters densely packed (SEM).
Remarks
The rugulae in Quercus sp. 2 are much longer than the rugulae in Quercus sp. 3. The rugulae in Quercus sp. 2 are forming a single layer, but are clustered in Quercus sp. 2 and the clusters are showing different relief.
Quercus sp. 4 (Quercus Group Ilex)
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 20–23 µm long in LM, 17–19 µm long in SEM, equatorial diameter 14–16 µm wide in LM, 11–13 µm wide in SEM; tricolporate, pori small, indistinct; exine 0.8–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, microrugulate, perforate in SEM, microrugulate sometimes forming clusters, cluster irregularly distributed (SEM).
Remarks
The rugulae in Quercus sp. 2 are much longer than the rugulae in Quercus sp. 4. The rugulae in Quercus sp. 2 are forming a single layer, but are partly clustered in Quercus sp. 4, and the clusters are showing different relief. Quercus sp. 3 shows clear pori (LM) that are not visible in Quercus sp. 4. The rugulae of Quercus sp. 4 are much shorter and narrower than the rugulae in Quercus sp. 3. The clusters in Quercus sp. 4 are also fewer, smaller and wider apart than observed in Quercus sp. 3.
Quercus sp. 5 (aff. Quercus Group Cyclobalanopsis)
(–)
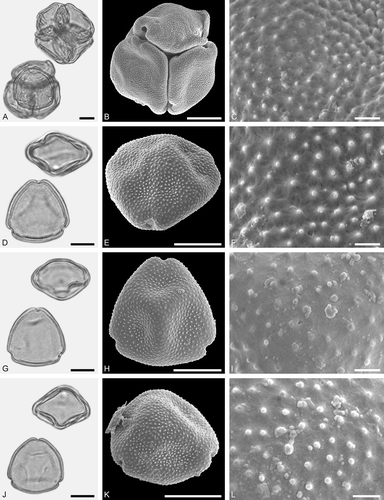
Figure 9. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Fagaceae pollen. A–L. Quercus sp. 6 (Quercus Group Quercus/Lobatae), close-ups of central mesocolpium. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
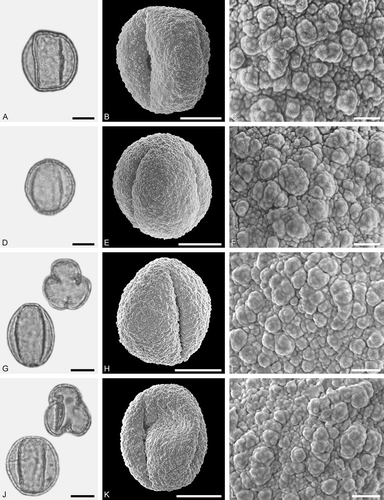
Figure 10. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Fagaceae pollen. A–C. Quercus sp. 7 (Quercus Group Quercus/Lobatae), close-ups of area around colpi. D–F. Quercus sp. 5 (aff. Quercus Group Cyclobalanopsis), close-up of mesocolpium. G–L. Trigonobalanopsis sp., close-up of mesocolpium. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 20–22 µm long in LM, 19–21 µm long in SEM, equatorial diameter 12–14 µm wide in LM, 12–14 µm wide in SEM; tricolporate; exine 0.6–0.7 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, rugulate, perforate, fossulate in SEM, rugulae with a microechinate suprasculpture (SEM).
Remarks
Pollen morphology (LM and SEM) of Quercus Group Cyclobalanopsis has been described by Miyoshi (Citation1982), Wei (Citation2003), Wang and Pu (Citation2004), Fujiki and Ozawa (Citation2007), Denk and Grimm (Citation2009b), Makino et al. (Citation2009), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The pollen ultrastructure (TEM) of this group has been presented by Wang and Pu (Citation2004) and Denk and Tekleva (Citation2014).
Fossil record
A cupule likely representing Quercus Group Cyclobalanopsis and associated with Quercus acorns of unknown affinity has been found in the nuts beds of the middle Eocene Clarno Formation of western North America (Manchester Citation1994), and represents the oldest record of this otherwise purely East Asian clade of oaks. Oldest records in East Asia date to the late Eocene of Japan (leaves and fruits; Huzioka & Takahashi Citation1970), and have been occasionally reported from the Oligocene onwards in Chinese and Japanese macrofossil assemblages (e.g. Ishida Citation1970; Manning Citation1978; Yabe Citation2008). The only pollen grains so far documented using SEM were from the Pleistocene of Nepal (Nakagawa et al. Citation1996) and, possibly, Miocene of eastern China (Liu et al. Citation2007). The Lavanttal pollen would represent the first Eurasian record of Group Cyclobalanopsis outside its modern distribution range, indicating that this today (tropical-)subtropical East Asian oak lineage extended into Europe in the middle to late Miocene. With respect to all currently available data on oaks, such an extension of the range appears unlikely, and it cannot be entirely ruled out that the pollen is underdeveloped pollen of a red oak (Group Lobatae; Solomon Citation1983).
Ecological implications
Members of the Group Cyclobalanopsis are usually found in the subtropical (meridional) regions of East Asia with hot summers and sufficient or excess precipitation during growing season (see Fang et al. Citation2009). Their main habitats are wet, mixed or broad-leaved evergreen forests, in lowlands, valleys and on mountain slopes; several species are also found in montane, nemoral mixed-mesophytic forests, including mountain tops, at altitudes of up to 2800 m (Huang et al. Citation1999). Most species of Group Cyclobalanopsis can be categorised as semihumid-meridional or meridio-nemoral elements (File S1). If oaks with affinities to Group Cyclobalanopsis thrived in Lavanttal, they would be indicative of wet, subtropical conditions in the lowlands, where they formed part of mixed evergreen-deciduous forests as today found in southern China.
Quercus sp. 6 (Quercus Group Quercus/Lobatae)
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 25–28 µm long in LM, 23–27 µm long in SEM, equatorial diameter 19–25 µm wide in LM, 18–24 µm wide in SEM; tricolporoidate; exine 0.9–1.2 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microverrucate, perforate in SEM, microverrucae often fused to form large agglomerates of different shape, microverrucae with a granulate suprasculpture (SEM).
Remarks
Pollen morphology (LM and SEM) of Quercus Group Quercus/Lobatae has been described and figured by Crepet and Daghlian (Citation1980), Lieux (Citation1980), Miyoshi (Citation1982), Jones et al. (Citation1995), Wei (Citation2003), Wang and Pu (Citation2004), Denk and Grimm (Citation2009b), Makino et al. (Citation2009), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The pollen ultrastructure (TEM) of these groups has been documented by Crepet and Daghlian (Citation1980) and Denk and Tekleva (Citation2014).
Fossil record
Oldest fossils that can be unambiguously linked to either Group Quercus, the ‘white oaks’, and/or Group Lobatae, the ‘red oaks’, are fossil pollen reported from the middle Eocene of Axel-Heiberg Island, Arctic Archipelago, north-eastern Canada (McIntyre Citation1991) and western Greenland (Grímsson et al. Citation2015b); and subsequently (Eocene–Oligocene boundary) from the eastern foothills of the Rocky Mountains (Bouchal et al. Citation2014) and the early Oligocene of Central Europe (Denk et al. Citation2012). Seeds with basal abortive ovules, today only found in the northern hemispheric Group Quercus and the exclusively Eurasian Groups Cerris and Ilex, have been reported from the middle Miocene of Washington, north-western United States (Borgardt & Pigg Citation1999), in association with potential white oak foliage. Four of the eight Quercus pollen morphotaxa figured by Liu et al. (Citation2007) from the Miocene of China are representing this lineage and this pollen type has also been found in Holocene of the Sea of Japan (Tekleva et al. Citation2014). Pollen of this lineage has also been reported from the middle to late Miocene of Iceland, indicating episodic migration of oaks across the North Atlantic Land Bridge (Denk et al. Citation2010, Citation2011).
Ecological implications
Both groups of oaks are ecologically and taxonomically highly diverse in North America (c. 200 species) including evergreen species adapted to diverse habitats (such as chaparral, wood steppe, humid-subtropical forests) as well as deciduous species populating mesic to dry subtropical to boreal forests (e.g. Nixon Citation1997), including such dominated by or including beech trees (Fagus; e.g. Maycock Citation1994). Particularly in Group Lobatae, species are found that thrive along river beds and in back-swamp forests (Nixon Citation1997). In the south-western part of the United States and across Mexico, white and red oaks are found in distinctly dry (semi-arid) settings (e.g. Baja California, lee-ward parts of the Sierra Madre). Red and white oaks are co-dominant in several North American vegetation zones such as the Oak-Hickory and Oak-Chestnut regions (southern United States) and the Oak-Pine Region (south-eastern United States) and Oak-Chestnut Region (Atlantic coast states; Maycock Citation1994) In Eurasia, Group Lobatae is extinct (see Denk et al. Citation2012), and Group Quercus is limited to deciduous species of temperate (nemoral to boreal) forests (Schroeder Citation1998). About five species can be found in East Asia, the most widespread being Quercus mongolica Fischer ex Ledeb., which ranges from Siberia and Mongolia to Japan, Sakhalin and the Kuril Islands. In China, the species are restricted to mixed mesophytic deciduous forests at 0–2700 m a.s.l. (Huang et al. Citation1999).
Although the Eurasian species of Group Quercus are typically found in fully humid warm-temperate to snow climates (Cfa, Cfb, Dfb), similar to Fagus and Corylus, they include a few species in western Eurasia that have adapted to summer-dry Mediterranean and near-steppe climate conditions (Cs- and BS-climates; e.g. subsection Galliferae; Tschan & Denk Citation2012). The northern limit of white oaks in North America and Eurasia marks the boundary between snow climates with warm summers (Dfb) and cool (short) summers (Dfc). Species of Groups Quercus and Lobatae can be categorised as tropical-meridional, eurytropical, semihumid-meridional, meridio-nemoral, nemoral or boreal elements (File S1). Given the high variation of habitats and climate conditions covered by individual members of Quercus Groups Quercus and Lobatae, particular in North America, no discrete conclusions can be drawn on their role in the Lavanttal biota at this point.
Quercus sp. 7 (Quercus Group Quercus/Lobatae)
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 32–34 µm long in LM, 31–33 µm long in SEM, equatorial diameter 26–28 µm wide in LM, 22–24 µm wide in SEM; tricolporoidate; exine 1.1–1.3 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microverrucate in SEM, microverrucae often forming small rounded clusters, clusters irregularly distributed (SEM).
Remarks
Quercus sp. 7 has a clear poroid (LM) germination area that is not observed in Quercus sp. 6. The fused microverrucae in Quercus sp. 6 form large agglomerates that are not comparable to the small ones observed in Quercus sp. 7. The agglomerates in Quercus sp. 7 do not show the granulate suprasculpture observed in Quercus sp. 6.
Genus Trigonobalanopsis Kvaček et H.Walther (extinct)
Trigonobalanopsis sp.
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 16–21 µm long in LM, 13–18 µm long in SEM, equatorial diameter 11–16 µm wide in LM, 9–12 µm wide in SEM; tricolporate, colpi long; exine 0.7–1.1 µm thick (LM), nexine as thick or slightly thicker than sexine; tectate; sculpturing psilate in LM, rugulate to microrugulate, perforate in SEM, parallel rugulae forming irregularly arranged groups, rugulae segmented (SEM).
Remarks
Fossil pollen showing LM-based morphological affinities to pollen of the extinct Trigonobalanopsis have sometimes been assigned to the pollen form-species Cupuliferoipollenites oviformis (Potonié) Potonié ex Potonié (e.g. Stuchlik et al. Citation2014).
Fossil record
Trigonobalanopsis fossils (leaves, cupules, fruits) are numerous in the European late Cainozoic record. Trigonobalanopsis exacantha (Mai) Kvaček et H.Walther cupules/fruits have been documented from the Miocene of Germany and the Czech Republic (e.g. Kvaček & Walther Citation1988). Leaves of T. rhamnoides (Rossm.) Kvaček et H.Walther have been documented from the late Eocene of the Czech Republic, the Oligocene of Germany, the Miocene of Poland, Germany, Austria and the Czech Republic (e.g. Kvaček & Walther Citation1988, Citation1989). Pollen assigned to T. schmidtii H.Walther et Zetter has been described from laminar surface of fossil T. rhamnoides leaves from the Miocene of Germany (Walther & Zetter Citation1993). Dispersed Trigonobalanopsis pollen has been described, using combined LM and SEM, from the early Oligocene of Germany (Denk et al. Citation2012), the early Miocene of Austria (Meller et al. Citation1999) and the late Miocene of Iceland (Denk et al. Citation2011).
Ecological implications
Mai (Citation1995) and Meller et al. (Citation1999) listed Trigonobalanopsis in several Eocene to Miocene floras in Europe, in association with predominately subtropical as well as distinctly temperate elements. In the Oligocene of Central Europe, Trigonobalanopsis is found part of a flora that supposedly thrived in a humid, warm temperate climate (Denk et al. Citation2012). In Iceland, pollen of Trigonobalanopsis is found in the 10 Ma (in a similar setting) and 4.2–3.8 Ma floras (after substantial local cooling; Denk et al. Citation2011, Citation2013). More precise ecological and climatic preferences of this extinct Fagaceae have yet to be established.
Family Juglandaceae DC. ex Perleb
Subfamily Engelhardioideae Iljinsk.
Engelhardioideae gen. et sp. indet.
(–)
Figure 11. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Juglandaceae pollen. A–L. Engelhardioideae gen. et sp. indet. (Alfaroa vel Engelhardia vel Oreomunnea), pollen in tetrad (A), single grains (D, G, J), close-ups of polar area. Scale bars – 10 μm (A, B, D, E, G, H, J, K), 1 μm (C, F, I, L).
Description
Pollen, monad (rarely in tetrads), oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 17–19 µm long in LM, equatorial diameter 23–27 µm wide in LM, 18–26 µm wide in SEM; triporate; exine 0.8–1.1 µm thick, nexine thinner than sexine; tectate; sculpturing psilate to scabrate in LM, microechinate in SEM, microechini at regular interval, microstriate to microrugulate pattern radiating from base of echini (SEM).
Remarks
The subfamily Engelhardioideae includes four well-defined extant genera that are supported by both molecular and morphological data; Alfaroa, Alfaropsis, Engelhardia (often misspelled as ‘Engelhardtia’; see Dilmy Citation1955) and Oreomunnea (e.g. Manchester Citation1987b; Manos & Stone Citation2001; Manos et al. Citation2007; Zhang et al. Citation2013). The most comprehensive work on Juglandaceae pollen including species representing every extant genera of the family is that of Stone and Broome (Citation1971, Citation1975). Pollen of Rhoiptelea, a genus recently transferred into the family (APG Citation2009: subfamily Rhoipteleoideae), has also been studied in detail by Skarpy et al. (Citation2009). Alfaroa, Alfaropsis (as Engelhardia roxburghiana Wall.), Engelhardia and Oreomunnea pollen has been studied and figured using LM, SEM and TEM by Whitehead (Citation1965) and Stone and Broome (Citation1975). Fossil pollen showing LM-based morphological affinities to pollen of modern Engelhardioideae have commonly been assigned to the pollen form-genus Momipites (e.g. Nichols Citation1973; Stuchlik et al. Citation2009). Based on the morphology of the pollen from the Lavanttal Basin, in comparison to pollen from extent genera of Engelhardioideae, it could be affiliated with Alfaroa, Engelhardia or Oreomunnea. Based on the complete fossil record of Engelhardioideae detailed most recently by Manchester (Citation1987b), the pollen probably originates from either Oreomunnea or Engelhardia, but is more likely affiliated with Oreomunnea since it has a much richer and widespread European macrofossil record during the Miocene than Engelhardia.
Fossil record
Engelhardioideae have a rich and well-documented fossil record in the Cainozoic of the Northern Hemisphere composed of fruits, leaves, wood, catkins and in situ and dispersed pollen (see partly table 4 in Manchester Citation1987b). Pollen of the Engelhardioideae-type has been reported from the Upper Cretaceous and Paleocene of North America and the Paleocene of Europe (e.g. Muller Citation1981; Manchester Citation1989b). Based on correlation with macrofossils, all Engelhardioideae pollen records prior to the Eocene are questioned by Manchester (Citation1987b, Citation1989b) and considered to represent extinct lineages that have not reached the level of extant genera. Engelhardioideae-type catkins/inflorescences (Eokachyra aeolius Crepet, Dilcher et Potter; Eoengelhardia puryearensis Crepet, Daghlian et Zavada) with in situ pollen characteristic for the subfamily have been described from the middle Eocene of Tennessee, east south-central United States (Crepet et al. Citation1975, Citation1980). Different Engelhardioideae-type fruits, Palaeocarya puryearensis (Berry) Manchester and Paraengelhardtia eocenica Berry, have been reported from the same locality (e.g. Manchester Citation1987b, Citation1989b). The earliest records of Engelhardioideae-type wood (Engelhardioxylon nutbedensis Manchester; E. texana Manchester) come also from North America, i.e. the middle Eocene of south-central and north-western United States (Manchester Citation1983). Wood of Engelhardioideae has also been recorded from the late Eocene of Europe (E. macrocrystallosum H.Gottwald; Gottwald Citation1992), and from the Oligocene of the Russian Far East (E. mameticum Blokh. et Snezhk.; Blokhina et al. Citation2002; Blokhina Citation2004, Citation2007). Fossil Engelhardioideae fruits are considered the most reliable and easily affiliated organs of the subfamily.
According to Manchester (Citation1987b), Alfaroa-type fruits are not known from the fossil record, but Oreomunnea-like (Palaeocarya sect. Palaeocarya) and Alfaropsis-/Engelhardia-like (Palaeocarya sect. Monocosta) types of fruits are numerous throughout the Cainozoic of North America and Eurasia. Approximately eight different fruit species, four occurring in the middle to late Eocene of the United States (California, Oregon, Kentucky, Tennessee, Washington), one from the Oligocene (Oregon) and one from the Miocene (Idaho) of the United States, one from the Miocene of East Asia (Japan, Korea), and one ranging from the middle Eocene to Pliocene of Europe, have been affiliated with Oreomunnea and assigned to sect. Palaeocarya (e.g. Jähnichen et al. Citation1977, Citation1984; Tanai & Uemura Citation1983; Manchester Citation1987b). Fossils of sect. Monocosta are rare in the fossil record and only three species, two from the Eocene of the United States (Mississippi, Utah), and one from the late Oligocene of Europe (France), have been affiliated with Alfaropsis/Engelhardia and assigned to this section (e.g. Jähnichen et al. Citation1977; Manchester Citation1987b). Newly described fruit fossils (Oreomunnea grahamii Manchester et Herrera) from the early Miocene of Panama represent the earliest macrofossil record of Oreomunnea (Herrera et al. Citation2014). Alfaropsis/Engelhardia-type leaves are hard to identify because of convergences with leaves of other genera/families and their record is minute and unreliable (e.g. Manchester Citation1987b). Fossil Oreomunnea/Alfaroa-like leaflets are frequent in the Cainozoic record of Europe (Kvaček Citation1972; Jähnichen et al. Citation1977; Citation1984; Jähnichen Citation1991) and south-eastern United States (Dilcher & Manchester Citation1986). These have mostly been assigned to two species, Oreoroa orsbergensis (Wessel et Weber) Dilcher et Manchester, from the middle Eocene to late Miocene of Europe, and Oreoroa claibornensis Dilcher et Manchester, from the middle Eocene of east south-central United States (Dilcher & Manchester Citation1986).
Ecological implications
The genera of Engelhardioideae are all relatively small, Alfaroa comprises five species, Alfaropsis is composed of only a single species, Engelhardia includes c. six species, and Oreomunnea has two species (e.g. Manning Citation1966, Citation1978; Stone Citation1968, Citation1977, Citation2010; Lu et al. Citation1999). Oreomunnea and Alfaroa occur in the Americas, Alfaropsis and Engelhardia in East Asia. In the Americas, Oreomunnea has a more restricted distribution than Alfaroa. Oreomunnea mexicana (Standl.) J.-F.Leroy ranges from southern Mexico to Costa Rica, occurring at altitudes of 600 to 1900 m (Stone Citation1977). Oreomunnea pterocarpa Oerst. is endemic to Costa Rica, growing at altitude of 200 to 1500 m. This species is mostly restricted to the rainforests of the Cartago Province, where it is a typical canopy tree at mid-elevation sites where the annual rainfall approaches 7000 mm/yr (Stone Citation1977). Alfaroa has a wider distribution occurring from Mexico to Columbia and the West Indies. Alfaroa costaricensis Standl. is occurring in Mexico, Guatemala, Costa Rica and Panama, growing at altitude of 600 to 3200 m (Stone Citation1977). Alfaroa guatemalensis (Standl.) L.O.Williams et Ant.Molina is endemic to Guatemala; there it occurs at altitude of 1200 to 2200 m and is often growing on rich volcanic soil in the mountain forests (Stone Citation2010). Alfaroa manningii J.León is endemic to Costa Rica, growing at altitude of 300 to 1300 m in the premontane rainforest (Stone Citation1977). Alfaroa mexicana D.E.Stone is occurring in Mexico and Mesoamerica, and can be found in rich deciduous forests at altitudes of 730 to 1700 m (Stone Citation1968). Alfaroa williamsii Ant.Molina is occurring in Mesoamerica and Colombia, growing at an altitude of 1100 to 2300 m.
In conclusion, both Alfaroa and Oreomunnea thrive in wet habitats under subtropical to tropical climates (A-, Cwa-, Cfa-climates). In Asia, Engelhardia has a wide distribution ranging from India over eastern Asia and into Melanesia (A-climates, hot Cwa-climates; e.g. Manning Citation1966, Citation1978; Lu et al. Citation1999). In China, the three Engelhardia species, E. hainanensis P.Y.Chen, E. serrata Blume and E. spicata Blume, occur in rich valley forests and/or on mountain slopes, and depending on the species can be found from sea level to an altitude of 2100 m (Lu et al. Citation1999). Alfaropsis has a more discontinuous distribution occurring in hilly and mountain regions of eastern Pakistan, southern China, Taiwan, Vietnam, Sumatra and Borneo. Alfaropsis roxburgiana (Wall.) Iljinsk., the sole species of the genus, occurs in mixed broad-leaved and/or evergreen forest in China at an altitude of 200 to 1500 m (e.g. Manning Citation1966, Citation1978; Lu et al. Citation1999). Except for Alfaropsis, all other modern members of Engelhardioideae have a restricted distribution with focus on the tropical-subtropical climate zones (A-climates, and warmest variants of Cwa-/Cfa-climates). Species of Alfaroa and Oreomunnea occur in the premontane rainforests of Central America (tropical A-climates) as well as in the succeeding temperate virgin and cloud forests at elevations of up to 1700 m in (southern) Mexico and 2200 m in Costa Rica (Cfa-, Cwb-climates; Stone Citation1977, Citation2010) and could be categorised as tropical-meridional or meridio-nemoral elements (File S1).
The distribution in the past was, however, much less restricted (Kvaček Citation2007, addressed as ‘Engelhardia’), and fossils with affinities to Engelhardioideae are frequently found in association with fully temperate elements indicating that they were more tolerant regarding temperature regimes than their modern relatives (e.g. Kvaček Citation2007). Also, Manchester (Citation1987b) concluded that based on the diversity of Engelhardioideae from the middle Eocene of North America, that included different species of Palaeocarya, and also Paleooreomunnea and Paraengelhardtia, suggested a primary radiation of this group under a subtropical climate. In conclusion, fossil Engelhardioideae should be scored as (A+)Cfa+Cfb+Cwa+Cwb taxa regarding their ‘Köppen signature’; and possibly included also nemoral elements following the definition of Denk et al. (Citation2013).
Subfamily Juglandoideae Eaton
Genus Carya Nutt.
Carya sp.
(–)
Description
Pollen, monad (rarely tetrads), oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 28–33 µm long in LM, equatorial diameter 41–49 µm wide in LM, 41–44 µm wide in SEM; triporate, one or more pori subequatorially positioned; exine 1.3–1.8 µm thick (LM), nexine thinner than sexine, exine thinner in distal central polar area, thinning circular in outline; tectate; sculpturing scabrate in LM, microechinate in SEM, microechini at regular interval, microstriate to microrugulate pattern radiating from base of echini (SEM).
Remarks
The subfamily Juglandoideae includes five well-defined extant genera that are supported by both molecular and morphological data: Carya (incl. Annamocarya), Juglans and Pterocarya; and the monotypic Cyclocarya and Platycarya (e.g. Manchester Citation1987b; Manos & Stone Citation2001; Manos et al. Citation2007; Zhang et al. Citation2013). Pollen morphology (LM and SEM) and ultrastructure (TEM) of Carya has been presented in detail by Stone and Broome (Citation1975). Pollen of various extant Carya species has also been studied (LM, SEM, rarely TEM) by Stone (Citation1963), Whitehead (Citation1963, Citation1965), Stone et al. (Citation1964), Lieux (Citation1980), Liu (Citation1987), Jones et al. (Citation1995), Wang et al. (Citation1995), Fritz and Allesch (Citation1999), Beug (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). Fossil pollen showing LM-based morphological affinities to pollen of modern Carya have commonly been assigned to the pollen form-genus Caryapollenites (e.g. Krutzsch Citation1961; Stuchlik et al. Citation2009).
Fossil record
Carya has a fairly rich fossil record in the Cainozoic of the Northern Hemisphere composed of fruits, leaves, wood, catkins and in situ and dispersed pollen (see table 5 in Manchester Citation1987b). Carya-like pollen is known from several localities from the late Paleocene of North America and Europe (e.g. Muller Citation1981; Manchester Citation1987b, Citation1989b; Zetter et al. Citation2011). It has been pointed out that these pollen grains are smaller than pollen of extant Carya and evidence from the fruit record also indicates that the modern Carya had not yet diverged. Fossil pollen comparable to pollen of extant Carya, in both size and morphology, is first known from the Eocene and/or Oligocene of North America, Europe and western Siberia, and from the middle Miocene of eastern Asia. The pollen record further suggests that Carya thrived in Europe until the late Pleistocene (e.g. Muller Citation1981; Manchester Citation1987b, Citation1989b).
The fossil record of Carya fruits has been summarised and revised by Mai (Citation1981a) and Manchester (Citation1987b). These studies acknowledge 14 species from the late Eocene to late Pliocene of Europe, two species from the Oligocene and Miocene of western Siberia, one from the Miocene of China, four from the Miocene and Pliocene of Japan, and two species from the early Oligocene and Miocene of the Unites States (Mai Citation1981a; Manchester Citation1987b). Carya catkins/inflorescences (C. florissantensis Manchester) with in situ Carya-type pollen have been described from the early Oligocene of Colorado, USA (Manchester Citation1987b). Fossil wood affiliated with Carya is rare, but has been documented from the late Cainozoic of Europe and Japan. These include Eucaryoxylon crystallophorum W. R. Müller-Stoll et E. Mädel from the Miocene of Hungary (Müller-Stoll & Mädel Citation1960), E. moenanum W. R. Müller-Stoll et E. Mädel-Angeliewa from the late Pliocene of Germany (Müller-Stoll & Mädel-Angeliewa Citation1983) and C. protojaponica Watari from the Miocene of Japan (Watari Citation1952). There are numerous findings of fossil Carya leaves/leaflets from the Cainozoic of North America and Eurasia, but Carya leaves are hard to identify with certainty because of the overlap with leaves/leaflets of Cyclocarya, Juglans and Pterocarya. In his monograph on fossil Juglandaceae, Manchester (Citation1987b) does not revise the leaf record of Carya or Carya-like fossils, but names five species based on leaf material, which he considered representing extant Carya. These include C. sessilis MacGinite from the Eocene of California (USA; MacGinite Citation1941), C. cashmanensis Wolfe and C. pugetensis Wolfe from the Eocene of Washington (USA; Wolfe Citation1968), C. libbeyi (Lesq.) MacGinite from the early Oligocene of Colorado (Unites States; MacGinite Citation1953) and C. serraefolia (Göpp.) Kräusel from the late Oligocene and Miocene of the Czech Republic (Europe; Knobloch Citation1961; Bůžek Citation1971). Fossil Carya leaves/leaflets have also been described from East Asia (e.g. Tanai Citation1992). It has been hypothesised that Carya originated in North America during the early Paleocene and dispersed into Europe during late Paleocene or early Eocene time over the North Atlantic Land Bridge and probably dispersed further into Asia following the closure of the Turgai Seaway in the early Oligocene (Zhang et al. Citation2013). The two major clades within Carya, corresponding to the extant lineages of eastern Asia and eastern North America, are thought to have split latest in the Miocene (Zhang et al. Citation2013).
Ecological implications
Carya is composed of c. 15 tree species showing a disjunct distribution in subtropical to mild temperate areas of eastern North America (11 native species; Stone Citation1997) and eastern Asia (four native species; Lu et al. Citation1999). Carya is considered a monophyletic genus composing two well supported clades that correspond to the eastern Asian group and the eastern North American group (e.g. Zhang et al. Citation2013). The species ranging in habitat from temperate mixed mesophytic forests to mountain rain forests (Stone Citation1997; Lu et al. Citation1999). The climatic and ecological niche of East Asian and North American Carya is relatively equivalent, the distribution focus lies in the subtropical, warm temperate climates with hot summers with sufficient precipitation during growing season (Cfa, Cwa), and most species thriving close to water bodies and floodplains (Stone Citation1997; Lu et al. Citation1999). Most species can be categorised as meridio-nemoral elements (File S1). In the Lavanttal, Carya may have thrived in a habitat similar to its modern representatives in the south-eastern quarter of the United States, as part of thermophilic wetland forest.
Genus Juglans L.
Juglans sp.
(–)
Figure 13. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Juglandaceae pollen. A–L. Juglans sp., pollen with varying number of pori (A, D, G, J), close-ups of distal polar area (C, L), and proximal polar area (F, I). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
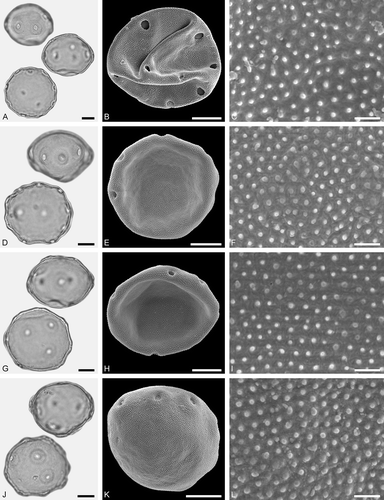
Description
Pollen, monad, oblate, heteropolar, outline circular to polygonal in polar and equatorial view; polar axis 30–35 µm long in LM, equatorial diameter 36–43 µm wide in LM, 33–40 µm wide in SEM; stephanoporate (10–12), often 1–3 pori positioned outside the equator on the distal polar side, pori annulate; exine 1.0–1.3 µm thick, nexine thinner than sexine; tectate; sculpturing scabrate in LM, microechinate in SEM, microechini numerous and at regular interval, microstriate to microrugulate pattern circling the base of echini (SEM).
Remarks
The LM and SEM based pollen morphology of many Juglans species has been investigated by Stone et al. (Citation1964), Whitehead (Citation1965), Ueno (Citation1967), Huang (Citation1972), Stone and Broome (Citation1975), Lieux (Citation1980), Jones et al. (Citation1995), Fritz and Allesch (Citation1999), Wei (Citation2003), Beug (Citation2004), Mert (Citation2010), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). The pollen ultrastructure (TEM) of Juglans has been presented by Stone et al. (Citation1964), and Stone and Broome (Citation1975). Fossil pollen showing LM-based morphological affinities to pollen of modern Juglans have commonly been assigned to the pollen form-genus Juglanspollenites (e.g. Raatz Citation1937; Stuchlik et al. Citation2009).
Fossil record
Juglans has a rich fossil record in the Cainozoic of the Northern Hemisphere composed mostly of fruits and dispersed pollen, but also leaves (see partly table 6 in Manchester Citation1987b). The LM-based pollen record of the genus has been summarised by Muller (Citation1981) and Manchester (Citation1987b, Citation1989b). The earliest pollen records of Juglans are from the Eocene of North America, but pollen of this genus first became widespread in North America and Eurasia during the Oligocene and Miocene. The fossil fruit record of Juglans is extensive with numerous North American and Eurasian records extending back to the Eocene.
Juglans fruits of sect. Rhysocaryon (black walnuts) include among others J. clarnensis Scott from the middle Eocene of Oregon (north-western United States), J. calvertiana Berry from the Miocene of Maryland and Virginia (north-eastern United States), J. nevadensis Berry from the Miocene of Nevada (south-western United States), J. siouxensis (Barbour) Berry from the Miocene of Nebraska (mid-western United States) and J. linkii Brown from the late Miocene/early Pliocene of north-western Ecuador that is the only megafossil of Juglans recorded from South America (Manchester Citation1987b). Juglans fruits of section Cardiocaryon (butternuts) include among others J. lacunosa Manchester from the early to middle Oligocene of Washington, J. tephrodes Unger from the middle/late Oligocene to Pliocene of Europe, J. megacinerea Miki ex Chaney from the Miocene to Pleistocene of Japan, J. siberica P.I.Dorof. from the Miocene western Siberia, J. eocinerea L.V.Hills, Klovan et A.R.Sweet from the Miocene of Banks Island in Arctic Canada (e.g. Kirchheimer Citation1957; Hills et al. Citation1974; Nirei Citation1975; Manchester Citation1987b).
There are many reports of fossil Juglans leaves/leaflets from the Cainozoic of North America and Eurasia, but Juglans leaves are hard to identify with certainty because of overlap with leaves/leaflets of Carya, Cyclocarya and Pterocarya. According to Manchester (Citation1987b), the leaf/leaflet record of Juglans needs to be revised.
Ecological implications
Juglans is the largest genus within the Juglandaceae, composed of c. 21 species, occurring in eastern and western North America, Central America, the West Indies and western South America, across Asia and south-eastern Europe (Manning Citation1978; Stanford et al. Citation2000). The genus is divided into four sections. Section Cardiocaryon includes three species, J. mandshurica Maxim., occurring in China, Taiwan and Korea; J. sigillata Dode, occurring in China and Korea; and J. ailantifolia Carrière, occurring in Japan. Section Dioscaryon includes only a single species, J. regia L., ranging from south-eastern Europe over to Iran and the Himalayas and into China. Section Rhysocaryon includes 16 species, all occurring in the Americas and the West Indies. Section Trachycaryon has only a single species, J. cinerea L., occurring in the eastern United States.
The genus shows a wide range of habitats, occurring in various different forest types, thriving in temperate to tropical climates (e.g. Manning Citation1978; Stone Citation1997; Lu et al. Citation1999; Stanford et al. Citation2000). Species of Juglans can be addressed as semihumid-meridional, meridio-nemoral or nemoral elements (File S1). The plants producing Juglans pollen could have been part of various lowland to mid-altitude habitats at Lavanttal, but are overall indicative of a climate characterised by a pronounced summer.
Genus Pterocarya Kunth.
Pterocarya sp.
(–)
Figure 14. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Juglandaceae pollen. A–L. Pterocarya sp., pollen with varying number of pori (A, D, G, J), close-ups of aperture membrane (C), distal polar area (E, L), and proximal polar area (J). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
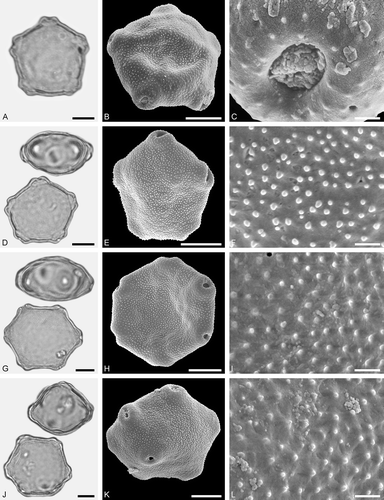
Description
Pollen, monad, oblate, sometimes heteropolar, outline polygonal in polar view, elliptic in equatorial view; polar axis 18–31 µm long in LM, equatorial diameter 29–43 µm wide in LM, 27–39 µm wide in SEM; stephanoporate (5–8); sometimes one pori positioned outside the equator on the distal polar side, pori annulate; exine 0.9–1.3 µm thick, nexine thinner than sexine; tectate; sculpturing scabrate in LM, microechinate in SEM, microechini at regular interval, microstriate to microrugulate pattern radiating from base of echini, pattern inconspicuous (SEM).
Remarks
pollen has been studied and figured using LM, SEM and TEM by Stone and Broome (Citation1975). Several other researchers have also investigated and figured LM and SEM based micrographs showing Pterocarya pollen from different extant species, e.g. Whitehead (Citation1965), Liu (Citation1987), Wang et al. (Citation1995), Fritz and Allesch (Citation1999), Beug (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). Fossil pollen showing LM-based morphological affinities to pollen of modern Pterocarya hase commonly been assigned to the pollen form-genus Polyatriopollenites (e.g. Pflug Citation1953; Stuchlik et al. Citation2009).
Fossil record
The macrofossil record of Pterocarya has been summarised by Kirchheimer (Citation1957) and Manchester (Citation1987b). The LM-based palynological record of Pterocarya and Pterocarya-like pollen has been summarised by Muller (Citation1981) and Manchester (Citation1987b, Citation1989b). Pterocarya-like pollen has been documented from the uppermost Cretaceous of Russia and North America, but as pointed out by Manchester (Citation1987b, Citation1989b), these grains are much smaller than pollen of extant Pterocarya and have only been studied using LM; all these records are considered doubtful and need further validation. The first Pterocarya-like pollen grains corresponding to pollen of extant Pterocarya are from the Paleocene of the Russian Far East and the United States; the oldest European record is from early Eocene. By the late Eocene, Pterocarya pollen are documented from most areas of the Northern Hemisphere, but come much more abundant in the Oligocene and Miocene (e.g. Muller Citation1981; Manchester Citation1987b).
Pterocarya fruits are well-documented from the Cainozoic of the Northern Hemisphere, with the earliest fruit, P. macginitti Manchester et Dilcher, occurring in the middle Eocene of Wyoming, USA (Manchester & Dilcher Citation1982). Additional North American species based on fruits include P. occidentalis Manchester from the Oligocene of California (western United States), P. eomacroptera Manchester and P. smiley Manchester from the Miocene of Idaho (north-western United States; Manchester Citation1987b). The European record of Pterocarya fruits has been summarised by Kirchheimer (Citation1957), suggesting that several species were present during the late Cainozoic in this part of the world. Pterocarya fruits have also been described from the Miocene of Iceland (Denk et al. Citation2011). According to Manchester (Citation1987b), the European Pterocarya fruit record needs to be revised. Various different Pterocarya fruits have also been described from the Oligocene and Miocene of Asia (e.g. Manchester Citation1987b). The earliest Pterocarya fruits are from the middle Eocene of North America and during the Oligocene the genus dispersed across North America, Europe and Asia, as documented by fossil fruits, dispersed pollen and leaves. During the Miocene it had a wide distribution across most of the Northern Hemisphere.
Ecological implications
Pterocarya is a small genus composing only six species of deciduous trees, five are native to East Asia and one occurs mainly in the Caucasus region (e.g. Manning Citation1978; Lu et al. Citation1999; Denk et al. Citation2001). The five East Asian species, P. hupehensis Skan, P. macroptera Batalin, P. rhoifolia Siebold et Zucc., P. stenoptera C.DC., P. tonkinensis Dode, distributed in China, Korea and Japan, occur mostly in moist forests, in lowlands, valleys or on mountain slopes. They often grow along riverbanks or streams and, depending on the species and the geographical region, ranging from sea level to an altitude of 3500 m (Lu et al. Citation1999). Pterocarya fraxinifolia (Poir.) Spach has a restricted distribution confined to the Caucasus region and Asia Minor, occurring mostly in the Hyrcanian mixed forests along the seashores of the Black and Caspian Seas at an altitude below 1000 m. Typically found in lowland riparian forests and on mountain slopes in rich mixed broadleaved deciduous forests, it occurs also at an altitude up to 1700 m in the Zagros Mountains of Iran (e.g. Akhani & Salimian Citation2003; Sheykholislami & Ahmadi Citation2009). Most East Asian species of the genus have also a (very) restricted distribution. The two species with non-restricted distributions (P. rhoifolia, P. stenoptera) are nemoral elements (File S1). Based on the modern habitat of Pterocarya, it is likely that the fossil Pterocarya pollen from the Lavanttal Basin originate from trees growing in and around the lowland or the surrounding hillsides and mountain slopes, especially along the banks of streams and rivers running into the basin.
Family Myricaceae A.Rich. ex Kunth
Myricaceae gen. et spec. indet. (Morella vel Myrica)
(–)
Figure 15. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Myricaceae and Cannabaceae pollen. A–F. Myricaceae gen. et spec. indet. (Morella vel Myrica), close-ups of polar area. G–L. Celtis sp. 1, close-ups of polar area. Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
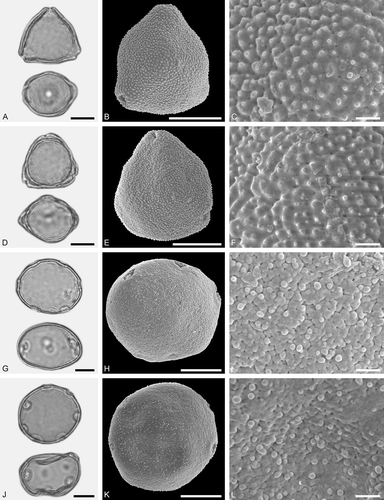
Description
Pollen, monad, oblate, outline convex triangular in polar view, elliptic in equatorial view; polar axis 19–21 µm long in LM, equatorial diameter 22–24 µm wide in LM, 20–23 µm wide in SEM; triporate, exine protruding in areas of apertures; exine 0.9–1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microechinate, perforate in SEM, numerous perforations in-between microechini.
Remarks
Pollen morphology (LM and SEM) and ultrastructure (TEM) of all four Myricaceae genera have been studied by Sundberg (Citation1985). Numerous other pollen studies include LM and SEM documentation of all Myrica and most Morella species (e.g. Huang Citation1972; Lieux Citation1980; Jones et al. Citation1995; Wang et al. Citation1995; Fritz & Allesch Citation1999; Punt et al. 2002; Beug Citation2004; Fujiki et al. Citation2005; Fujiki & Ozawa Citation2007; Li et al. 2011a; Miyoshi et al. Citation2011). Based on these studies, the distinctive feature of Myricaceae pollen is the so-called ‘Myrica’ type aperture. In the vicinity of the pores, the nexine is lacking and the sexine is thickened forming an annulus (Sundberg Citation1985). Pollen in Myricaceae is rather homogeneous and only the pollen of Canacomyrica and Comptonia are distinctive (Sundberg Citation1985). Pollen of the two extant Myrica species and the c. 47 Morella species overlap both in LM morphology and SEM sculpturing and cannot be set apart. The fossil Myricaceae type pollen from Lavanttal could therefore originate from plants belonging to Myrica or Morella.
Fossil pollen showing LM-based morphological affinities to pollen of modern Morella and Myrica has commonly been assigned to the pollen form-genus Myricipites -(e.g. Wodehouse Citation1933; Stuchlik et al. Citation2009).
Fossil record
Myricaceae comprise four genera, the monotypic Comptonia and Canacomyrica, the small Myrica (two species) and Morella with c. 47 species (e.g. Wilbur Citation1994; Huguet et al. Citation2005; Herbert Citation2005a, Citation2005b; Herbert et al. Citation2006). With the exception of Comptonia and Canacomyrica, most Myricaceae species have traditionally been assigned to Myrica. Pollen affiliated to Myricaceae has been documented from the Late Cretaceous of North America and the Paleocene of Eurasia, becoming more abundant during the Eocene and Oligocene of Europe. This type of pollen is also very frequent in Miocene sediments of Europe (e.g. Muller Citation1981). The nomenclatural problems associated with Myrica versus Morella (see later) have resulted in a confusing fossil record of the two genera. Even though leaves of Myrica (sunken stomata) can be distinguished from those of Morella, and the structure of their fruits are also distinguishable, most authors have assigned Morella fossils to Myrica (see Herbert Citation2005b). According to Mai (Citation1995), Myrica (s.l., incl. Morella) has an Eocene to Miocene fossil record in Europe. For an accurate circumscription of the fossil record of these two genera previous documentations including leaf fossils and carpological fossils needs to be revised.
Ecological implications
There are clear differences between the two deciduous species, the cold-tolerant Myrica gale L. found in warm temperate and snow climates with warm and cold summers (Cfb-, Cfc-, Dfb-, Dfc-climates) and the summer-draught tolerant, temperate Myrica hartwegii S.Watson (mostly Csb-climates), and the remaining predominately subtropical to tropical – thriving in the equatorial A-climates and warm temperate Cfa-, Cwa-, Csa-climates – evergreen species that are now all included in the genus Morella (cf. Huguet et al. Citation2005; Herbert Citation2005a, Citation2005b). The two Myrica species are deciduous shrubs with dry fruits adapted for dispersal by water. Myrica hartwegii is endemic to the northern and central Sierra Nevada of California, where it occurs along the borders of streams at elevation of 250 to 1800 m (Bornstein Citation1997). Myrica gale is widespread and has an almost circum-boreal distribution. Depending on the geographical region, it extends from sea level to c. 700 m a.s.l. It is a lowland wetland plant occurring along streams and lake margins, in ponds and bogs, wet heaths and fens, in marshes and sea lochs, and it is abundant in swampy environments (e.g. Bornstein Citation1997; Skene et al. Citation2000).
Morella comprises evergreen shrubs and small trees producing papillose fleshy fruits that are dispersed mostly by birds (Herbert Citation2005b). The shrubs or small trees are found in many habitats growing from sea level to c. 3000 m a.s.l., occurring in lowland valley forests as well as mountain slope forests. The plants are part of various different forest types, occurring in mixed evergreen conifer and broad-leaved forests, mixed deciduous-evergreen broad-leaved forests and evergreen broad-leaved forests (e.g. Bornstein Citation1997; Lu & Bornstein Citation1999). Most of the Morella species are, however, growing in wetland habitats, thriving near streams and rivers, in coastal swamps and marshes, around lakes margins, in bogs and around ponds or in wet meadows.
The two species of Myrica could be addressed as boreal and semihumid-meridional elements (File S1), whereas species of Morella could be categorised as tropical-meridional, semihumid-meridional, meridio-nemoral, or nemoral. Nevertheless, both Myrica and Morella require wetland habitats or sufficient inflow of water through their substrate; hence a similar habitat is suggested for the plants producing the fossil pollen from Lavanttal.
Order Rosales Bercht. et J.Presl
Family Cannabaceae Martynov
Genus Celtis L.
Celtis sp. 1
(–)
Description
Pollen, monad, spheroidal to oblate, outline circular to elliptic in polar view, elliptic in equatorial view; polar axis 21–25 µm long in LM, equatorial diameter 30–35 µm wide in LM, 27–30 µm wide in SEM; stephanoporate (5–9), pori sometimes subequatorially positioned, circular to elliptic, sexine slightly thickened around pori; exine 0.9–1.1 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, microrugulate, perforate, fossulate, microechinate in SEM, microechini irregularly distributed, apex of microechini blunt, pori membrane microrugulate, microechinate (SEM).
Remarks
The pollen morphology and ultrastructure of Cannabaceae has been studied in detail by Zavada (Citation1983) and Takahashi (Citation1989). Celtis pollen from numerous North American, European, African and Asian species have been investigated and figured using both LM and SEM, and sometimes TEM, by Huang (Citation1972), Zavada (Citation1983), Zavada and Dilcher (Citation1986), Takahashi (Citation1989), Lieux (Citation1980), Jones et al. (Citation1995), Stafford (Citation1995), Beug (Citation2004), Sattarian et al. (Citation2006), Zarafshar et al. (Citation2010), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). Fossil pollen showing LM-based morphological affinities to pollen of modern Celtis have commonly been assigned to the pollen form-genus Celtipollenites (Nagy Citation1969; Stuchlik et al. Citation2009).
Fossil record
The macrofossils record of Celtis dates back to the Paleocene of the Americas and East Asia with leaves and fruits of C. aspera (Newb.) Manchester, Akhmetiev et Kodrul, early Paleocene of western North America, eastern China and the Russian Far East, and endocarps from the late Paleocene of Brazil. Earliest macrofossil records in Europe are from the Eocene (see Manchester et al. Citation2002). The genus becomes a relatively common element of fossil assemblages (leaves, endocarps, flowers) in the Miocene of Europe (e.g. in Austria, Meller Citation1998, Citation2011; Kovar-Eder et al. Citation2004; Gross et al. Citation2014). Since the endocarps of Celtis have a high content of biogenic carbonate (e.g. Wang et al. Citation1997), they have a high preservation potential and can be found in vertebrate-bearing sediments lacking other plant remains (e.g. Gregor Citation1986). Fossil records from Africa are rare but endocarps have been reported from the Miocene of Kenya (Chesters Citation1957) and Pliocene hominid sites (Bonnefille Citation2010).
Ecological implications
Celtis is widely distributed in tropical and temperate regions of the Northern and Southern Hemisphere and comprises c. 60 species (Sherman-Broyles et al. Citation1997; Fu et al. Citation2003). The six North American species can be divided into two ecological groups. The eastern North American C. laevigata Willd., C. occidentalis L. and C. tenuifolia Nutt. occur along streams, in flood plains, forested hillsides and woodlands at low elevations, essentially in warm temperate, fully humid climates with hot summers (Cfa). Celtis lindheimeri Engelm. ex K.Koch, C. pallida Torr. (ranging from southernmost United States to northern Argentina) and C. reticulata Torr. occur in warm semi-dry to desert conditions, in canyons, on mesas, along washes, in brushland and grassland, often preferring hard, rocky substrate, occurring at altitudes of up to 2300 m (Sherman-Broyles et al. Citation1997). The four western Eurasian species are found in the meridional zone (see Schroeder Citation1998), the two widespread species C. australis L. and C. occidentalis L. in both dry and humid habitats (e.g. Meusel et al. Citation1965; Browics & Zieliński Citation1982). The 11 species of Celtis occurring in China are typically found in tropical monsoon rain forests (and extending into south Asia, southeast Asia and Australasia) and subtropical and mixed mesophytic forests from sea level up to 2400 m (Fu et al. Citation2003). In Africa, Celtis is part of several semi-humid to humid vegetation types such as the tropical and subtropical broad-leaved forests of eastern Guinea, Nigeria and Cameroon and the semi-deciduous forests of central and western Africa (e.g. Fayolle et al. Citation2014; Miller & Gosling Citation2014).
Most northern hemispheric species of Celtis can be categorised as meridio-nemoral or nemoral elements, three as tropical-meridional or tropical, and two as eurytropical (restricted to dry climates). In the light of the Fagales diversity, it can be assumed that the Celtis pollen found in the Lavanttal Basin originate from trees with similar habitat preferences as the modern eastern North American and East Asian species, thriving in the lowland wetlands and surrounding hillside mixed broad-leaved deciduous and evergreen forests.
Celtis sp. 2
(–)
Figure 16. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Cannabaceae and Elaeagnaceae pollen. A–C. Celtis sp. 2, close-up of polar area. D–F. Cannabaceae gen. et spec. indet., close-up of polar area. G–L. Elaeagnus sp., close-ups of area around colpus (I), and mesocolpium (L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
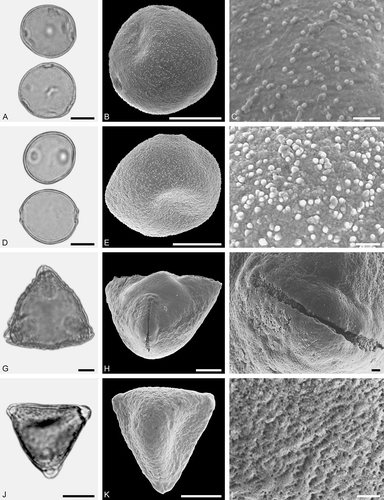
Description
Pollen, monad, spheroidal to oblate, outline circular to elliptic in polar view, elliptic in equatorial view; polar axis 21–23 µm long in LM, equatorial diameter 22–24 µm wide in LM, 20–22 µm wide in SEM; triporate, pori circular, sexine slightly thickened around pori; exine 0.9–1.1 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, microechinate, perforate in SEM, microechini irregularly distributed, pori membrane microechinate (SEM).
Remarks
The main difference between Celtis sp. 1 and Celtis sp. 2 is that the former is characterised by 5–9 pori that are sometimes at irregular intervals, but Celtis sp. 2 is triporate. The sculpturing (SEM) in Celtis sp. 1 is characterised by numerous perforations and fossulae, but these are only rarely occurring in Celtis sp. 2.
Cannabaceae gen. et spec. indet.
(–)
Description
Pollen, monad, spheroidal to oblate, outline elliptic in polar and equatorial views; polar axis 21–23 µm long in LM, equatorial diameter 23–25 µm wide in LM, 22–24 µm wide in SEM; diporate, pori circular, sexine thickened around pori, pori annulate; exine 0.8–0.9 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, microechinate, granulate, perforate in SEM, microechini irregularly distributed (SEM).
Remarks
Since we cannot affiliate this pollen type with any particular genus in the Cannabaceae, we refrain from drawing any further interpretation based on this pollen type.
Family Elaeagnaceae Juss.
Genus Elaeagnus L.
Elaeagnus sp.
(–)
Description
Pollen, monad, oblate, heteropolar, outline triangular in polar view, elliptic in equatorial view; equatorial diameter 27–52 µm wide in LM, 26–47 µm wide in SEM; tricolporate, colpi short, widening towards distal ends; exine 1.2–2.4 thick (LM), nexine thinner than sexine, sexine protruding around pori forming an atrium, area around colpi also protruding; tectate; sculpturing scabrate in LM, microrugulate, granulate, perforate in LM, colpus membrane microverrucate (SEM).
Remarks
We were unable to find any comprehensive comparative palynological study of extant Elaeagnaceae/Elaeagnus species. Pollen from individual Elaeagnus species have been documented using LM and SEM by several authors (e.g. Huang Citation1972; Wang et al. Citation1995; Beug Citation2004; Fujiki & Ozawa Citation2007; Li et al. 2011a; Miyoshi et al. Citation2011). The fossil pollen grains from Lavanttal are comparable to extant pollen of E. glabra Thunb. as figured by Fujiki and Ozawa (Citation2007) and Miyoshi et al. (Citation2011). Fossil pollen showing LM-based morphological affinities to pollen of modern Elaeagnus have commonly been assigned to the pollen form-species Slovakipollis elaeagnoides Krutzsch (Krutzsch Citation1962; Stuchlik et al. Citation2014).
Fossil record
The pollen and macrofossil record of Eleagnaceae/Elaeagnus was briefly mentioned by Friis et al. (Citation2011) and recently summarised by Su et al. (Citation2014). According to these authors, the Late Cretaceous and Paleogene pollen records of Elaeagnaceae and/or Elaeagnus are all uncertain and need to be studied in more detail. The only reliable macrofossil records of Elaeagnus are wood remains (E. semiannulipora Watari) from the early Miocene of Japan (Watari Citation1952), leaves (E. tibetensis T.Su et Z.K.Zhou) from the late Miocene of eastern Tibet (Su et al. Citation2014) and a flower (E. orchidioides [Straus] H.J.Gregor) from the Pliocene of Germany (Gregor & Mai Citation1984). In Europe, comparable Elaeagnus-type pollen grains have been reported from the Miocene of Poland (Stuchlik et al. Citation2014), Germany (Krutzsch Citation1962; Thiele-Pfeiffer Citation1980) and Austria (Zetter Citation1988).
Ecological implications
Elaeagnus comprises c. 70–90 species of shrubs, sometimes climbing, or small trees, deciduous or evergreen, native to North America, Eurasia and north-eastern Australia. The dominant part of the species is native to the Qinghai-Tibet Plateau and surrounding regions (Qin & Gilbert Citation2007; Sun & Lin Citation2010; Su et al. Citation2014). In China, Elaeagnus thrives in various temperate to subtropical forest types. It can be found along streams and small rivers and lakes; it grows in open areas, in thickets and bushland, and in open woodlands as well as in dense forests. Elaeagnus is found in valley forests and on hills, forested mountain slopes and mountains, and it occurs at elevation ranging from sea level to c. 2800 m a.s.l. (Qin & Gilbert Citation2007; Sun & Lin Citation2010).
Most species of Elaeagnus can be categorised as meridio-nemoral or nemoral elements, few species are distinct in being restricted to seasonally (Cwa; semihumid-meridional: e.g. E. conferta Roxb., E. gonyanthes Benth.) or generally dry climates (B-climates; eurytropical elements; e.g. E. angustifolia L., E. oxycarpa Schltdl.; File S1). Because of the vast range of habitats of extant Elaeagnus, it is hard to pinpoint where the plants that produced the fossil pollen were growing during accumulation of the sediments. Based on the fossil pollen and their affiliation to E. glabra Thunb., it is possible that they originate from evergreen shrubs that were growing along highland and mountain streams within the dense mixed forests surrounding the lowland wetland basins.
Family Rhamnaceae Juss.
Rhamnaceae gen. et spec. indet.
(–)
Figure 17. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Rhamnaceae and Rosaceae pollen. A–C. Rhamnaceae gen. et spec. indet., close-up of area around colpus and mesocolpium. D–F. Prunus sp. 1, close-up of colpus membrane and area around colpus. G–L. Prunus sp. 2, close-ups of mesocolpium (I), and the sexine bridge arching over pori (L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
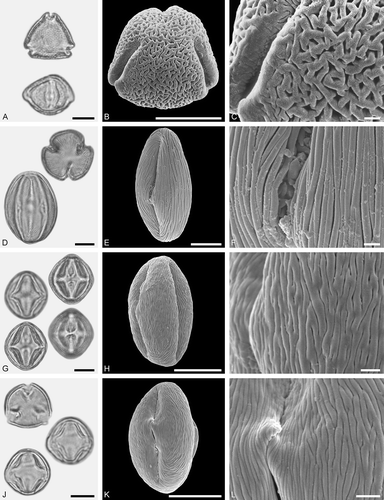
Description
Pollen, monad, oblate, outline convex-triangular in polar view, elliptic in equatorial view; polar axis 17–18 µm long in LM, equatorial diameter 21–23 µm wide in LM, 17–18 µm wide in SEM; tricolporate, colpi conspicuous (deep, SEM), sexine protruding in area of colpi, margins of endopori thickened in corners where crossing colpi (LM); exine 1.3–1.4 µm thick (LM), nexine as thick as sexine; tectate; sculpturing reticulate in LM, microreticulate to reticulate in SEM, muri high and psilate, muri fused along colpi forming a margo, lumina varying in size and outline, often elongated (SEM).
Remarks
The pollen morphology (LM and SEM) of Rhamnaceae has been presented among others by Zhang and Chen (Citation1986, Citation1992), Schirarend and Köhler (Citation1993), Schirarend (Citation1996), Punt et al. (2003) and Perveen and Qaiser (Citation2005). The general morphology of the Lavanttal pollen clearly fits this family (form, outline, arrangement of colpi/pori, thickenings, margo, etc.). The fossil pollen is mostly similar to pollen from extant species of Paliurus, Ziziphus and Rhamnus, but we are unable to affiliate this pollen type to a particular genus with any certainty based on the SEM sculpturing. Despite the apparent number of studies on pollen of extant Rhamnaceae, only a fraction of the c. 900 species have been published. More comparable material showing pollen of extant taxa is needed to reliably affiliate this pollen type with a particular extant genus. Fossil pollen showing LM-based morphological affinities to pollen of modern Rhamnaceae have commonly been assigned to the pollen form-genus Rhamnaceaepollenites (Thiele-Pfeiffer Citation1980; Stuchlik et al. Citation2014).
Fossil record
The biogeographic history and fossil record of the family has been discussed by Richardson et al. (Citation2000b, Citation2004), Burge and Manchester (Citation2008; genus Paliurus) and Friis et al. (Citation2011). Based on these authors, only a single reliable record dates back to the Late Cretaceous, a flower, Coahuilanthus belinda Calvillo-Canadell et Cevallos-Ferriz from Mexico (Calvillo-Canadell & Cevallos-Ferriz Citation2007). No reliable records of Rhamnaceae are known from the Paleocene. Various fossil Paliurus fruits occur from the middle Eocene to late Miocene in North America, the late Eocene to Pleistocene in Asia, and from the Oligocene to Pliocene in Europe (e.g. Burge & Manchester Citation2008). The fossil pollen record of Rhamnaceae was last summarised by Muller (Citation1981), showing that pollen of this type occurs in Oligocene and younger sediments.
Ecological implications
The Rhamnaceae are a moderately large family composed of 50 genera and c. 900 species of shrubs, trees, climbers and one herb. The family has a cosmopolitan distribution and is occurring mostly in warm temperate to tropical environments (Richardson et al. Citation2000a, Citation2000b; Chen & Schirarend Citation2007). Since we are unable to affiliate the fossil pollen with any certainty to a particular genus, we refrain from giving any detailed assumptions on the ecology/habit of the plant that produced this pollen. Based on the similarities of the fossil pollen to those of extant Paliurus, Ziziphus and Rhamnus, it is, however, likely that it represents an evergreen or deciduous shrub or small tree growing in the understory, or in marginal sheltered areas, possibly along small streams, in the dense mixed hillside forests surrounding the wetland basin.
Family Rosaceae Juss.
Genus Prunus L.
Prunus sp. 1
(–)
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 37–38 µm long in LM, 38–39 µm long in SEM, equatorial diameter 27–28 µm wide in LM, 19–20 µm wide in SEM; tricolporate, colpi long, endopori circular, margin of endopori slightly thickened, endopori not wider than the width of colpi; exine 1.1–1.3 µm thick (LM), nexine thinner than sexine; tectate; sculpturing striate in LM and SEM, striae long, extending between polar areas, rarely dividing, striae 0.5–0.6 µm wide, sexine partly arching over pori forming a bridge, colpus membrane microechinate (SEM).
Remarks
The Rosaceae include about 90 genera and c. 3000 species; recent phylogenetic studies recognise three subfamilies: Rosoideae, Dryadoideae and Spiraeoideae. The subfamily Spiraeoideae includes the tribe Amygdaleae, which comprises the genus Prunus (Potter et al. Citation2007). The basic pollen morphology of all three rosaceous subfamilies (see Potter et al. Citation2007) and many genera/species of the tribes and subtribes within Rosaceae have been studied to some extent. Pollen types of the subfamily Rosoideae have been studied using mostly LM and SEM by Huang (Citation1972), Hebda et al. (Citation1988a, Citation1988b), Hebda and Chinnappa (Citation1990), Jones et al. (Citation1995), Wang et al. (Citation1995), Beug (Citation2004), Tahir (Citation2005), Fujiki and Ozawa (Citation2007), Chung et al. (Citation2010), Li et al. (Citation2011a), Miyoshi et al. (Citation2011), Wrońska-Pilarek (Citation2011), Wrońska-Pilarek and Jagodziński (Citation2011), Wrońska-Pilarek et al. (Citation2012), Perveen and Qaiser (Citation2014) and Faghir et al. (Citation2015). The subfamily Dryadoideae has been studied by Hebda et al. (Citation1988b) and Beug (Citation2004). The pollen of the subfamily Spiraeoideae has been studied by Huang (Citation1972), Hebda et al. (Citation1988a, Citation1988b), Jones et al. (Citation1995), Wang et al. (Citation1995), Beug (Citation2004), Bednorz et al. (Citation2005), Dönmez (Citation2008), Joneghani (Citation2008), Sorkheh et al. (Citation2008), Vafadar et al. (Citation2010), Zamani et al. (Citation2010), Li et al. (Citation2011a), Miyoshi et al. (Citation2011), Geraci et al. (Citation2012), Ćalić et al. (Citation2013), Shi et al. (Citation2013) and Perveen and Qaiser (Citation2014). Based on these studies, the Rosaceae pollen grains from Lavanttal can be affiliated with Prunus.
Rosaceae pollen grains are rarely correctly identified/affiliated in LM-based palaeopalynological studies. They have sometimes been misidentified as Cyrillaceae and/or Clethraceae, or simply overlooked. Some fossil pollen showing LM-based morphological affinities to pollen of modern Rosaceae has been assigned to different form-species under the pollen form-genus Tricolporopollenites (e.g. Thomson & Pflug Citation1953; Stuchlik et al. Citation2014).
Fossil record
The Rosaceae have an extensive Cainozoic fossil record starting in the early Eocene composed of leaves, endocarps, fruits, wood, flowers and pollen (LM), that has been summarised by Kirchheimer (Citation1973), Muller (Citation1981), Mai (Citation1984, Citation1995), Manchester (Citation1999), Zhichen et al. (Citation2004), DeVore and Pigg (Citation2007), Friis et al. (Citation2011), Benedict et al. (Citation2011), Li et al. (Citation2011a) and Liu et al. (Citation2013). Prunus has been reported from the Eocene onwards of North America, Asia and Europe. In Asia, Prunus wutuensis Y.Li, Thierry Sm., Chang-Jiang Liu, N.Awasthi, Jian Yang, Yu-Fei Wang et Cheng-Sen Li endocarps have been documented from the early Eocene Wutu Formation of Wutu, Shandong Province, east China (Li et al. Citation2011b). Prunus-type of wood has been described from the Oligocene to Miocene of Japan (Suzuki Citation1984; Takahashi & Suzuki Citation1988) and several different Prunus endocarp types are known from the late Miocene to Pliocene of Japan (Miki Citation1936, Citation1938; Tanai & Onoe Citation1961). In North America, flowers, fruits and in situ pollen of P. cathybrownae J.C.Benedict, DeVore et Pigg have been described from the latest early Eocene (c. 49.4 Ma) Republic Flora of north-eastern Washington State (USA; Benedict et al. Citation2011). Prunus-type leaves are also frequent in the Republic Flora (DeVore & Pigg Citation2007). Prunus endocarps are known from the Eocene Princeton Chert of British Columbia (Canada; Cevallos-Ferriz & Stockey Citation1991) and the Clarno Nut Beds of Oregon (USA; P. weinsteinii Manchester, P. olsonii Manchester; Manchester Citation1994). Prunus-type of wood has been reported from the Princeton Chert, the Clarno Nut Beds and from the Eocene of Yellowstone National Park, Wyoming (USA; Wheeler et al. Citation1978; Cevallos-Ferriz & Stockey Citation1990; Wheeler & Manchester Citation2002). In Europe, fossil Prunus endocarps are frequent components of fruit and seed floras and are known from the Eocene onwards. At least 23 different Prunus species have been described based on endocarps from the Eocene to Pliocene fossil record of Europe (e.g. Kirchheimer Citation1973; Mai Citation1984, Citation1995). Fossil Prunus-type of pollen has been described using SEM from the middle Miocene of Iceland (Denk et al. Citation2011).
Ecological implications
Prunus consists of c. 200 species growing mostly in the temperate parts of the Northern Hemisphere, but with many representatives in tropical and subtropical regions (e.g. Yü et al. Citation1986; Lee & Wen Citation2001; Gu & Bartholomew Citation2003; Mabberley Citation2008). Native Prunus shrubs and trees in China and East Asia thrive at elevations between 200–3800 m a.s.l. and occur in various temperate to subtropical and sometimes tropical forest types, including deciduous broad-leaved, evergreen broad-leaved, mixed deciduous and evergreen broad-leaved, and mixed broad-leaved conifer forests. They grow in sparse forests or at forests margins, in thickets and scrub vegetation and in dense forests, and are often found along stream sides in valleys or on gravely slopes in forested areas (Yü et al. Citation1986; Gu & Bartholomew Citation2003; Wen & Shi Citation2012). In North and Central America, the native, arborescent P. serotina Ehrh. is widely distributed within the Deciduous Forest Region of eastern United States and Canada, ranging south from Nova Scotia and New Brunswick to Florida, and west to the Lake Superior, Minnesota, South Dakota, eastern Nebraska, eastern Kansas, eastern Oklahoma and eastern Texas. It also has disjunct populations in Arizona and New Mexico as well as in Mexico and Guatemala (McVaugh Citation1951). Depending on the geographical region, P. serotina occurs from sea-level to mountain tops at elevations of c. 2000 m a.s.l. It grows in thickets, sparse woodlands, at forest margins and in dense forests, it is also growing on lowland floodplains, in canyons and along mountain sides and on high summits (McVaugh Citation1951).
Distribution data available for 12 species of Prunus reflect the climate variance of the genus, covering most subtypes of warm temperate and snow climates, and including species that can be categorised as generalist, meridio-nemoral, nemoral and boreal elements (File S1). Based on the habitat range of extant Prunus, the pollen from Lavanttal could have originated from trees/shrubs growing along streams in the lowland wetlands or on the floodplain. The Prunus plants could also have been part of the dense mixed forests surrounding the lowland and reaching into the highland and mountains.
Prunus sp. 2
(–)
Description
Pollen, monad, prolate to spheroidal, outline circular to convex-triangular in polar view, elliptic in equatorial view; polar axis 19–24 µm long in LM, 20–24 µm long in SEM, equatorial diameter 19–22 µm wide in LM, 13–15 µm wide in SEM; tricolporate, endopori lalongate, margins of endopori perpendicular to polar axis slightly thickened; exine 0.8–1.1 µm thick (LM), nexine thinner than sexine, sexine protruding around pori; tectate; sculpturing psilate to weakly striate in LM, striate in SEM, striae longitudinally arranged, often merging and diverging, striae variable in thickness, narrowing towards colpi, sexine arching over pori forming a distinct bridge (SEM).
Remarks
Prunus sp. 1 is much larger than Prunus sp. 2. The latter also show a more distinct bridge over the pori (LM). The striae in Prunus sp. 1 are much longer and straighter than the striae observed in Prunus sp. 2. The striae in Prunus sp. 2 are merging and diverging more often than in Prunus sp. 1.
Family Ulmaceae Mirb.
Genus Cedrelospermum Saporta (extinct)
Cedrelospermum sp.
(–)
Figure 18. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Ulmaceae pollen. A–L. Cedrelospermum sp., pollen with varying number of pori (A, D, G, J), close-ups of area around porus (C), distal polar area (I), and proximal polar area (L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
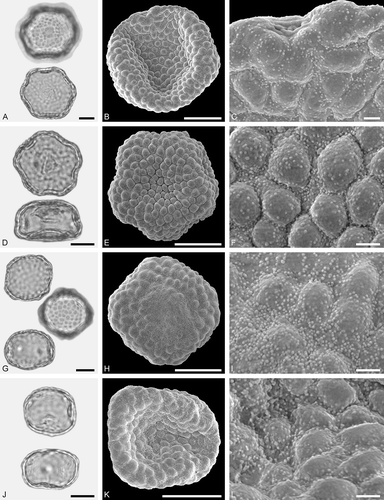
Description
Pollen, monad, oblate, outline quadrangular to hexagonal in polar view, elliptic in equatorial view; polar axis 17–22 µm long in LM, equatorial diameter 24–36 µm wide in LM, 24–32 µm wide in SEM; stephano-(4–6)porate, sexine slightly protruding in area of endopori; exine 1.5–2.6 µm thick, nexine thinner than sexine, sexing slightly thicken around endopori, nexine thinner in distal polar area; tectate; sculpturing verrucate in LM, verrucate, microechinate in SEM, verrucate similar in size, mostly closely space, microechini numerous, occurring on both verrucate and in-between (SEM).
Remarks
This genus is diagnosed on the basis of fruits (e.g. Manchester Citation1987a, Citation1989a; Hably & Thiébaut Citation2002). In situ pollen from staminate flowers of Cedrelospermum attached to leaves and fruits have been described from the middle Eocene Parachute Creek Member of the Green River Formation (Manchester Citation1989b). Dispersed fossil pollen showing LM-based morphological affinities to pollen of the extinct Cedrelospermum have been assigned to the pollen form-species Polyporopollenites verrucatus Thiele-Pfeiffer (Thiele-Pfeiffer Citation1980) and the form-genus Ulmipollenites (e.g. Wolff Citation1934; Stuchlik et al. Citation2009).
Fossil record
Cedrelospermum leaves and fruits have been documented from the Eocene to Miocene of Europe. The oldest European records are from the middle Eocene of Germany (Wilde & Manchester Citation2003) and Hungary (Erdei & Rákosi Citation2009). The middle Miocene fruits from Romania (Paraschiv Citation2008) and the pollen from the Lavanttal Basin are thus far the youngest records in Europe. In North America, Cedrelospermum has been documented from the middle Eocene to Oligocene, whereas records from southern Mexico are of uncertain age, but probably not older than Oligocene and younger than Pleistocene (Magallón-Puebla & Cevallos-Ferriz Citation1994). The distribution of the genus is strictly restricted to these two areas.
Manchester and Tiffney (Citation2001) have discussed the phylogeography of Ulmaceae, based on morphological phylogenetic analyses, and regarded Cedrelospermum as a link between the southern (Phyllostylon, Ampelocera) and northern genera (Ulmus, Zelkova, Hemiptelea). In Austria, fruits of C. aquense (Saporta) Saporta are documented from the early to middle Miocene Styrian sites Leoben and Schönegg (Hably & Thiébaut Citation2002) and the early Oligocene Tyrolian site Häring. For the early/middle Miocene fruits from Leoben, Schönegg and Parschlug (Styria), the binomen C. stiriacum (Ettingshausen) Kovar-Eder et Kvaček (Kovar-Eder et al., 2004) has been established, because Kovar-Eder et al. (2004) regarded the Miocene records as clearly distinguishable from the Paleogene records. Leaves of C. flichei (Saporta) Hably et M.Thiébaut are described from Häring and the nearby locality Duxer Köpfl (Butzmann et al. Citation2009), those of C. ulmifolium (Unger) Kovar-Eder et Kvaček from Parschlug and Weingraben (Burgenland; Jechorek & Kovar-Eder Citation2004).
Ecological implications
Cedrelospermum fossils have been found in several Eocene to Miocene floras in association with numerous tropical to temperate elements (e.g. Rüffle Citation1963; Mai Citation1995; Manchester Citation1999; Meller et al. Citation1999; Hably & Thiébaut Citation2002; Kovar-Eder et al. Citation2004; Collinson et al. Citation2012). Mai (Citation1995) regarded the species as a pioneer element in open habitats. The probable ecology of Cedrelospermum is still not known. For instance, various interpretations have been provided for the Cedrelospermum-rich Céreste Flora (France, Oligocene): swamp forest (Théobald Citation1937), seasonal climate with mainly open, herbaceous vegetation (Lutz Citation1984) and unknown palaeoecology (Hably & Thiébaut Citation2002). In the Eocene of Messel, Cedrelospermum is found in association with a very rich carpoflora including numerous (humid) tropical-subtropical elements (Collinson et al. Citation2012).
The association of Cedrelospermum with a highly diverse Fagales flora including many meridio-nemoral and nemoral elements that are not tolerating summer-draught (such as Corylus, Fagus; restricted to Cfa-, Cfb-, Dfb-climates) or have only recently adapted to such situations (Quercus Group Ilex; mostly Cfa-, Cwa-, Cwb-climates) at the Lavanttal locality, is in agreement with the hypothesis of Gregor (Citation1986; Cfa-climate) for the Parschlug flora (Austria, Miocene). Notably, the only evergreen species of the widespread and diverse genus Ulmus is restricted to low and mid-altitudes of the Himalayas and montane regions of southern China, where it thrives in a subtropical, monsoonal Cwa-climate, with high summer precipitation (see later). We assume that the Lavanttal Cedrelospermum thrived in similar habitats: humid, mixed broad-leaved and evergreen lowland forests.
Genus Ulmus L.
Ulmus sp. 1
(–, –)
Figure 19. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Ulmaceae pollen. A–F. Ulmus sp. 1, same grain with six pori, distal side of grain (B, C), and proximal side of grain (E, F). G–L. Ulmus sp. 1, same grain with five pori, distal side of grain (B, C), and proximal side of grain (E, F). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
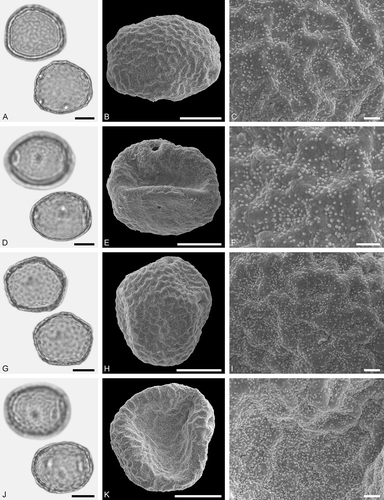
Figure 20. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Ulmaceae pollen. A–F. Ulmus sp. 1, same grain with five pori, distal side of grain (B, C), and proximal side of grain (E), close-up (F) showing porus. G–L. Ulmus sp. 2, same grain, distal side of grain (H, I), and proximal side of grain (K, L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
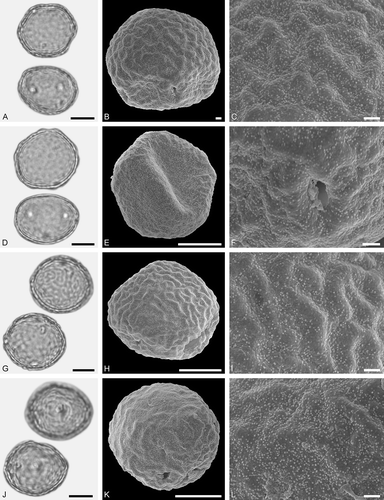
Description
Pollen, monad, oblate, outline quadrangular to hexagonal in polar view, elliptic in equatorial view; polar axis 20–24 µm long in LM, equatorial diameter 26–31 µm wide in LM, 25–28 µm wide in SEM; stephano-(4–6)porate, sexine slightly protruding in area of endopori; exine 1.0–1.4 µm thick, nexine thinner than sexine, sexing slightly thicken around endopori, nexine thinner in distal polar area; tectate; sculpturing rugulate in LM, rugulate, microechinate, perforate in SEM, rugulae 1.0–1.2 µm wide, rugulae often fused and surrounding depressions (SEM).
Remarks
The pollen morphology and ultrastructure of extant Ulmaceae has been studied in detail by Zavada (Citation1983) and Takahashi (Citation1989). Pollen from various extant North American and Eurasian Ulmus species has been studied and figured using LM, SEM and sometimes TEM by Huang (Citation1972), Zavada (Citation1983), Zavada and Dilcher (Citation1986), Takahashi (Citation1989), Lieux (Citation1980), Xin et al. (Citation1993), Jones et al. (Citation1995), Stafford (Citation1995), Wang et al. (Citation1995), Morita et al. (Citation1998), Beug (Citation2004), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). For characters on how to distinguish between Ulmus and Zelkova pollen, see Morita et al. (Citation1998), amongst others. Fossil pollen showing LM-based morphological affinities to pollen of modern Ulmus has commonly been assigned to the pollen form-genus Ulmipollenites (e.g. Wolff Citation1934; Stuchlik et al. Citation2009).
Fossil record
The early Ulmus-like fossil record of the Paleocene-Eocene has been summarised by Denk and Dillhoff (Citation2005). Some of the best preserved early fossils are associated and attached fruits and leaves of U. okanaganensis Denk et Dillhoff from the early Eocene McAbee and co-eval floras of British Columbia, western North America (Denk & Dillhoff Citation2005). The Eocene to Oligocene fossil record of Ulmus has been summarised by Manchester (Citation1989c) suggesting that unambiguous Ulmus remains are not found in Eurasia before the Oligocene. Ulmus is very abundant in European Miocene leaf assemblages (Mai Citation1995; Meller & Hably Citation2014), and fruits have been found from the late early/early middle Miocene Parschlug site (Kovar-Eder et al. Citation2004). From the Lavanttal Basin, Berger (Citation1955) mentioned Ulmus leaves from Messensach (early middle Miocene) and St Stefan (late middle Miocene).
Ecological implications
Ulmus is a large and widespread northern hemispheric genus and common element of broad-leaved deciduous forests of the temperate zone with a preference for warm temperate climates with warm or hot summers and sufficient precipitation during growing season (Cfa-, Cfb-, Cwa-, Cwb-climates). Some species are tolerant against generally low temperatures, e.g. U. glaucescens Franch., U. pumila L., U. laciniata Mayr, extending into regions with snow climates and short (cold) summers (Fu et al. Citation2003; Fang et al. Citation2009); one widespread species, U. minor Mill. extends into summer-dry climates (Csa, Csb).
The mainly deciduous trees, rarely shrubs, occur in different forest types. About 35 to 45 species are described, with a centre of distribution in central and northern Asia (Todzia & Panero Citation1998). In China, 21 species are known, 14 of them are endemic (Fu et al. Citation2003), the others are also growing in Russia, Korea and Japan. The trees occur in lowland, subtropical mixed evergreen broad-leaved forests at 200–900 m a.s.l. and in montane mixed mesophytic forests at 700–1800 (–2900) m a.s.l. Most species occur along river banks and mountain ravines, in wetlands near streams, on mountain slopes, and often on limestone. The only evergreen species, Ulmus lanceifolia Roxb., is growing between 300–1500 m a.sl., in some provinces in China and also in Bhutan, India, Thailand and Vietnam (Fu et al. Citation2003). In North America, about ten species are native, also in southern Mexico (Todzia & Panero Citation1998), but none in western North America. In the south-eastern United States, they are growing in different types of woodland habitats, in floodplains and lowland woodlands as well as in upland forests, along banks of streams, and in wet to moist rich woodlands (Godfrey & Wooten Citation1981). Ulmus is often growing on limestone and may occur up to 900 m a.s.l. (Sherman-Broyles et al. Citation1997). In riparian forests, Ulmus prefers nutrient-rich and alkaline soil (Mai Citation1995), but may tolerate poor soil conditions (Elias Citation1980). Ulmus species can be categorised as generalists, semihumid-meridional (U. lanceifolia), meridio-nemoral, nemoral and boreal (U. laciniata) elements (File S1). At Lavanttal, Ulmus may have been part of the riparian forests, growing close to lakes and along rivers/streams and reaching into the (mesic) slope forests surrounding the basin.
Ulmus sp. 2
(–, –)
Figure 21. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Ulmaceae pollen. A–F. Ulmus sp. 2, same grain with five pori, distal side of grain (B, C), and proximal side of grain (E, F). G–I. Zelkova sp., grain with four pori, distal side (H, I). J-L. Zelkova sp., grain with four pori, proximal side of grain (K), close-up of pori showing membrane (L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
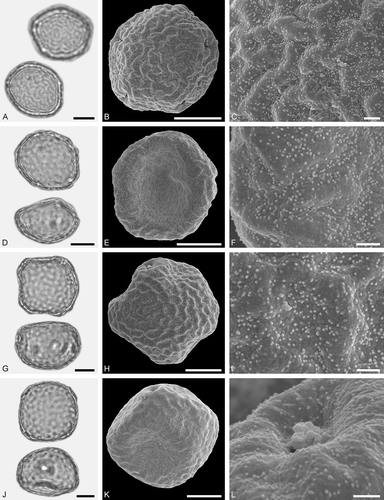
Description
Pollen, monad, oblate, outline quadrangular to hexagonal in polar view, elliptic in equatorial view; polar axis 20–25 µm long in LM, equatorial diameter 27–33 µm wide in LM, 25–29 µm wide in SEM; stephano-(4–6)porate; exine 0.8–1.1 µm thick, nexine thinner than sexine, sexing slightly thicken around endopori, nexine thinner in distal polar area; tectate; sculpturing rugulate in LM, rugulate, microechinate in SEM, rugulae 0.8–0.9 µm wide, rugulae often running parallel, flanked by elongated depressions (SEM).
Remarks
In Ulmus sp. 1, the rugulae are fused and surrounding depressions (LM and SEM), but in Ulmus sp. 2, the rugulae are running parallel and flanked by elongated depressions.
Genus Zelkova Spach
Zelkova sp.
(–, –)
Figure 22. LM (A, D, G, J) and SEM (B, C, E, F, H, I, K, L) micrographs of dispersed fossil Ulmaceae pollen. A–F. Zelkova sp., same grain with four pori, distal side of grain (B, C), and proximal side of grain (E, F). G–L. Zelkova sp., same grain with four pori, distal side (H, I), and proximal side (K, L). Scale bars – 10 µm (A, B, D, E, G, H, J, K), 1 µm (C, F, I, L).
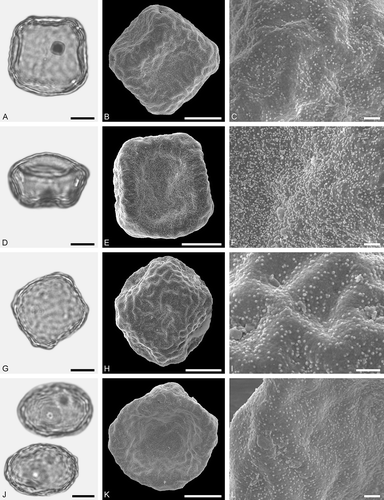
Description
Pollen, monad, oblate, outline quadrangular to hexagonal in polar view, elliptic in equatorial view; polar axis 23–26 µm long in LM, equatorial diameter 31–36 µm wide in LM, 30–32 µm wide in SEM; stephano-(4–6)porate; exine 1.2–1.7 µm thick, nexine thinner than sexine, sexing slightly thicken around endopori, nexine thinner in distal polar area; tectate; sculpturing rugulate in LM, rugulate, microechinate in SEM, rugulae 1.0–1.2 µm wide, rugulae often fused and surrounding depressions (SEM).
Remarks
Zelkova pollen from all of the six extant western and east Asian and Mediterranean species has been described and figured based on LM and/or SEM investigations by Huang (Citation1972), Takahashi (Citation1989), Stafford (Citation1995), Morita et al. (Citation1998), Nakagawa et al. (Citation1998), Li et al. (Citation2011a) and Miyoshi et al. (Citation2011). Studies on the ultrastructure (TEM) of Zelkova pollen are rare (e.g. Takahashi Citation1989). Fossil pollen showing LM-based morphological affinities to pollen of modern Zelkova has commonly been assigned to the pollen form-genus Zelkovaepollenites (e.g. Nagy Citation1969; Stuchlik et al. Citation2009).
Fossil record
Zelkova was widely distributed in the Northern Hemisphere during Paleogene and Neogene times (Wang et al. Citation2001; Denk & Grimm Citation2005). In North America, Zelkova leaves have been described from the late Paleocene/early Eocene and fruits have been documented from Oligocene sites (Burnham Citation1986; Manchester Citation1989c). Denk and Grimm (Citation2005) concluded – based on molecular, morphological and fossil evidence – that the genus originated in the northern Pacific region and migrated towards Europe in the late Oligocene after the closure of the Turgai Strait. In Eurasia, the genus has a solid fossil record from the Eocene onwards (Mai Citation1995; Lui et al. Citation1996) and has been documented from the late Pliocene of the Netherlands and the Eemian interglacial period in south-eastern Europe (Lang Citation1994). Zelkova leaves are widespread in the Eurasian leaf fossil record and typically addressed as Z. zelkovifolia (Unger) Bůžek et Kotl., e.g. late Oligocene of Rott, Germany, middle Miocene of Parschlug, Austria, late Miocene of Vegora, Greece, and Pliocene of Mogi, Japan (see Denk & Grimm Citation2005, table 7). From the Lavanttal Basin, Zelkova leaves have been mentioned by Berger (Citation1955) from the Ölbach locality and from Schönweg by Hofmann (Citation1929; as ‘Planera ungeri Kovats’).
Ecological implications
Today, Zelkova is represented in the Northern Hemisphere by three species in East Asia (two widespread, one highly restricted), one in the Caucasus-Hyrcanian area, one in Crete and one in Sicily. The latter can be regarded as relict trees, which survived the Quaternary glaciations in the eastern Mediterranean area (e.g. Wang et al. Citation2001; Christe et al. Citation2014). The trees are up to 30–60 m high or are of shrubby growth in unfavourable conditions (dry stands). In China, Zelkova occurs between 200 to 2500 m a.s.l. in valleys along streams (Fu et al. Citation2003) within the mixed northern hardwood forests, the deciduous broad-leaved forests, and as minor element also within the mixed mesophytic forests (Wang Citation1961). In central Japan, Zelkova serrata (Thunb.) Makino is one of the few tall tree species that are found in forests dominated by Fagus. Along river valleys on unstable ground, Z. serrata is found within mixed evergreen broad-leaved forests typically associated with Acer (Miyawaki & Suzuki Citation1980). Zelkova carpinifolia (Pall.) K.Koch occurs in the Hyrcanian forests and the Caucasus; typical habitats are the lowland forests south of the Caspian Sea in an essentially fully humid, warm temperate climate (Cfa, Cfb) associated with mainly deciduous elements and some evergreen shrubs or lianas (e.g. Akhani et al. Citation2010) and somewhat drier stands in Georgia (periphery of the Colchic area and eastern central Georgia) associated with evergreen species and mean annual precipitation of > 1000 mm/yr (Denk et al. Citation2001). Akhani et al. (Citation2010) stress the lack or only short dry period and that the mean temperature of the coldest month is above 0 °C. In the summer-dry Mediterranean climates, Z. abelicea (Lam.) Boiss. is restricted to the relatively humid mountains of Crete, where it grows on limestone and thrives in the vicinity of dolines (sinkholes in karst areas) acting as natural water reservoirs (Søndergaard & Egli Citation2006), whereas the population of the Sicilian endemic Z. sicula Di Pasquale, Garfi et Quézel is threatened by annihilation.
Despite their restricted distribution at present, the four putative relict species (three in western Eurasia and Zelkova sinica C.K.Schneid. in China) share the distinct preference for warm temperate climates with the two relatively wide spread species in East Asia (Z. schneideriana Hand.-Mazz., southern and central China; Z. serrata, China to Kuril Islands; e.g. Fu et al. Citation2003; Kvavadze & Connor Citation2005). Overall, species of Zelkova can be categorised as semihumid-meridional, meridio-nemoral, and nemoral elements (File S1).
Discussion
Occurrence and identification of angiosperm pollen
Most of the angiosperm pollen types described here represent genera producing very distinct pollen; pollen types that are usually identifiable using LM. These include commonly reported Miocene elements in European palynofloras such as Alnus, Betula, Fagus, Quercus, Carya, Juglans and Pterocarya. Most of the families (Betulaceae, Fagaceae, Juglandaceae, Myricaceae, Ulmaceae) and genera dealt with here were previously mentioned by Klaus (Citation1984). Pollen of the families Rosaceae (Prunus) and Rhamnaceae have not been reported before, and additional pollen types affiliated with previously identified families like the Betulaceae, Fagaceae and Ulmaceae/Cannabaceae are also presented for the first time (). Since Klaus (Citation1984) made most of his investigations using only LM, we are sometimes not sure if the pollen, we describe/figure, represent the same taxon as mentioned in the study by Klaus ().
Even though many of the pollen types represent commonly reported elements, the individual taxa (pollen types) described in this paper rarely make up more than 0.5% of the entire palynomorph spectrum (F. Grímsson, pers. observation based on four years of studying this sample). Of the taxa described here, pollen of Fagus is by far the most common type encountered and appears in various, albeit non-specific (hence all addressed as Fagus sp.) forms and shapes. The rarest pollen grains are those of Corylus, Castanea, Quercus sp. 2, Quercus sp. 5, Quercus sp. 7, Cannabaceae gen. et spec. indet., Elaeagnus, Rhamnaceae gen. et spec. indet., Prunus sp. 1 and Prunus sp. 2.
Palaeoecological interpretations and palaeoenvironmental reconstructions – preliminary results
Previous accounts on the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin (Grímsson et al. Citation2011, Citation2015a; Grímsson & Zetter Citation2011) have shown that spores and pollen accumulated in a freshwater environment. Species richness and habitat of possible modern analogues suggests that the spores and pollen originate from plants growing directly within the lowland wetland and are reflecting various units of the autochthonous vegetation, and also the allochthonous vegetation surrounding the main basin. Many of the previously described spores and pollen were dispersed by plants occupying various microsites within the lowland wetland forest (lake margins, along rivers, swamps, on levees, hammocks, floodplains, etc.) or at its periphery. These include Carex, Cercidiphyllum, Dryopteris, Ephedra, Ginkgo, Glyptostrobus, Larix, Liquidambar, Lycopodium, Magnolia, Osmunda, Parthenocissus, Picea, Pinus, Platanus, Ranunculaceae, Salix, Sciadopitys, Selaginella, Sequoia, Sparganium, Sphagnum, Typha and Vitis. Based on the ecological preferences of potential modern analogues a large part of the pollen was apparently dispersed by plants thriving in different habitats of the surrounding lowland, highland or mountain forests. These include Abies, Buxus, Cathaya, Cedrus, Cercidiphyllum, Cryptomeria, Daphnyphyllum, Distylium, Fortunearia, Ginkgo, Keteleeria, Larix, Lycopodium, Parrotia, Parthenocissus, Picea, Pinus, Pteris, Sequoia, Trochodendron, Tsuga and Vitis.
The angiosperm pollen presented here may also originate from plants that could have thrived in various habitats and vegetation units of the lowland, hinterland, and surrounding highland and mountains. Alnus, Betula, Carpinus, Carya, Cedrelospermum, Celtis, Elaeagnus, Juglans, Myrica/Morella, Ostrya, Prunus, Pterocarya, Quercus Group Quercus/Lobatae, Trigonobalanopsis and Ulmus were most likely growing in the lowland wetland forests (mixed evergreen/deciduous broad-leaved/conifer forests), some in swampy areas, or around lake margins and along streams and rivers, or on floodplains and on levees, and growing mostly in riparian forests and mesophytic forests bordering the basin. Some of these plants (Prunus, Betula, Pterocarya, Myrica/Morella, Elaeagnus) were probably also growing along streams reaching into the surrounding highland and/or mountains. Corylus, Quercus Group Cyclobalanopsis, Zelkova, Engelhardioidea and Rhamnaceae were most likely growing in lowland/hinterland forests outside the wetlands, along the periphery of the wetland basin reaching into hillside forests surrounding the basin. Castanea and Quercus Group Cerris and Group Ilex were probably confined to the mixed hillside and mountain forest at some distance from the main accumulating area.
Based on the climatic preferences, expressed by their ‘Köppen signatures’, which the Fagales and Rosales lineages present at the Lavanttal site (; ), tropical (A-)climates and climates with pronounced (summer) draught (B-, Cs-, Ds-climates) can be ruled out for this timescale and region. The same holds for boreal climates with short but humid summers (Cfc, Dfc, Dfd, Dwc). Especially the diversity of Fagales, including genera that are today composed predominately or exclusively of nemoral and meridio-nemoral elements (), points to climate conditions not unlike those found today in the lowlands and adjacent mountain regions of the (south-)eastern United States, the humid-meridional region of western Eurasia (e.g. northern Italy, Black Sea region, western Caucasus), central and southern China, or Honshu (Japan). These regions are characterised by subtropical conditions at lower elevations (Cfa-, Cwa-climates) and subsequent altitudinal successions: Cfa → Cfb/Dfa → Dfb in eastern United States, western Eurasia, central China and Japan, or Cwa → Cwb → Dwb in southern China (the same situation can be found in the southern foothills of the Himalayas). The climax vegetation in these areas are mixed mesophytic forests and various forms of the ‘Laurisilva’ (mixed evergreen/deciduous broad-leaved forests), forest types characteristic for the humid and semi-humid, summer-rain areas of the meridional and nemoral zone (Walter Citation1973; Schroeder Citation1998; see Velitzelos et al. Citation2014, table 23). Dominant and common genera in these forests are the various members of the northern hemispheric Fagales, which are represented by 23 different lineages at the Lavanttal site. The most important taxa for palaeovegetation and climate reconstruction are Fagus, a genus with a relatively narrow climatic and ecological niche and one of the most dominant genera in mixed mesophytic forests of North America, China and Japan (Maycock Citation1994; Cao Citation1995; Peters Citation1997), and Quercus Group Ilex, a co-dominant group in the East Asian monsoon influenced, winter-dry or fully humid southern foothills of the Himalayas and montane regions of south-western and central China (Huang et al. Citation1999; see also distribution maps in Fang et al. Citation2009). Equally informative is Corylus, with a similar ecological and climatic niche as Fagus, and the co-occurrence of Carya, Juglans, Pterocarya and Engelhardioideae, pinpointing towards rich mixed evergreen-broad leaved forests as today only found in south-western China (Fang et al. Citation2009) and the warm subtropical parts of the south-eastern United States (see distribution maps in Thompson et al. Citation1999a, Citation1999b, Citation2001; eFloras Citation2008). The high Fagales diversity further indicates a varying landscape with a relatively high variety of niches including riparian, dry and mesic forests (; showing habitat preferences of the potential modern analogues of the Lavanttal Fagales and Rosales).
Table II. Angiosperm pollen described in this study in comparison to Klaus (Citation1984).
Table III. Summarised ‘Köppen signatures’ for Fagales and Rosales of Lavanttal.
Table IV. Preferred general forest types of extant species of genera represented among the Lavanttal Fagales and Rosales.
Figure 23. Köppen signatures of potential modern analogues of Fagales and Rosales lineages found at the Lavanttal site. The bar chart shows the proportion of extant species part of the modern genus/lineage categorised for generalised climate-vegetation types (see Denk et al. Citation2013; see Material and Methods section). Generalists – no distinct climatic preference; arctic-alpine elements – occurring in tundra (ET) and adjacent climates; boreal elements – preference for D-climates, occurring in climates with cold and short summers but not ET climates; nemoral elements – preference for warm temperate and/or snow climates with warm summers (Cfb, Cwb, Csb, Dfb, Dwb, Dsb); meridio-nemoral elements – preference for warm temperate climates with hot, but not warm, summers (Cfa- and Cwa-climates); semihumid-meridional elements – preference for semihumid warm temperate climates with hot (and warm) summers; tropical-meridional – preference for tropical (A-climates) and warm temperate climates with hot but not warm summers; tropical – species restricted to tropical (A-climates); eurytropical – preference for non-tropical climates with summer draught and generally dry climates (B- and Cs-climates).
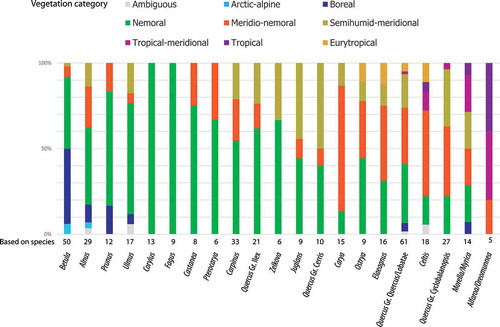
The angiosperm taxa described here as well as spores and pollen previously mentioned by Grímsson et al. (Citation2011), Citation2015a) and Grímsson and Zetter (Citation2011) represent only a part of the well preserved and species rich palynoflora from the phosphoritic nodules of the Lavanttal Basin. Additional angiosperm pollen types will be described in following contributions. Fully detailed interpretation of the palaeovegetation, ecology and climate awaits further notice until all the angiosperm pollen have been described.
FileS1 Extracted data from the updated ClimGrim database v. 0.3
Download MS Excel (98.9 KB)Acknowledgements
The authors thank Martina Weber, Steven R. Manchester and an anonymous reviewer for their nice and careful reviews of this long manuscript.
Disclosure statement
No potential conflict of interest was reported by the author.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Accorsi CA, Mazzanti MB, Torri P, Venturi L. 1991. Flora Palinologica Italiana, Sezione Aeropalinologica – S 235: Ostrya carpinifolia Scop. Aerobiologia 7: 81–90. doi:10.1007/BF02450023.
- Akhani H, Djamali M, Ghorbanalizadeh A, Ramezani E. 2010. Plant biodiversity of Hyrcanian relict forests, N Iran: An overview of the flora, vegetation, palaeoecology and conservation. Pakistan Journal Botany 42: 231–258.
- Akhani H, Salimian M. 2003. An extant disjunct stand of Pterocarya fraxinifolia (Juglandaceae) in the central Zagros Mountains, W Iran. Willdenowia 33: 113–120. doi:10.3372/wi.33.33111.
- Akhondnezhad S, Nejadsattari T, Sattarian A, Asri Y, Bagheriieh Najjar MB. 2011. Pollen morphology of the genus Carpinus L. (Corylaceae) in Iran. Iranian Journal of Botany 17: 233–237.
- Angiosperm Phylogeny Group (APG). 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. doi:10.1111/(ISSN)1095-8339.
- Axelrod DI. 1983. Biogeography of oaks in the Arcto-Tertiary Province. Annals of the Missouri Botanical Garden 70: 629–657. doi:10.2307/2398982.
- Bednorz L, Maciejewska-Rutkowska I, Wrońska-Pilarek D, Fujiki T. 2005. Pollen morphology of the Polish species of the genus Sorbus L. Acta Societatis Botanicorum Poloniae 74: 315–322.
- Benedict JC, DeVore ML, Pigg KB. 2011. Prunus and Oemleria (Rosaceae) Flowers from the Late Early Eocene Republic Flora of Northeastern Washington State, U.S.A. International Journal of Plant Sciences 172: 948–958. doi:10.1086/660880.
- Berger W. 1953. Studien zur Systematik und Geschichte der Gattung Carpinus. Botaniska Notiser 1: 1–47.
- Berger W. 1955. Jungtertiäre Pflanzenreste aus dem unteren Lavanttal in Ostkärnten. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 100: 402–430.
- Beug H-J. 2004. Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. München: Dr. Friedrich Pfeil.
- Birks HJB. 1968. The identification of Betula nana pollen. New Phytologist 67: 309–314. doi:10.1111/nph.1968.67.issue-2.
- Bisht VK, Kuniyal CP, Nautiyal BP, Prasad P. 2013. Spatial distribution and regeneration of Quercus semecarpifolia and Quercus floribunda in a subalpine forest of western Himalaya, India. Physiology and Molecular Biology of Plants 19: 443–448. doi:10.1007/s12298-013-0189-z.
- Blackmore S, Steinmann JAJ, Hoen PP, Punt W. 2003. The northwest European pollen flora, 65. Betulaceae and Corylaceae. Review of Palaeobotany and Palynology 123: 71–98. doi:10.1016/S0034-6667(02)00156-2.
- Blokhina NI. 2004. On some aspects of systematics and evolution of the Engelhardioideae (Juglandaceae) by wood anatomy. Acta Palaeontologica Romaniae 4: 13–21.
- Blokhina NI. 2007. Fossil wood of the Juglandaceae: Some questions of taxonomy, evolution, and phylogeny in the family based on wood anatomy. Paleontological Journal 41: 1040–1053.
- Blokhina NI, Popov AM, Snezhkova SA. 2002. Fossil wood of Engelhardioxylon mameticum sp. nov. (Juglandaceae) from the Paleogene of Kamchatka. Paleontological Journal 36: 429–437.
- Bonnefille R. 2010. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global and Planetary Change 72: 390–411. doi:10.1016/j.gloplacha.2010.01.015.
- Borgardt SJ, Pigg KB. 1999. Anatomical and developmental study of petrified Quercus (Fagaceae) fruits from the Middle Miocene, Yakima Canyon, Washington, USA. American Journal of Botany 86: 307–325. doi:10.2307/2656753.
- Bornstein AJ. 1997. Myricaceae Blume. In: Flora of North America Editorial Committee, eds. Flora of North America north of Mexico, Volume 3, Magnoliophyta: Magnoliidae and Hamamelidae, 429–435. New York: Oxford University Press.
- Bouchal J, Zetter R, Grímsson F, Denk T. 2014. Evolutionary trends and ecological differentiation in early Cenozoic Fagaceae of western North America. American Journal of Botany 101: 1332–1349. doi:10.3732/ajb.1400118.
- Browics K, Zieliński J. 1982. Chorology of trees and shrubs in south-west Asia and adjacent regions. Warsaw: Polish Scientific Publishers.
- Budantsev L. 1982. Ulmaceae–Betulaceae. In: Takhtajan A, Zhilin S, eds. Fossil flowering plants of the USSR, Volume 2, 120–128. Leningrad: Nauka.
- Burge DO, Manchester SR. 2008. Fruit morphology, fossil history, and biogeography of Paliurus (Rhamnaceae). International Journal of Plant Sciences 169: 1066–1085. doi:10.1086/522648.
- Burnham RJ. 1986. Foliar morphological analysis of the Ulmoideae (Ulmaceae) from the Early Tertiary of Western North America. Palaeontographica B 201: 135–167.
- Butzmann R, Fischer TC, Rieber E. 2009. Makroflora aus dem inneralpinen Fächerdelta der Häring-Formation (Rupelium) vom Duxer Köpfl bei Kufstein/Unterinntal, Österreich. Zitteliana A48/49: 129–163.
- Bůžek Č. 1971. Tertiary flora from the northern part of the Pětipsy area (North-Bohemian Basin). Rozpravy Ústředního Ústavu Geologického 36: 1–118.
- Ćalić D, Devrnja N, Kostić I, Kostić M. 2013. Pollen morphology, viability, and germination of Prunus domestica cv. Požegača. Scientia Horticulturae 155: 118–122. doi:10.1016/j.scienta.2013.03.017.
- Calvillo-Canadell L, Cevallos-Ferriz SRS. 2007. Reproductive structures of Rhamnaceae from the Cerro del Pueblo (Late Cretaceous, Coahuila) and Coatzingo (Oligocene, Puebla) formations, Mexico. American Journal of Botany 94: 1658–1669. doi:10.3732/ajb.94.10.1658.
- Cao K-F. 1995. Fagus dominance in Chinese montane forests. PhD Thesis, Landbouwuniversiteit te Wageningen, Wageningen, The Netherlands.
- Cevallos-Ferriz SRS, Stockey RA. 1990. Vegetative remains of the Rosaceae from the Princeton Chert (middle Eocene) of British Columbia. International Association of Wood Anatomists Bulletin, New Series 11: 261–280.
- Cevallos-Ferriz SRS, Stockey RA. 1991. Fruits and seeds from the Princeton Chert (middle Eocene) of British Columbia: Rosaceae (Prunoideae). Botanical Gazette 152: 369–379. doi:10.1086/bg.1991.152.issue-3.
- Chen Y, Schirarend C. 2007. Rhamnaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Volume 12, Hippocastanaceae through Theaceae, 115–168. Beijing: Science Press.
- Chen Z, Li J. 2004. Phylogenetics and biogeography of Alnus (Betulaceae) inferred from sequences of nuclear ribosomal DNA ITS region. International Journal of Plant Sciences 165: 325–335. doi:10.1086/ijps.2004.165.issue-2.
- Chen Z-D. 1991. Pollen morphology of the Betulaceae. Acta Phytotaxonomica Sinica 29: 494–503.
- Chesters K. 1957. The Miocene flora of Rusinga Island, Lake Victoria, Kenya. Palaeontographica B 101: 30–71.
- Christe C, Kozlowski G, Frey D, Bétrisey S, Maharramova E, Garfi G, Pirintsos S, Naciri Y. 2014. Footprints of past intensive diversification and structuring in the genus Zelkova (Ulmaceae) in south-western Eurasia. Journal of Biogeography 41: 1081–1093.
- Chung K-S, Elisens WJ, Skvarla JJ. 2010. Pollen morphology and its phylogenetic significance in tribe Sanguisorbeae (Rosaceae). Plant Systematics and Evolution 285: 139–148. doi:10.1007/s00606-009-0262-9.
- Chung TF, Huang TC. 1972. Paleoecological study of Taipei Basin. Taiwania 17: 117–141.
- Clegg BF, Tinner W, Gavin DG, Hu FS. 2005. Morphological differentiation of Betula (birch) pollen in northwest North America and its palaeoecological application. The Holocene 15: 229–237.
- Coladonato M. 1993. Corylus americana. In: USDA Forest Service, ed. Fire effects information system (FEIS database), http://www.feis-crs.org/beta/; accessed January–Mai 2015.
- Collinson ME, Manchester SR, Wilde V. 2012. Fossil fruits and seeds of the middle Eocene Messel Biota, Germany. Abhandlungen der Senckenberg Gesellschaft für Naturforschung 570: 1–251.
- Crane PR. 1989. Early fossil history and evolution of the Betulaceae. In: Crane PR, Blackmore S, eds. Evolution, systematics, and fossil history of the Hamamelidae, Volume 2: ‘Higher’ Hamamelidae. Systematics Association Special Volume No. 40B, 87–116. Oxford: Clarendon Press.
- Crepet WL, Daghlian CP. 1980. Castaneoid inflorescences from the middle Eocene of Tennessee and the diagnostic value of pollen (at the subfamily level) in the Fagaceae. American Journal of Botany 67: 739–757. doi:10.2307/2442667.
- Crepet WL, Daghlian CP, Zavada M. 1980. Investigations of angiosperms from the Eocene of North America: A new juglandaceous catkin. Review of Palaeobotany and Palynology 30: 361–370. doi:10.1016/0034-6667(80)90019-6.
- Crepet WL, Dilcher DL, Potter FW. 1975. Investigations of angiosperms from the Eocene of North America: A catkin with juglandaceous affinities. American Journal of Botany 62: 813–823. doi:10.2307/2441892.
- Dane F, Lang P, Huang H, Fu Y. 2003. Intercontinental genetic divergence of Castanea species in eastern Asia and eastern North America. Heredity 91: 314–321. doi:10.1038/sj.hdy.6800300.
- Dašková J. 2008. In situ pollen of Alnus kefersteinii (Göpp.) Unger (Betulales: Betulaceae) from the Oligocene of Bechlejovice, Czech Republich. Journal of the National Museum (Prague), Natural History Series 177: 27–31.
- Delzon S, Urli M, Samalens J-C, Lamy J-B, Lischke H, Sin F, Zimmermann NE, Porté AJ. 2013. Field evidence of colonisation by Holm Oak, at the northern margin of its distribution range, during the Anthropocene period. PLoS ONE 8: e80443.
- Denk T. 2003. Phylogeny of Fagus L. (Fagaceae) based on morphological data. Plant Systematics and Evolution 240: 55–81.
- Denk T. 2004. Revision of Fagus from the Cenozoic of Europe and southwestern Asia and its phylogenetic implications. Documenta Naturae 150: 1–72.
- Denk T, Dillhoff RM. 2005. Ulmus leaves and fruits from the Early–Middle Eocene of northwestern North America: Systematics and implications for character evolution within Ulmaceae. Canadian Journal of Botany 83: 1663–1681. doi:10.1139/b05-122.
- Denk T, Frotzler N, Davitashvili N. 2001. Vegetational patterns and distribution of relict taxa in humid temperate forests and wetlands of Georgia (Transcaucasia). Biological Journal of the Linnean Society 72: 287–332. doi:10.1111/j.1095-8312.2001.tb01318.x.
- Denk T, Grimm GW. 2005. Phylogeny and biogeography of Zelkova (Ulmaceae sensu stricto) as inferred from leaf morphology, ITS sequence data and the fossil record. Botanical Journal of the Linnean Society 147: 129–157. doi:10.1111/boj.2005.147.issue-2.
- Denk T, Grimm GW. 2009a. The biogeographic history of beech trees. Review of Palaeobotany and Palynology 158: 83–100. doi:10.1016/j.revpalbo.2009.08.007.
- Denk T, Grimm GW. 2009b. Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus (Fagaceae). International Journal of Plant Sciences 170: 926–940. doi:10.1086/592012.
- Denk T, Grimm GW. 2010. The oaks of western Eurasia: Traditional classifications and evidence from two nuclear markers. Taxon 59: 351–366.
- Denk T, Grimm GW, Grímsson F, Zetter R. 2013. Evidence from “Köppen signatures” of fossil plant assemblages for effective heat transport of Gulf Stream to subarctic North Atlantic during Miocene cooling. Biogeosciences 10: 7927–7942.
- Denk T, Grimm GW, Hemleben V. 2005. Patterns of molecular and morphological differentiation in Fagus (Fagaceae): Phylogenetic implications. American Journal of Botany 92: 1006–1016. doi:10.3732/ajb.92.6.1006.
- Denk T, Grímsson F, Zetter R. 2010. Episodic migration of oaks to Iceland: Evidence for a North Atlantic “land bridge” in the latest Miocene. American Journal of Botany 97: 276–287.
- Denk T, Grímsson F, Zetter R. 2012. Fagaceae from the early Oligocene of Central Europe: Persisting New World and emerging Old World biogeographic links. Review of Palaeobotany and Palynology 169: 7–20. doi:10.1016/j.revpalbo.2011.09.010.
- Denk T, Grímsson F, Zetter R, Símonarson LA. 2011. Late Cainozoic floras of Iceland – 15 million years of vegetation and climate history in the northern North Atlantic. Springer: Dordrecht.
- Denk T, Güner HT, Grimm GW. 2014. From mesic to arid: Leaf epidermal features suggest preadaptation in Miocene dragon trees (Dracaena). Review of Palaeobotany and Palynology 200: 211–228. doi:10.1016/j.revpalbo.2013.09.009.
- Denk T, Meller B. 2001. Systematic significance of the cupule/nut complex in living and fossil Fagus. International Journal of Plant Sciences 162: 869–897. doi:10.1086/ijps.2001.162.issue-4.
- Denk T, Tekleva MV. 2014. Pollen morphology and ultrastructure of Quercus with focus on Group Ilex (= Quercus Subgenus Heterobalanus (Oerst.) Menitsky): Implications for oak systematics and evolution. Grana 53: 255–282. doi:10.1080/00173134.2014.918647.
- DeVore M, Pigg KB. 2007. A brief review of the fossil history of the family Rosaceae with a focus on the Eocene Okanogan Highlands of eastern Washington State, USA, and British Columbia, Canada. Plant Systematics and Evolution 266: 45–57. doi:10.1007/s00606-007-0540-3.
- Dilcher DL, Manchester SR. 1986. Investigations of angiosperms from the Eocene of North America: Leaves of the Engelhardieae (Juglandaceae). Botanical Gazette 147: 189–199. doi:10.1086/bg.1986.147.issue-2.
- Dillhoff RM, Leopold EB, Manchester SR. 2005. The McAbee flora of British Columbia and its relation to the early-middle Eocene Okanagan Highlands flora of the Pacific Northwest. Canadian Journal of Earth Sciences 42: 151–166.
- Dilmy A. 1955. Valid names of the genera Bischoffia Bl. and Engelhardia Lesch. Rimba Indonesia 4: 29–37.
- Dönmez EO. 2008. Pollen morphology in Turkish Crataegus (Rosaceae). Botanica Helvetica 118: 59–70. doi:10.1007/s00035-008-0823-5.
- eFloras. 2008. Flora of North America. http://www.efloras.org; accessed January–Mai 2015.
- Elias TE. 1980. Field guide to North American trees. Danbury: Grolier Book Clubs.
- Erdei B, Rákosi L. 2009. The Middle Eocene flora of Csordakút (N Hungary). Geologica Carpathica 60: 43–57. doi:10.2478/v10096-009-0005-4.
- Faghir MB, Attar F, Shahvon RS, Mehrmanesh A. 2015. Pollen morphology of the genus Alchemilla L. (Rosaceae) in Iran. Turkish Journal of Botany 39: 267–279. doi:10.3906/bot-1406-23.
- Fang J, Wang Z, Tang Z. 2009. Atlas of woody plants in China. Distribution and climate index and volumes 1–3. Beijing: Higher Education Press.
- Fayolle A, Picard N, Doucet J-L, Swaine M, Bayol N, Bénédict F, Gourlet-Fleury S. 2014. A new insight in the structure, composition and functioning of central African moist forests. Forest Ecology and Management 329: 195–205.
- Ferguson DK, Pingen M, Zetter R, Hofmann C-C. 1998. Advances in our knowledge of the Miocene plant assemblage from Kreuzau, Germany. Review of Palaeobotany and Palynology 101: 147–177. doi:10.1016/S0034-6667(97)00074-2.
- Forest F, Savolainen V, Chase MW, Lupia R, Bruneau A, Crane PR. 2005. Teasing apart molecular- versus fossil-based error estimates when dating phylogenetic trees: A case study in the birch family (Betulaceae). Systematic Botany 30: 118–133. doi:10.1600/0363644053661850.
- Friis EM, Crane PR, Pedersen KR. 2011. Early flowers and angiosperm evolution. New York: Cambridge University Press.
- Fritz A, Allesch K. 1999. Pollenmorphologische Bilddokumentation ausgewählter Sippen der ehemals Kätzchenblütigen. Carinthia II 189/109: 471–490.
- Fryer JL. 2007. Corylus cornuta. In: USDA Forest Service, ed. Fire effects information system (FEIS database), http://www.feis-crs.org/beta/; accessed January–Mai 2015.
- Fu L, Xin Y, Whittemore A. 2003. Ulmaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Volume 5, Ulmaceae through Basellaceae, 1–19. Beijing: Science Press.
- Fujiki T, Ozawa T. 2007. The pollen flora of Ryukyu, Japan. Ginowan: Akua Koraru Kikaku.
- Fujiki T, Zhou Z, Yasuda Y. 2005. Asian environmental history 1. The pollen flora of Yunnan, China. Vol. I. New Delhi: Roli Books Pvt.
- Furlow JJ. 1979. The systematics of the American species of Alnus (Betulaceae). Rhodora 81: 1–121, 151–248.
- Furlow JJ. 1997. Betulaceae Gray. In: Flora of North America Editorial Committee, eds. Flora of North America north of Mexico, Volume 3, Magnoliophyta: Magnoliidae and Hamamelidae, 507–538. New York: Oxford University Press.
- Geraci A, Polizzano V, Marino P, Schicchi R. 2012. Investigation on the pollen morphology of traditional cultivars of Prunus species in Sicily. Acta Societatis Botanicorum Poloniae 81: 175–184. doi:10.5586/asbp.2012.017.
- Godfrey RK, Wooten JW. 1981. Aquatic and wetland plants of southeastern United States. Dicotyledons. Athens: University of Georgia Press.
- Gottwald H. 1992. Hölzer aus marinen Sanden des oberen Eozän von Helmstedt (Niedersachsen). Palaeontographica B 225: 27–103.
- Graham A. 1999. Late Cretaceous and Cenozoic history of North Amercian vegetation. New York: Oxford University Press.
- Gregor H-J. 1986. Zur Flora des Randecker Maares (Miozän, Baden-Württemberg). Stuttgarter Beiträge zur Naturkunde Serie B 122: 1–29.
- Gregor HJ, Mai DH. 1984. Subtropische Elemente im europäischen Tertiär IV (Onagraceae, Rutaceae, Vitaceae, Theaceae, Elaeagnaceae). Documenta Naturae 16: 1–37.
- Grimm GW, Denk T. 2012. Reliability and resolution of the coexistence approach – A revalidation using modern-day data. Review of Palaeobotany and Palynology 172: 33–47. doi:10.1016/j.revpalbo.2012.01.006.
- Grimm GW, Renner SS. 2013. Harvesting GenBank for a Betulaceae supermatrix, and a new chronogram for the family. Botanical Journal of the Linnean Society 172: 465–477.
- Grímsson F, Denk T. 2005. Fagus from the Miocene of Iceland: Systematics and biogeographical considerations. Review of Palaeobotany and Palynology 134: 27–54. doi:10.1016/j.revpalbo.2004.11.002.
- Grímsson F, Meller B, Bouchal J, Zetter R. 2015a. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part III. Magnoliophyta 1 – Magnoliales to Fabales. Grana 54: 85–128. doi:10.1080/00173134.2015.1007081.
- Grímsson F, Zetter R. 2011. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part II. Pinophyta (Cupressaceae, Pinaceae and Sciadopityaceae). Grana 50: 262–310.
- Grímsson F, Zetter R, Baal C. 2011. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part I. Bryophyta, Lycopodiophyta, Pteridophyta, Ginkgophyta and Gnetophyta. Grana 50: 102–128. doi:10.1080/00173134.2011.585804.
- Grímsson F, Zetter R, Grimm GW, Pedersen GK, Pedersen AK, Denk T. 2015b. Fagaceae pollen from the early Cenozoic of West Greenland: Revisiting Engler’s and Chaney’s Arcto-Tertiary hypotheses. Plant Systematics and Evolution 301: 809–832. doi:10.1007/s00606-014-1118-5.
- Gross M, Böhme M, Havlik P, Aiglstorfer M. 2014. The Late Middle Miocene (Sarmatian s. str.) fossil site Gratkorn – the first decade of research, geology, stratigraphy and vertebrate fauna. Palaeobiodiversity and Palaeoenvironments 94: 5–20. doi:10.1007/s12549-013-0149-1.
- Gu C, Bartholomew B. 2003. Prunus Linnaeus. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Volume 9, Pittosporaceae through Connaraceae, 401–403. Beijing: Science Press.
- Hably L, Thiébaut M. 2002. Revision of Cedrelospermum (Ulmaceae) fruits and leaves from the Tertiary of Hungary and France. Palaeontographica B 262: 71–90.
- Hebda RJ, Chinnappa CC. 1990. Studies on pollen morphology of Rosaceae in Canada. Review of Palaeobotany and Palynology 64: 103–108. doi:10.1016/0034-6667(90)90123-Z.
- Hebda RJ, Chinnappa CC, Smith BM. 1988a. Pollen morphology of the Rosaceae of western Canada I. Agrimonia to Crataegus. Grana 27: 95–113. doi:10.1080/00173138809432836.
- Hebda RJ, Chinnappa CC, Smith BM. 1988b. Pollen morphology of the Rosaceae of western Canada II. Dryas, Fragaria, Holodiscus. Canadian Journal of Botany 66: 595–612. doi:10.1139/b88-086.
- Herbert J. 2005a. New combinations and new species in Morella (Myricaceae). Novon 15: 293–295.
- Herbert J. 2005b. Systematics and biogeography of Myricaceae. PhD thesis, University of St Andrews, St Andrews, UK.
- Herbert J, Chase MW, Möller M, Abbott RJ. 2006. Nuclear and plastid DNA sequences confirm the placement of the enigmatic Canacomyrica monticola in Myricaceae. Taxon 55: 349–357. doi:10.2307/25065582.
- Herrera F, Manchester SR, Koll R, Jaramillo C. 2014. Fruits of Oreomunnea (Juglandaceae) in the early Miocene of Panama. In: Stevens WD, Montiel OM, Raven PH, eds. Paleobotany and biogeography: A Festschrift for Alan Graham in his 80th year, 124–133. St. Louis, MO: Missouri Botanical Garden Press.
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. 2009. Pollen terminology – An illustrated handbook. Wien: Springer.
- Hills LV, Klovan JE, Sweet AR. 1974. Juglans eocinerea n. sp., Beaufort Formation (Tertiary), southwestern Banks Island, Arctic Canada. Canadian Journal of Botany 52: 65–90. doi:10.1139/b74-011.
- Hofmann C-C, Zetter R, Draxler I. 2002. Pollen- und Sporenvergesellschaftungen aus dem Karpatium des Korneuburger Beckens (Niederösterreich). Beiträge zur Paläontologie 27: 17–43.
- Hofmann E. 1929. Fossile Pflanzenreste aus dem Tertiär des Lavanttales in Kärnten. Verhandlungen Geologische Bundesanstalt Wien 4: 101–120.
- Huang C, Zhang Y, Bartholomew B. 1999. Fagaceae. In: Wu ZY, Raven PH, eds. Flora of China, Volume 4, Cycadaceae through Fagaceae, 314–400. Beijing: Science Press.
- Huang T-C. 1972. Pollen flora of Taiwan. Taipei: National Taiwan University Botany Department Press.
- Huguet V, Gouy M, Normand P, Zimpfer JF, Fernandez MP. 2005. Molecular phylogeny of Myricaceae: A reexamination of host-symbiont specificity. Molecular Phylogenetics and Evolution 34: 557–568. doi:10.1016/j.ympev.2004.11.018.
- Huzioka K, Takahashi E. 1970. The Eocene flora of the Ubo coal-field, southwest Honshu, Japan. Journal of the Mining College, Akita University, Series A (Mining Geology) 4: 1–88.
- International Organization for Plant Information (IOPI). 1996–2007. Global plant checklist [online database]. http://www.bgbm.org/iopi/gpc/default.asp; accessed February 2015.
- Ishida S. 1970. The Noroshi flora of Noto Peninsula, Central Japan. Memoirs of the Faculty of Sciences, Kyoto University, Series of Geology and Minerology 37: 1–112.
- Jähnichen H. 1991. Engelhardioid leaves and fruits (Juglandaceae) from the European Tertiary, part III. Tertiary Research 12: 159–164.
- Jähnichen H, Friedrich WL, Takác W. 1984. Engelhardioid leaves and fruits from the European Tertiary, part II. Tertiary Research 6: 109–134.
- Jähnichen H, Mai DH, Walther H. 1977. Blätter und Früchte von Engelhardia Lesch. ex. Bl. (Juglandaceae) aus dem europäischen Tertiär. Feddes Repertorium 88: 323–363.
- Jechorek H, Kovar-Eder J. 2004. Neue Taxa aus der Flora von Weingraben (Burgenland, Miozän, Badenium). Annalen des Naturhistorischen Museums Wien A 106: 327–343.
- Jentys-Szaferowa J. 1975. Studies on the epidermis of recent and fossil fruits of Carpinus and Ostrya and its significance in the systematics and history of these genera. Acta Palaeobotanica 16: 3–70.
- Joneghani VN. 2008. Pollen morphology of the genus Malus (Rosaceae). Iranian Journal of Science and Technology, Transaction A 32/A2: 89–97.
- Jones GD, Bryant VM, Lieux MH, Jones SD, Lindgren PD. 1995. Pollen of the southeastern United States, with emphasis on melissopalynology and entomopalynology. AASP Contribution Series 30: 1–104.
- Jones JH, Dilcher DL. 1988. A study of the “Dryophyllum” leaf forms from the Paleogene of southeastern North America. Palaeontographica B 208: 53–80.
- Karlsdóttir L, Hallsdóttir M, Thórsson ÆT, Anamthawat-Jónsson K. 2008. Characteristics of pollen from natural triploid Betula hybrids. Grana 47: 52–59. doi:10.1080/00173130801927498.
- Karlsdóttir L, Thórsson ÆT, Hallsdóttir M, Sigurgeirsson A, Eysteinsson T, Anamthawat-Jónsson K. 2007. Differentiating pollen of Betula species from Iceland. Grana 46: 78–84.
- Kirchheimer F. 1957. Die Laubgewächse der Braunkohlenzeit; mit einem kritischen Katalog ihrer Früchte und Samen. Halle (Saale): VEB Wilhelm Knapp.
- Kirchheimer F. 1973. Rosaceae. Fossilium Catalogus II. Plantae. Pars 25. The Hague: Amsterdam.
- Klaus W. 1984. Zur Mikroflora des Unter-Sarmat am Alpen-Südostrand. Beiträge zur Paläontologie von Österreich 11: 289–419.
- Kmenta M. 2011. Die Mikroflora der untermiozänen Fundstelle Altmittweida, Deutschland. MSc Thesis, University of Vienna, Vienna, Austria.
- Knobloch E. 1961. Die oberoligozäne Flora des Pirskenbergens bei Šluknov in Nord-Böhmen. Sborník Ústředního Ústavu Geologického, Paleontologie 26: 241–315.
- Köppen W. 1936. Das geographische System der Klimate. In: Köppen W, Geiger R, eds. Handbuch der Klimatologie, Band 1, Teil C, 1–44). Berlin: Borntraeger.
- Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. 2006. World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15: 259–263. doi:10.1127/0941-2948/2006/0130.
- Kovar-Eder J, Kvaček Z, Ströbitzer-Hermann M. 2004. The Miocene flora of Parschlug (Styria, Austria) – revision and synthesis. Annalen des Naturhistorischen Museums Wien A 105: 45–159.
- Krutzsch W. 1961. Beitrag zur Sporenpaläontologie der präoberoligozänen kontinentalen und marinen Tertärablegerungen Brandenburgs. Berichte der Geologischen Gesellschaft 4: 290–343.
- Krutzsch W. 1962. Stratigraphisch bzw. botanisch wichtige neue Sporen- und Pollenformen aus dem deutschen Tertiär. Geologie 11: 265–308.
- Kvaček Z. 1972. Engelhardia leaves in the European Tertiary. Časopis pro Mineralogii a Geologii 17: 25–31.
- Kvaček Z. 2007. Do extant nearest relatives of thermophile European Cenozoic plant elements reliably reflect climatic signal? Palaeogeography, Palaeoclimatology, Palaeoecology 253: 32–40. doi:10.1016/j.palaeo.2007.03.032.
- Kvaček Z, Walther H. 1988. Revision der mitteleuropäischen tertiären Fagaceae nach blattepidermalen Charakteristiken II. Teil: Castanopsis (D. Don) Spach, Trigonobalanus Forman, Trigonobalanopsis Kvaček & Walther. Feddes Repertorium 99: 395–418.
- Kvaček Z, Walther H. 1989. Paleobotanical studies in Fagaceae of the European Tertiary. Plant Systematics and Evolution 162: 213–229. doi:10.1007/BF00936918.
- Kvaček Z, Walther H. 1991. Revision der mitteleuropäischen tertiären Fagaceen nach blattepidermalen Charakteristiken. IV. Teil Fagus Linné. Feddes Repertorium 102: 471–534.
- Kvaček Z, Walther H. 1998. The Oligocene volcanic flora of Kundratice near Litoměřice, Česke Středohoří Volcanic Complex (Czech Republic) – a review. Acta Musei Nationalis Pragae, Series B, Historia Naturalis 54: 1–42.
- Kvaček Z, Walther H. 2010. European Tertiary Fagaceae with chinquapin-like foliage and leaf epidermal characteristics. Feddes Repertorium 121: 248–267. doi:10.1002/fedr.v121.7/8.
- Kvavadze EV, Connor SE. 2005. Zelkova carpinifolia (Pallas) K. Koch in Holocene sediments of Georgia – an indicator of climatic optima. Review of Palaeobotany and Palynology 133: 69–89. doi:10.1016/j.revpalbo.2004.09.002.
- Lang G. 1994. Quartäre Vegetationsgeschichte Europas. Jena: Fischer.
- Lee S, Wen J. 2001. A phylogenetic analysis of Prunus and the Amygdaloideae (Rosaceae) using ITS sequences of nuclear ribosomal DNA. American Journal of Botany 88: 150–160. doi:10.2307/2657135.
- Leopold EB, Birkebak J, Reinink-Smith L, Jayachandar AP, Narváez P, Zaborac-Reed S. 2012. Pollen morphology of the three subgenera of Alnus. Palynology 36: 131–151. doi:10.1080/01916122.2012.657876.
- Li P, Skvortsov AK. 1999. Betulaceae. In: Wu ZY, Raven PH, eds. Flora of China, Volume 4, Cycadaceae through Fagaceae, 286–313. Beijing: Science Press.
- Li T-Q, Cao H-J, Kang M-S, Zhang Z-X, Zhao N, Zhang H. 2011a. Pollen flora of China woody plants by SEM. Beijing: Science Press.
- Li Y, Smith T, Liu C-J, Awasthi N, Yang J, Wang Y-F, Li C-S. 2011b. Endocarps of Prunus (Rosaceae: Prunoideae) from the early Eocene of Wutu, Shandong Province, China. Taxon 60: 555–564.
- Lieux MH. 1980. An atlas of pollen of trees, shrubs, and woody vines of Louisiana and other southeastern states, part II. Platanaceae to Betulaceae. Pollen et Spores 22: 191–243.
- Lin L, Yao Q, Xu H, Mu H, Jiang J. 2013. Characteristics of the staminate flower and pollen from autotetraploid Betula platyphylla. Dendrobiology 69: 3–11. doi:10.12657/denbio.069.001.
- Liu C. 1987. Studies of pollen morphology in the Rhoipteaceae and the relative families. Acta Botanica Yunnanica 9: 181–186.
- Liu X, Manchester SR, Jin J. 2014. Alnus subgenus Alnus in the Eocene of Western North America based on leaves, associated catkins, pollen, and fruits. American Journal of Botany 101: 1925–1943. doi:10.3732/ajb.1400228.
- Liu X-L, Wen J, Nie Z-L, Johnson G, Liang Z-S, Chang Z-Y. 2013. Polyphyly of the Padus group of Prunus (Rosaceae) and the evolution of biogeographic disjunctions between eastern Asia and eastern North America. Journal of Plant Research 126: 351–361. doi:10.1007/s10265-012-0535-1.
- Liu YS. 1996. Foliar architecture of Betulaceae and a revision of Chinese betulaceous megafossils. Palaeontographica B 239: 23–57.
- Liu Y-S, Zetter R, Ferguson DK, Mohr BAR. 2007. Discriminating fossil evergreen and deciduous Quercus pollen: A case study from the Miocene of eastern China. Review of Palaeobotany and Palynology 145: 289–303. doi:10.1016/j.revpalbo.2006.12.001.
- Lu A, Bornstein AJ. 1999. Myricaceae. In: Wu ZY, Raven PH, eds. Flora of China, Volume 4, Cycadaceae through Fagaceae, 275–276. Beijing: Science Press.
- Lu A, Stone DE, Grauke LJ. 1999. Juglandaceae. In: Wu ZY, Raven PH, eds. Flora of China, Volume 4, Cycadaceae through Fagaceae, 277–285. Beijing: Science Press.
- Lui YS, Guo SX, Ferguson DK. 1996. Catalogue of Cenozoic megafossil plants in China. Palaeontographica B 238: 141–179.
- Lutz H. 1984. Beitrag zur Kenntnis der Unteroligozänen Insektenfauna von Cereste (Süd-Frankreich). Documenta Naturae 21: 1–26.
- Mabberley DJ. 2008. Mabberley’s Plant-Book: A portable dictionary of plants, their classification, and uses. Cambridge, UK: Cambridge University Press.
- MacGinite HD. 1941. A middle Eocene flora from the central Sierra Nevada. Contributions to Paleontology, 1–178. Washington: Carnegie Institution, Publication 534.
- MacGinite HD. 1953. Fossil plants of the Florissant beds, Colorado, 1–198. Washington: Carnegie Institution, Publication 599.
- Magallón-Puebla S, Cevallos-Ferriz SRS. 1994. Latest occurrence of the extinct genus Cedrelospermum (Ulmaceae) in North America: Cedrelospermum manchesteri from Mexico. Review of Palaeobotany and Palynology 81: 115–128. doi:10.1016/0034-6667(94)90102-3.
- Mai DH. 1981a. Der Formenkreis der Vietnam-Nuß (Carya poilanei (Chev.) Leroy) in Europa. Feddes Repertorium 92: 339–385. doi:10.1002/fedr.19810920502.
- Mai DH. 1981b. Entwicklung und klimatische Differenzierung der Laubwaldflora Mitteleuropas im Tertiär. Flora 171: 525–582.
- Mai DH. 1984. Karpologische Untersuchungen der Steinkerne fossiler und recenter Amygdalaceae (Rosales). Feddes Repertorium 95: 299–329.
- Mai DH. 1987. Neue Arten von Früchten und Samen aus dem Tertiär von Nordwestsachsen und der Lausitz. Feddes Repertorium 98: 105–126.
- Mai DH. 1995. Tertiäre Vegetationsgeschichte Europas. Jena: Gustav Fischer.
- Mai DH, Walther H. 1991. Die oligozänen und untermiozänen Floren Nordwestsachsens und des Bitterfelder Raumes. Schriften des Staatlichen Museums für Mineralogie und Geologie Dresden 38: 61–63.
- Mäkelä EM. 1996. Size distinctions between Betula pollen types — A review. Grana 35: 248–256. doi:10.1080/00173139609430011.
- Makino M, Hayashi R, Takahara H. 2009. Pollen morphology of the genus Quercus by scanning electron microscope. Scientific Reports of Kyoto Prefectural University, Life and Environmental Sciences 61: 53–81.
- Manchester SR. 1983. Fossil Wood of the Engelhardieae (Juglandaceae) from the Eocene of North America: Engelhardioxylon gen. nov. Botanical Gazette 144: 157–163. doi:10.1086/bg.1983.144.issue-1.
- Manchester SR. 1987a. Extinct ulmaceous fruits from the Tertiary of Europe and western North America. Review of Palaeobotany and Palynology 52: 119–129.
- Manchester SR. 1987b. The fossil history of the Juglandaceae. Monographs in Systematic Botany from the Missouri Botanical Garden 21: 1–137.
- Manchester SR. 1989a. Attached reproductive and vegetative remains of the extinct American-European genus Cedrelospermum (Ulmaceae) from the early Tertiary of Utah and Colorado. American Journal of Botany 76: 256–276.
- Manchester SR. 1989b. Early history of the Juglandaceae. Plant Systematics and Evolution 162: 231–250. doi:10.1007/BF00936919.
- Manchester SR. 1989c. Systematic and fossil history of the Ulmaceae. In: Crane PR, Blackmore S, eds. Evolution, systematics, and fossil history of the Hamamelidae, Volume 2: ‘Higher’ Hamamelidae. Systematics Association Special Volume No. 40B, 221–251. Oxford: Clarendon Press.
- Manchester SR. 1994. Fruits and seeds of the middle Eocene Nut Beds Flora, Clarno Formation, Oregon. Palaeontographica Americana 58: 1–205.
- Manchester SR. 1999. Biogeographical relationships of North American Tertiary floras. Annals of the Missouri Botanical Garden 86: 472–522. doi:10.2307/2666183.
- Manchester SR, Akhmetiev MA, Kodrul TA. 2002. Leaves and fruits of Celtis aspera (Newberry) comb. nov. (Celtidaceae) from the Paleocene of North America and eastern Asia. International Journal of Plant Sciences 163: 725–736.
- Manchester SR, Dilcher DL. 1982. Pterocaryoid fruits (Juglandaceae) in the Paleogene of North America and their evolutionary and biogeographic significance. American Journal of Botany 69: 275–286. doi:10.2307/2443015.
- Manchester SR, Dillhoff RM. 2004. Fagus (Fagaceae) fruits, foliage, and pollen from the Middle Eocene of Pacific Northwestern North America. Canadian Journal of Botany 82: 1509–1517. doi:10.1139/b04-112.
- Manchester SR, Pigg KB, Crane PR. 2004. Palaeocarpinus dakotensis sp.n. (Betulaceae: Coryloideae) and Associated Staminate Catkins, Pollen, and Leaves from the Paleocene of North Dakota. International Journal of Plant Sciences 165: 1135–1148. doi:10.1086/ijps.2004.165.issue-6.
- Manchester SR, Guo S-X. 1996. Palaeocarpinus (extinct Betulaceae) from northwestern China: new evidence for paleocene floristic continuity between Asia, North America, and Europe. International Journal of Plant Sciences 157: 240–246. doi:10.1086/ijps.1996.157.issue-2.
- Manchester SR, Tiffney BH. 2001. Integration of paleobotanical and neobotanical data in the assessment of phytogeographic history of Holarctic angiosperm clades. International Journal of Plant Sciences 162, S6: S19–S27. doi:10.1086/ijps.2001.162.issue-s6.
- Manning WE. 1966. New combinations and notes on Engelhardia (Juglandaceae) of the Old World. Bulletin of the Torrey Botanical Club 93: 34–52. doi:10.2307/2483884.
- Manning WE. 1978. The classification within the Juglandaceae. Annals of the Missouri Botanical Garden 65: 1058–1087. doi:10.2307/2398782.
- Manos PS, Soltis PS, Soltis DE, Manchester SR, Oh S-H, Bell CD, Dilcher DL, Stone DE. 2007. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. Systematic Biology 56: 412–430. doi:10.1080/10635150701408523.
- Manos PS, Stone DE. 2001. Evolution, phylogeny, and systematics of the Juglandaceae. Annals of the Missouri Botanical Garden 88: 231–269. doi:10.2307/2666226.
- May L. 2011. Morphological differentiation of Alnus pollen from western North America. MSc Thesis, University of Victoria, Victoria, BC.
- May L, Lacourse T. 2012. Morphological differentiation of Alnus (alder) pollen from western North America. Review of Palaeobotany and Palynology 180: 15–24.
- Maycock PF. 1994. The ecology of beech (Fagus grandifolia Ehrh.) forests of the deciduous forests of southeastern North America, and a comparison with the beech (Fagus crenata) forests of Japan. In: Miyawaki A, Iwatsuki K, Grandtner MM, eds. Vegetation in eastern North America, 351–410. Tokyo: University of Tokyo Press.
- McIntyre DJ. 1991. Pollen and spore flora of an Eocene forest, eastern Axel Heiberg Island, NWT. Geological Survey of Canada Bulletin 403: 83–97.
- McVaugh R. 1951. A revision of the North American black cherries (Prunus serotina Ehrh., and relatives). Brittonia 7: 279–315. doi:10.2307/2804698.
- Meller B. 1998. Systematisch-taxonomische Untersuchungen von Karpo-Taphocoenosen des Köflach-Voitsberger Braunkohlenrevieres (Untermiozän; Steiermark, Österreich) und ihre paläoökologische Bedeutung. Jahrbuch der Geologischen Bundesanstalt Wien 140: 497–654.
- Meller B. 2011. Wetland vegetation types in the Late Miocene Alpine Molasse Basin in Upper Austria. Palaeontographica B 287: 57–155.
- Meller B, Hably L. 2014. Wasser- und Sumpfplanzenvergesellschaftungen vom NW-Rand des Steirischen Beckens (Sarmatian, oberes Mittelmiozän). Berichte der Geologischen Bundesanstalt Wien 105: 14.
- Meller B, Kovar-Eder J, Zetter R. 1999. Lower Miocene diaspore, leaf and palynomorph assemblages from the base of the lignite-bearing sequence in the opencast mine Oberdorf, N Voitsberg (Styria, Austria) as an indication of a “Younger Mastixioid” vegetation. Palaeontographica B 252: 123–179.
- Mert C. 2010. Anther and pollen morphology and anatomy in walnut (Juglans regia L.). HortScience 45: 757–760.
- Meusel H, Jäger EJ, Weinert E. 1965. Vergleichende Chorologie der zentraleuropäischen Flora. Text und Karten, Band 1. Jena: VEB Fischer.
- Meyer HW, Manchester SR. 1997. The Oligocene Bridge Creek Flora of the John Day Formation, Oregon. University of California Publications in Geological Sciences 141: 91–94.
- Miki S. 1936. Plant fossils from the Stegodon beds and Elephas beds near Akashi. Japanese Journal of Botany 8: 303–341.
- Miki S. 1938. On the change of flora of Japan since the upper Pliocene and the floral composition at the present. Japanese Journal of Botany 9: 213–251.
- Miller C, Gosling W. 2014. Quaternary forest associations in lowland tropical West Africa. Quaternary Science Reviews 84: 7–25. doi:10.1016/j.quascirev.2013.10.027.
- Mindell RA, Stockey RA, Beard G. 2009. Permineralized Fagus nuts from the Eocene of Vancouver Island, Canada. International Journal of Plant Sciences 170: 551–560. doi:10.1086/592009.
- Miyawaki A, Suzuki K. 1980. Natürliche und ihre sommergrünen Sekundär-Wälder in Mitteljapan. Über die pflanzensoziologische Stellung der Quercetalia serrato-grosseserratae. Phytocoenologia 7: 492–506.
- Miyoshi N. 1982. Pollen morphology by means of scanning electron microscope 4. Fagaceae (Angiospermae). Bulletin of the Hiruzen Research Institute, Okayama University of Science 7: 55–60.
- Miyoshi N, Fujiki T, Kimura H. 2011. Pollen flora of Japan. Sapporo: Hokkaido University Press.
- Morita Y, Fujiki T, Kataoka H, Miyoshi N. 1998. Identification of Ulmus and Zelkova pollen. Japanese Journal of Palynology 44: 11–18.
- Muller J. 1981. Fossil pollen records of extant angiosperms. The Botanical Review 47: 1–142. doi:10.1007/BF02860537.
- Müller-Stoll WR, Mädel E. 1960. Juglandaceen-Hölzer aus dem Tertiär des pannonischen Beckens. Senckenbergiana Lethaea 41: 255–295.
- Müller-Stoll WR, Madel-Angeliewa E. 1983. Fossile Hölzer mit schmalen apotrachealen Parenchymbändern I. Arten aus der Gattung Eucaryoxylon Müller-Stoll & Mädel. Feddes Repertorium 94: 655–677.
- Murai S. 1964. Phytotaxonomical and geobotanical studies on gen. Alnus in Japan (III). Taxonomy of whole world species and distribution of each section. Bulletin of the Government Forest Experimental Station Meguro, Tokyo 171: 1–107.
- Nagy E. 1969. Palynological elaborations the Miocene layers of the Mecsek Mountains. Annales Instituti Geologici Publici Hungarici 52: 237–417.
- Nakagawa T, Garfì G, Reille M, Verlaque R. 1998. Pollen morphology of Zelkova sicula (Ulmaceae), a recently discovered relic species of the European Tertiary flora: Description, chromosomal relevance, and palaeobotanical significance. Review of Palaeobotany and Palynology 100: 27–37. doi:10.1016/S0034-6667(97)00062-6.
- Nakagawa T, Yasuda Y, Tabata H. 1996. Pollen morphology of Himalayan Pinus and Quercus and its importance in palynological studies in Himalayan area. Review of Palaeobotany and Palynology 91: 317–329. doi:10.1016/0034-6667(95)00072-0.
- Navarro E, Bousquet J, Moiroud A, Munive A, Piou D, Normand P. 2003. Molecular phylogeny of Alnus (Betulaceae), inferred from nuclear ribosomal DNA ITS sequences. Plant and Soil 254: 207–217. doi:10.1023/A:1024978409187.
- Ne’Eman G. 1993. Variation in leaf phenology and habit in Quercus ithaburensis, a Mediterranean deciduous tree. Journal of Ecology 81: 627–634.
- Nichols DJ. 1973. North American and European species of Momipites (“Engelhardtia”) and related genera. Geoscience and Man 7: 103–117. doi:10.1080/00721395.1973.9989740.
- Nikolaieva N, Brindza J, Garkava K, Ostrovsky R. 2014. Pollen features of hazelnut (Corylus avellana L.) from different habitats. Modern Phytomorphology 6: 53–58.
- Nirei H. 1975. A classification of fossil walnuts from Japan. Journal of Geosciences, Osaka City University 19: 31–62.
- Nixon KC. 1997. Fagaceae Dumortier. In: Flora of North America Editorial Committee, eds. Flora of North America north of Mexico, Volume 3, Magnoliophyta: Magnoliidae and Hamamelidae, 436–506. New York: Oxford University Press.
- Ohwi J. 1965. Flora of Japan. Washington: Smithsonian Institution.
- Paraschiv V. 2008. New Sarmatian plant macroremains from Oltenia region (Romania). Part 1. Acta Palaeontologica Romaniae 6: 279–286.
- Pehlivan S. 1987. A comparative study on the fine structures of the pollen walls and annuli in some Turkish Betulaceae, Moraceae, Cannabaceae, Haloragaceae. Communications, Faculty of Sciences, University of Ankara, Series C 5: 1–18.
- Perveen A, Qaiser M. 1999. Pollen flora of Pakistan – XXXI. Betulaceae. Pakistan Journal of Botany 31: 243–246.
- Perveen A, Qaiser M. 2005. Pollen flora of Pakistan – XLIV. Rhamnaceae. Pakistan Journal of Botany 37: 195–202.
- Perveen A, Qaiser M. 2014. Pollen flora of Pakistan – LXXI. Rosaceae. Pakistan Journal of Botany 46: 1027–1037.
- Peters R. 1997. Beech forests. Dordrecht: Kluwer.
- Petroselli A, Vessella F, Cavagnuolo L, Piovesan G, Schirone B. 2013. Ecological behavior of Quercus suber and Quercus ilex inferred by topographic wetness index (TWI). Trees 27: 1201–1215. doi:10.1007/s00468-013-0869-x.
- Pflug H. 1953. Zur Entstehung und Entwicklung des angiospermiden Pollens in der Erdgeschichte. Palaeontographica B 96: 60–171.
- Pigg KB, Manchester SR, Wehr WC. 2003. Corylus, Carpinus, and Palaeocarpinus (Betulaceae) from the Middle Eocene Klondike Mountain and Allenby Formations of Northwestern North America. International Journal of Plant Sciences 164: 807–822. doi:10.1086/ijps.2003.164.issue-5.
- Potonié R. 1931. Pollenformen aus tertiären Braunkohlen. III. Jahrbuch der Preussischen Geologischen Landesanstalt 52: 1–7.
- Potonié R. 1960. Synopsis der Gattungen der Sporae dispersae. III. Beihefte zum Geologischen Jahrbuch 39: 1–189.
- Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, Campbell CS. 2007. Phylogeny and classification of Rosaceae. Plant Systematics and Evolution 266: 5–43. doi:10.1007/s00606-007-0539-9.
- Praglowski J. 1982. Fagaceae L.: Fagoideae. World Pollen and Spore Flora 11. Stockholm: Almquist & Wiksell.
- Praglowski J. 1984. Fagaceae Dumort.: Castaneoideae Oerst. World Pollen and Spore Flora 13. Stockholm: Almquist & Wiksell.
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. doi:10.1016/j.revpalbo.2006.06.008.
- Punt W, Marks A, Hoen PP. 2003. The northwest European pollen flora, 66. Myricaceae. Review of Palaeobotany and Palynology 123: 99–105. doi:10.1016/S0034-6667(02)00157-4.
- Punt W, Marks A, Hoen PP. 2002. The northwest European pollen flora, 63. Rhamnaceae. Review of Palaeobotany and Palynology 123: 57–66. doi:10.1016/S0034-6667(02)00154-9.
- Qin H, Gilbert MB. 2007. Elaeagnaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Volume 13, Clusiaceae through Araliaceae, 251–273. Beijing: Science Press.
- Raatz GV. 1937. Mikrobotanisch-stratigraphische Untersuchung der Braunkohle des Muskauer Bogens. Abhandlungen der Preussischen Geologischen Landesanstalt, Neue Folge 183: 3–48.
- Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. 2004. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 1495–1508. doi:10.1098/rstb.2004.1537.
- Richardson JE, Fay MF, Cronk QCB, Bowman D, Chase, MW. 2000b. A phylogenetic analysis of Rhamnaceae using rbcL and trnL-F plastid DNA sequences. American Journal of Botany 87: 1309–1324. doi:10.2307/2656724.
- Richardson JE, Fay MF, Cronk QCB, Chase MW. 2000a. A revision of the tribal classification of Rhamnaceae. Kew Bulletin 55: 311–340. doi:10.2307/4115645.
- Richter SL, LePage BA. 2005. A high-resolution palynological analysis, Axel Heiberg Island, Canadian High Arctic. In: LePage BA, Williams CJ, Yang H, eds. The geobiology and ecology of Metasequoia, 137–158. Dordrecht: Springer.
- Rüffle L. 1963. Die obermiozäne (sarmatische) Flora vom Randecker Maar. Paläontologische Abhandlungen Abt. B 1: 139–298.
- Saito T. 1992. Pollen morphology and species-level distinction of the genus Fagus from the Hachiya Formation (lower Miocene), Mizunami Group, Japan. The Journal of Earth Planetary Sciences, Nagoya University 39: 31–46.
- Sattarian A, Van den Berg G, Van der Maesen LJG. 2006. Pollen morphology of African Celtis (Celtidaceae). Feddes Repertorium 117: 34–40. doi:10.1002/(ISSN)1522-239X.
- Schirarend C. 1996. Pollen morphology of the genus Paliurus (Rhamnaceae). Grana 35: 347–356. doi:10.1080/00173139609429093.
- Schirarend C, Köhler E. 1993. Rhamnaceae Juss. World Pollen and Spore Flora 17/18. Stockholm: Almquist & Wiksell.
- Schroeder G-F. 1998. Lehrbuch der Pflanzengeographie. Wiesbaden: Quelle & Meyer.
- Shaw K, Roy S, Wilson B. 2014a. Betula alnoides. The IUCN red list of threatened species. Version 2013.3. http://www.iucnredlist.org; accessed 24 April 2015.
- Shaw K, Roy S, Wilson B. 2014b. Ostrya carpinifolia. The IUCN red list of threatened species. Version 2013.3. http://www.iucnredlist.org; accessed 24 April 2015.
- Shen SEE. 1992. A monograph of the genus Fagus Thurn. ex L. (Fagaceae). New York: City University of New York.
- Sherman-Broyles SL, Barker WT, Schulz LM. 1997. Ulmaceae Mirbel. In: Flora of North America Editorial Committee, eds. Flora of North America north of Mexico, Volume 3, Magnoliophyta: Magnoliidae and Hamamelidae, 368–380. New York: Oxford University Press.
- Sheykholislami A, Ahmadi T. 2009. The study of Caucasian walnut (Pterocarya frexinifolia (Lam.) Spach.) in forests of Mashelak (Noshahr, Iran). Botany Research Journal 2: 28–33.
- Shi W, Wen J, Lutz S. 2013. Pollen morphology of the Maddenia clade of Prunus and its taxonomic and phylogenetic implications. Journal of Systematics and Evolution 51: 164–183. doi:10.1111/jse.v51.2.
- Skarpy A, Morbelli MA, Rowley JR. 2009. Structure of the pollen exine of Rhoiptelea chilantha. Taiwania 54: 101–112.
- Skene KR, Sprent JI, Raven JA, Herdman L. 2000. Myrica gale L. Journal of Ecology 88: 1079–1094. doi:10.1046/j.1365-2745.2000.00522.x.
- Solomon AM. 1983. Pollen morphology and plant taxonomy of red oaks in eastern North America. American Journal of Botany 70: 495–507. doi:10.2307/2443160.
- Søndergaard P, Egli BR. 2006. Zelkova abelicea (Ulmaceae) in Crete: Floristics, ecology, propagation and threats. Willdenowia 36: 317–322. doi:10.3372/wi.36.36126.
- Sorkheh K, Vezvaei A, Wirthensohn MG, Martínez-Gómez P. 2008. Pollen ultrastructure characterization in Californian and Australian almond cultivars. Journal of the American Pomological Society 62: 173–177.
- Srivastava SK. 1966. Upper Cretaceous microflora (Maestrichtian) from Scollard, Alberta, Canada. Pollen et Spores 8: 497–552.
- Stace CA. 1997. New flora of the British Isles. Cambridge: Cambridge University Press.
- Stafford PJ. 1995. The northwest European pollen flora, 53. Ulmaceae. Review of Palaeobotany and Palynology 88: 25–46. doi:10.1016/0034-6667(95)98770-8.
- Stanford AM, Harden R, Parks CR. 2000. Phylogeny and biogeography of Juglans (Juglandaceae) based on matK and ITS sequence data. American Journal of Botany 87: 872–882. doi:10.2307/2656895.
- Stone DE. 1963. Pollen size in hickories (Carya). Brittonia 15: 208–215. doi:10.2307/2805161.
- Stone DE. 1968. New World Juglandaceae: A new species of Alfaroa from Mexico. American Journal of Botany 55: 477–484. doi:10.2307/2440578.
- Stone DE. 1977. Juglandaceae. Fieldiana. Botany 40: 28–53.
- Stone DE. 1997. Juglandaceae A. Richard ex Kunth. In: Flora of North America Editorial Committee, eds. Flora of North America north of Mexico, Volume 3, Magnoliophyta: Magnoliidae and Hamamelidae, 416–428. New York: Oxford University Press.
- Stone DE. 2010. Review of New World Alfaroa and Old World Alfaropsis (Juglandaceae). Novon: A Journal for Botanical Nomenclature 20: 215–224. doi:10.3417/2009027.
- Stone DE, Broome CR. 1971. Pollen ultrastructure: Evidence for relationship of the Juglandaceae and the Rhoipteleaceae. Pollen et Spores 13: 5–14.
- Stone DE, Broome CR. 1975. Juglandaceae A. Rich. Ex Kunth. World Pollen and Spore Flora 4. Stockholm: Almquist & Wiksell.
- Stone DE, Reich J, Whitfield S. 1964. Fine structure of the walls of Juglans and Carya pollen. Pollen et Spores 6: 379–392.
- Stritch L. 2014. Carpinus tropicalis. The IUCN red list of threatened species. Version 2013.3. http://www.iucnredlist.org; accessed 24 April 2015.
- Stuchlik L, Ziembińska-Tworzydło M, Kohlman-Adamska A, Grabowska I, Słodkowska B, Ważyńska H, Sadowska A. 2009. Atlas of pollen and spores of the Polish Neogene. Volume 3. Angiosperms (1). Kraków: W. Szafer Institute of Botany, Polish Academy of Sciences.
- Stuchlik L, Ziembińska-Tworzydło M, Kohlman-Adamska A, Grabowska I, Słodkowska B, Worobiec E, Durska E. 2014. Atlas of pollen and spores of the Polish Neogene. Volume 4. Angiosperms (2). Kraków: W. Szafer Institute of Botany, Polish Academy of Sciences.
- Su T, Wilf P, Xu H, Zhou Z-K. 2014. Miocene leaves of Elaeagnus (Elaeagnaceae) from the Qinghai-Tibet Plateau, its modern center of diversity and endemism. American Journal of Botany 101: 1350–1361. doi:10.3732/ajb.1400229.
- Sun M, Lin Q. 2010. A revision of Elaeagnus L. (Elaeagnaceae) in mainland China. Journal of Systematics and Evolution 48: 356–390. doi:10.1111/jse.2010.48.issue-5.
- Sundberg MD. 1985. Pollen of the Myricaceae. Pollen et Spores 27: 15–27.
- Suzuki M. 1984. Some fossil woods from the Palaeogene of northern Kyushu, III. The Botanical Magazine, Tokyo 97: 457–468. doi:10.1007/BF02489578.
- Tahir SS. 2005. Pollen morphology of Sibbaldia species (Rosaceae). Pakistan Journal of Botany 37: 7–13.
- Takahashi A, Suzuki M. 1988. Two new fossil woods of Acer and a new combination of Prunus from the Tertiary of Japan. The Botanical Magazine, Tokyo 101: 473–481. doi:10.1007/BF02488089.
- Takahashi M. 1989. Pollen morphology of Celtidaceae and Ulmceae: A reinvestigation. In: Crane PR, Blackmore S, eds. Evolution, systematics, and fossil history of the Hamamelidae, Volume 2: ‘Higher’ Hamamelidae. Systematics Association Special Volume 40B, 253–265. Oxford: Clarendon Press.
- Tanai T. 1961. Neogene floral change in Japan. Journal of the Faculty of Science, Hokkaido University, Series IV: Geology and Mineralogy 11: 119–398.
- Tanai T. 1972. Tertiary history of vegetation in Japan. In: Graham A, ed. Floristics and paleofloristics of Asia and eastern North America, 235–255. Amsterdam: Elsevier.
- Tanai T. 1992. Juglandaceae from the Paleogene of Hokkaido, Japan. Bulletin of the National Science Museum, Tokyo, Series C 18: 13–41.
- Tanai T, Onoe T. 1961. A Mio–Pliocene flora from the Ningyo-Toge area on the border between Tottori and Okayama prefectures, Japan. Geological Survey of Japan Report 187: 1–62.
- Tanai T, Uemura K. 1983. Engelhardia fruits from the Tertiary of Japan. Journal of the Faculty of Science, Hokkaido University, Series IV 20: 249–260.
- Tekleva MV, Naryshkina NN, Evstigneeva TA. 2014. Fine structure of Quercus pollen from the Holocene sediments of the Sea of Japan. Plant Systematics and Evolution 300: 1877–1893. doi:10.1007/s00606-014-1014-z.
- Théobald N. 1937. Les insectes fossiles des terrains oligocènes de France. Nancy: G. Thomas.
- Thiele-Pfeiffer H. 1980. Die miozäne Mikroflora aus dem Braunkohlentagebau Oder bei Wackersdorf/Oberpfalz. Palaeontographica B 174: 95–224.
- Thiergart F. 1937. Die Pollenanalyse der Niederlausitzer Braunkohle, besonders im Profil der Grube Marga bei Senftenberg. Jahrbuch der Preussischen Geologischen Landesanstalt 58: 282–351.
- Thompson RS, Anderson KH, Bartlein PJ. 1999a. Atlas of relations between climatic parameters and distribution of important trees and shrubs in North America – Introduction and conifers. U.S. Geological Survey Professional Paper 1650–A: 1–269.
- Thompson RS, Anderson KH, Bartlein PJ. 1999b. Atlas of relations between climatic parameters and distribution of important trees and shrubs in North America – Hardwoods. U.S. Geological Survey Professional Paper 1650–B: 1–423.
- Thompson RS, Anderson KH, Bartlein PJ. 2001. Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America – Additional conifers, hardwoods, and monocots. U.S. Geological Survey Professional Paper 1650–C: 1–386.
- Thompson RS, Anderson KH, Strickland LE, Shafer SL, Pelltier RT, Bartlein PJ. 2006. Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America – Alaska species and ecoregions. U.S. Geological Survey Professional Paper 1650–D: 1–342.
- Thomson PW, Pflug H. 1953. Pollen und Sporen des mitteleuropäischen Tertiärs. Palaeontographica B 94: 1–158.
- Todzia CA, Panero JL. 1998. A new species of Ulmus (Ulmaceae) from southern Mexico and a synopsis of the species in Mexico. Brittonia 50: 343–347. doi:10.2307/2807778.
- Tralau H. 1962. Die spättertiären Fagus-Arten Europas. Botaniska Notiser 115: 147–176.
- Tschan G, Denk T. 2012. Trichome types, foliar indumentum and epicuticular wax in the Mediterranean gall oaks, Quercus subsection Galliferae (Fagaceae): Implications for taxonomy, ecology and evolution. Botanical Journal of the Linnean Society 169: 611–644. doi:10.1111/boj.2012.169.issue-4.
- Uemura K, Tanai T. 1993. Betulaceous leaves and fruits from the Oligocene of Kitami, Hokkaido, Japan. Memoirs of the National Science Museum, Tokyo 26: 21–29.
- Ueno J. 1967. On the pollen grains of Juglans mandaschurica Maxim. subsp. sieboldiana (Makino) Kitamura. Acta Phytotaxonomica Geobotanica 22: 175–182.
- Vafadar M, Attar F, Maroofi H, Mirtadzadini M. 2010. Pollen morphology of Amygdalus L. (Rosaceae) in Iran. Acta Societatis Botanicorum Poloniae 79: 63–71. doi:10.5586/asbp.2010.009.
- Van der Burgh J, Zetter R. 1998. Plant mega- and microfossil assemblages from the Brunssumian of “Hambach” near Düren, BRD. Review of Palaeobotany and Palynology 101: 209–256. doi:10.1016/S0034-6667(97)00076-6.
- Velitzelos D, Bouchal JM, Denk T. 2014. Review of the Cenozoic floras and vegetation of Greece. Review of Palaeobotany and Palynology 204: 56–117. doi:10.1016/j.revpalbo.2014.02.006.
- Walter H. 1973. Vegetation of the Earth in relation to the climate and the eco-physiological conditions. New York: Springer Verlag.
- Walther H, Zetter R. 1993. Zur Entwicklung der paläogenen Fagaceae Mitteleuropas. Palaeontographica B 230: 183–194.
- Wang CW. 1961. The forests of China. Cambridge: Harvard University.
- Wang F, Chien N, Zhang Y, Yang H. 1995. Pollen flora of China. Beijing: Science Press.
- Wang P, Pu F. 2004. Pollen morphology and biogeography of Fagaceae. Guangzhou: Guangdong Science and Technology Press.
- Wang Y, Jahren AH, Amundson R. 1997. Potential for 14C dating of biogenic carbonate in hackberry (Celtis) endocarps. Quaternary Research 47: 337–343. doi:10.1006/qres.1997.1894.
- Wang Y-F, Ferguson DK, Zetter R, Denk T, Garfi G. 2001. Leaf architecture and epidermal characters in Zelkova, Ulmaceae. Botanical Journal of the Linnean Society 136: 255–265. doi:10.1111/boj.2001.136.issue-3.
- Watari S. 1952. Dicotyledonous woods from the Miocene along the Japan-Sea side of Honsyu. Journal of the Faculty of Science, University of Tokyo, Section III, Botany 6: 97–134.
- Wei Z. 2003. Pollen of seed plants. Kunming, China: Yunnan Science and Technology Press.
- Wen J, Shi W. 2012. Revision of the Maddenia clade of Prunus (Rosaceae). PhytoKeys 11: 39–59. doi:10.3897/phytokeys.11.2825.
- Wheeler EA, Manchester SR. 2002. Woods of the Eocene Nut Beds Flora, Clarno Formation, Oregon, USA. International Association of Wood Anatomists Journal Supplement 3: 1–188.
- Wheeler EA, Scott RA, Barghoorn ES. 1978. Fossil dicotyledonous woods from Yellowstone National Park. II. Journal of the Arnold Arboretum 58: 280–306.
- Whitcher I, Wen J. 2001. Phylogeny and biogeography of Corylus (Betulaceae): Inferences from ITS sequences. Systematic Botany 26: 283–298.
- Whitehead DR. 1963. Pollen morphology in the Juglandaceae, I: Pollen size and pore number variation. Journal of the Arnold Arboretum 44: 101–110.
- Whitehead DR. 1965. Pollen morphology in the Juglandaceae, II: Survey of the family. Journal of the Arnold Arboretum 46: 369–410.
- Wilbur RL. 1994. The Myricaceae of the United States and Canada: Genera, subgenera, and series. Sida 16: 93–107.
- Wilde V, Manchester SR. 2003. Cedrelospermum-fruits (Ulmaceae) and related leaves from the middle Eocene of Messel (Hesse, Germany). Courier Forschungs-Institut Senckenberg 241: 147–157.
- Wodehouse RP. 1933. Tertiary Pollen II. The oil shales of the Eocene Green River Formation. Bulletin of the Torrey Botanical Club 60: 479–524. doi:10.2307/2480586.
- Wolfe JA. 1966. Tertiary plants from the Cook Inlet region, Alaska. United States Geological Survey Professional Paper 398 B: 1–32.
- Wolfe JA. 1968. Paleogene biostratigraphy of nonmarine rocks in King County, Washington. United States Geological Survey Professional Paper 571: 1–33.
- Wolfe JA, Wehr W. 1987. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. United State Geological Survey Bulletin 1597: 1–25.
- Wolff M. 1934. Mikrofossilien des pliozänen Humodils der älteren Schichten des Tertiärs sowie posttertiären Ablagerungen. Arbeiten aus dem Institut für Paläobotanik und Petrographie der Brennsteine 5: 55–95.
- Writing Group of Cenozoic Plants of China (WGCPC). 1978. Cenozoic plants of China, fossil plants of China. Volume Vol. 3. Beijing: Science Press.
- Wrońska-Pilarek D. 2011. Pollen morphology of Polish native species of the Rosa genus (Rosaceae) and its relation to systematics. Acta Societatis Botanicorum Poloniae 80: 221–232. doi:10.5586/asbp.2011.031.
- Wrońska-Pilarek D, Jagodziński AM. 2011. Systematic importance of pollen morphological features of selected species from the genus Rosa (Rosaceae). Plant Systematics and Evolution 295: 55–72.
- Wrońska-Pilarek D, Jagodziński AM, Maliński T. 2012. Morphological studies of pollen grains of the Polish endemic species of the genus Rubus (Rosaceae). Biologia 67: 87–96. doi:10.2478/s11756-011-0141-z.
- Xin Y, Zhang Y, Xi Y. 1993. Studies on the pollen morphology of the genus Ulmus L. in China and its taxonomic significance. Acta Botanica Sinica 35: 91–95.
- Yabe A. 2008. Early Miocene terrestrial climate inferred from plant megafossil assemblages of the Joban and Somaareas, Northeast Honshu, Japan. Bulletin of the Geological Survey of Japan 59: 397–413. doi:10.9795/bullgsj.59.397.
- Yoo K-O, Wen J. 2002. Phylogeny and biogeography of Carpinus and subfamily Coryloideae (Betulaceae). International Journal of Plant Sciences 163: 641–650. doi:10.1086/ijps.2002.163.issue-4.
- Yoo K-O, Wen J. 2007. Phylogeny of Carpinus and subfamily Coryloideae (Betulaceae) based on chloroplast and nuclear ribosomal sequence data. Plant Systematics and Evolution 267: 25–35. doi:10.1007/s00606-007-0533-2.
- Yü TT, Lu LT, Ku TC, Li CL, Chen SX. 1986. Rosaceae (3), Amygdaloideae. Flora Republicae Popularis Sinicae 38. Bejing, China: Science Press.
- Zamani A, Attar F, Maroofi H. 2010. Pollen morphology of the genus Pyrus (Rosaceae) in Iran. Acta Biologica Szegediensis 54: 51–56.
- Zarafshar M, Akbarinia M, Sattarian A, Josephus L, Van der Maesen G. 2010. Pollen morphology of Iranian Celtis (Celtidaceae-Ulmaceae). Botanica Serbica 34: 145–149.
- Zastawniak E, Walther H. 1998. Betulaceae from Sośnica near Wrocław (Poland) – a revision of Goepperts original materials and a study of more recent collections. Acta Palaeobotanica 38: 87–145.
- Zavada M. 1983. Pollen morphology of Ulmaceae. Grana 22: 23–30. doi:10.1080/00173138309429910.
- Zavada MS, Dilcher DL. 1986. Comparative pollen morphology and its relationship to phylogeny of pollen in the Hamamelidae. Annals of the Missouri Botanical Garden 73: 348–381. doi:10.2307/2399117.
- Zetter R. 1984. Morphologische Untersuchungen an Fagus-Blättern aus dem Neogen von Österreich. Beiträge zur Paläontologie von Österreich 11: 207–288.
- Zetter R. 1988. Bemerkungen zur Microflora der Kohleschichten im Bereich der süd-burgenländischen Schwelle. Biologisches Forschungsinstitut für Burgenland. Bericht 68: 159–166.
- Zetter R, Farabee MJ, Pigg KB, Manchester SR, DeVore ML, Nowak MD. 2011. Palynoflora of the late Paleocene silicified shale at Almont, North Dakota, USA. Palynology 35: 179–211. doi:10.1080/01916122.2010.501164.
- Zhang Y, Chen Y. 1986. Studies on the pollen morphology of tribe Zizipheae of Rhamnaceae in China. Acta Phytotaxonomica Sinica 24: 177–185.
- Zhang Y, Chen Y. 1992. A study on pollen morphology of tribe Rhamneae (Rhamnaceae) in Cina. Acta Phytotaxonomica Sinica 30: 73–81.
- Zhang J-B, Li R-Q, Manchester SR, Lin L, Wang W, Wen J, Chen Z-D. 2013. Integrated fossil and molecular data reveal the biogeographic diversification of the eastern Asian–eastern North American disjunct hickory genus (Carya Nutt.). PLoS ONE 8: e70449. doi:10.1371/journal.pone.0070449.
- Zhichen S, Weiming W, Fei H. 2004. Fossil pollen records of extant angiosperms in China. Botanical Review 70: 425–458. doi:10.1663/0006-8101(2004)070[0425:FPROEA]2.0.CO;2.
- Zhou G, Li X. 1994. Species composition and structure of Chinese beech forests. Natural History Research 3: 21–26.