Abstract
The middle Eocene Messel and Eckfeld localities are renowned for their excellently preserved faunas and diverse floras. Here we describe for the first time pollen from insect-pollinated plants found in situ on well-preserved ancient bees using light and scanning electron microscopy. There have been 140 pollen types reported from Messel and 162 pollen types from Eckfeld. Here we document 23 pollen types, six from Messel and 18 from Eckfeld (one is shared). The taxa reported here are all pollinated by insects and mostly not recovered in the previously studied dispersed fossil pollen records. Typically, a single or two pollen types are found on each fossil bee specimen, the maximum number of distinct pollen types on a single individual is five. Only five of the 23 pollen types obtained are angiosperms of unknown affinity, the remainder cover a broad taxonomic range of angiosperm trees and include members of several major clades: monocots (1 pollen type), fabids (7), malvids (4), asterids (5) and other core eudicots (1). Seven types each can be assigned to individual genera or infrafamilial clades. Since bees visit only flowers in the relative vicinity of their habitat, the recovered pollen provides a unique insight into the autochthonous palaeo-flora. The coexistence of taxa such as Decodon, Elaeocarpus, Mortoniodendron and other Tilioideae, Mastixoideae, Olax, Pouteria and Nyssa confirms current views that diverse, thermophilic forests thrived at the Messel and Eckfeld localities, probably under a warm subtropical, fully humid climate. Our study calls for increased attention to pollen found in situ on pollen-harvesting insects such as bees, which can provide new insights on insect-pollinated plants and complement even detailed palaeo-palynological knowledge obtained mostly from pollen of wind-pollinated plants in the dispersed pollen record of sediments. In the case of Elaeocarpus, Mortoniodendron, Olax and Pouteria the pollen collected by the middle Eocene bees represent the earliest unambiguous records of their respective genera.
The Messel Pit (southern Hesse, near Darmstadt) and Eckfeld Maar (Eifel, Rhineland-Palatia) of central and western Germany () are among the most species-rich middle Eocene sites in Europe. In addition to their well-known fauna (e.g. Schaal & Ziegler Citation1992; Lutz Citation1993; Lutz et al. Citation2000; Wappler & Engel Citation2003) and macro-flora and meso-flora (e.g. Wilde Citation1989; Wilde & Frankenhäuser Citation1998; Collinson et al. Citation2012), they harbour a highly diverse micro-flora with over 200 distinguished taxa of pollen and spores (Thiele-Pfeiffer Citation1988; Nickel Citation1994, Citation1996). In particular, the angiosperm pollen record documents diverse vegetation that was present in the middle Eocene of central Europe: A total of 114 pollen types with diagnostic features encompassing 38 families of angiosperms from 25 orders (Supplemental data File S1; Thiele-Pfeiffer Citation1988; Nickel Citation1996). Among many other insects, Messel and Eckfeld have yielded well-preserved fossils of bees, mostly of the extinct tribe Electrapini (Lutz Citation1993; Engel Citation2001a; Wappler & Engel Citation2003), although evidence for Megachilinae and a possible eucerine are also known (Wappler & Engel Citation2003; Wedmann et al. Citation2009). The Electrapini, are one of several highly eusocial corbiculate tribes among the Apinae, and one of several groups that form a grade leading to the more familiar tribes of honey bees and stingless bees (Lutz Citation1993; Schultz et al. Citation2001; Engel Citation2001a, Citation2001b; Wappler & Engel Citation2003). Given the presence of corbiculae (also known as metatibial ‘pollen baskets’, a specialised structure for the transport of moistened pollen back to the nest; Engel Citation2001a) and metatibial pollen presses among Electrapini (Engel Citation2001a), it is presumed that these taxa would have exhibited the same suite of behavioural repertoires necessary for collecting and manipulating pollen, and perhaps also similar harvesting preferences and strategies, as are known among their extant relatives. Fortunately, many of the bees from Messel and Eckfeld preserve on their bodies and particularly in their corbiculae the pollen they had collected and come into contact with during foraging bouts, thereby providing a means of inferring this aspect of their palaeo-biology. Naturally, before any such exploration of ancient bee pollen-collecting behaviour can be attempted, it is necessary to systematically survey the pollen of individual fossil bee specimens. Such an inventory provides an indication of the plants visited (either for nectar, pollen or oils) by the bee prior to death, and particularly those from which it actively collected pollen in its corbicula for transport to the nest and use as brood provisions. To date, there have been few studies aimed at retrieving and studying in situ pollen on fossils of pollen-harvesting/-feeding insects (Krassilov & Rasnitsyn Citation1982, Citation1996; Caldas et al. Citation1989; Ramírez et al. Citation2007). Here we document for the first time pollen found in situ on middle Eocene bees from the Messel and Eckfeld localities using a combination of light microscopy (LM) and scanning electron microscopy (SEM). The pollen grains recovered from these fossil insects are compared to those from earlier comprehensive LM studies of the sedimentary dispersed pollen record at Messel (Thiele-Pfeiffer Citation1988) and Eckfeld (Nickel Citation1996). Since insect-pollinated taxa commonly are not represented in the same way as wind-pollinated taxa in the dispersed pollen record, it can be assumed that pollen adhering to bees reflect a different spectrum of plants as pollen dispersed in the sediment. Therefore, this kind of study can provide an entirely new angle of looking into the Messel and Eckfeld ecosystems.
Material and methods
Fossil bees from two Eocene fossil Lagerstätten, the Grube Messel (‘Messel Pit’, maximum age 47.8± 0.2 Ma, deposition lasted for 1.6 Ma; Mertz & Renne Citation2005) and Eckfelder Maar (‘Eckfeld Maar’, 44.3 ± 0.4 Ma, 0.25 Ma of deposition; Mertz et al. Citation2000), were examined using a Leica MZ9.5 stereomicroscope equipped with standard incident ultraviolet (UV) illumination to detect pollen on the bees. Eleven bees, four from Messel and seven from Eckfeld, with pollen grains adhered to their bodies and representing species from two genera of Electrapini (Apidae: Apinae), Electrapis and Protobombus (Lutz Citation1993; Engel Citation2001a; Wappler & Engel Citation2003), were selected for study. For UV illumination, we used a 100 W high-pressure mercury burner in combination with a filter block consisting of an excitation filter (UV+ green: 425/60 nm), a dichromatic mirror (beam splitter, 470 nm) and a barrier (emission) filter (475 nm), providing a bright greenish fluorescence for the fluorescent material. The fossil pollen grains were extracted from the bee specimens with the help of wax sticky pads mounted at the end of a preparation needle. The wax was remobilised in 60 °C hot water and the pollen grains/clusters were fished out using a micromanipulator and placed into drops of glycerine. Using a micromanipulator, the grains and/or pollen clusters then were placed into drops of acetolysis liquid (9:1 mix of 99% acetic anhydrite and 95–97% sulphuric acid) on new glass slides. These slides were heated over a candle flame for a short time to dissolve extra organic material and to stain the pollen grains, which was afterwards transferred in fresh drops of glycerine to new glass slides. The pollen grains were first photographed under LM and transferred to SEM stubs by use of a micromanipulator and washed with drops of absolute ethanol to remove any remaining glycerine. The stubs were sputter coated with gold and the pollen grains were photographed under a JEOL 6400 SEM. Heteropolar pollen grains were turned/rotated on the stub surfaces, recoated with gold, and again photographed under the scanning electron microscope. The bee fossils were photographed with a Leica MZ16 Stereomicroscope, using either a JVC (model KY-F70B) or a Nikon Coolpix 4500 digital camera.
Systematic palaeontology
All pollen descriptions presented here include the most diagnostic features observed both in LM and SEM. The terminology follows mostly Punt et al. (Citation2007) and Hesse et al. (Citation2009). The classification (including informal clades) and author names of extant orders and families follow APG III (Citation2009). Taxa incertae sedis are listed at the end of each larger taxonomic group. Pollen grains from the same bee or body part are figured together and not according to the systematic affiliation, hence, the irregular figure numbers in the systematic section. In total, we found 23 pollen types, representing 12 angiosperm families from 11 orders and including five eudicot pollen types of unknown affinity (). Supplemental data File S2 includes a comprehensive list of the pollen obtained from the bee specimens and provides a summary of the taxonomic affinities (also provided in reduced form in ).
Table I. List of pollen taxa documented in this study, their systematic placement, and from which bee specimen they were obtained.
Clade Monocots
Order Asparagales Link
Family Iridaceae Juss.
Subfamily Iridoideae Eaton
Iridoideae gen. et sp. indet.
(, , , ; , , )
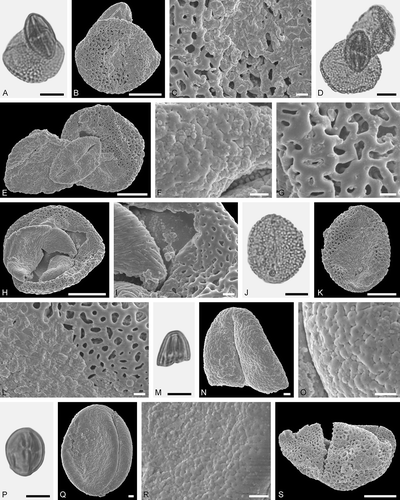
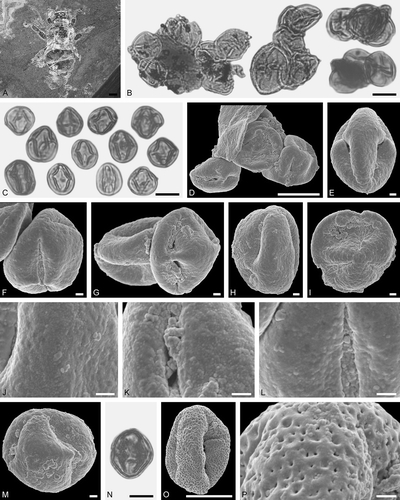
Figure 2. Electrapis sp. from Messel and associated pollen grains. A. Female (worker caste) FIS MeI 3300. B, C. LM micrographs. D‒N. SEM micrographs. B. Clumps of Nyssa sp. pollen grains. C. Nyssa sp. grains in equatorial view (left) and polar view (right). D, E. Clumps of Nyssa sp. pollen grains. F‒J. Nyssa sp. grains in equatorial view. K‒N. Nyssa sp., details of tectum surface. Scale bars – 2 mm (A), 10 µm (B‒J), 1 µm (K‒N).
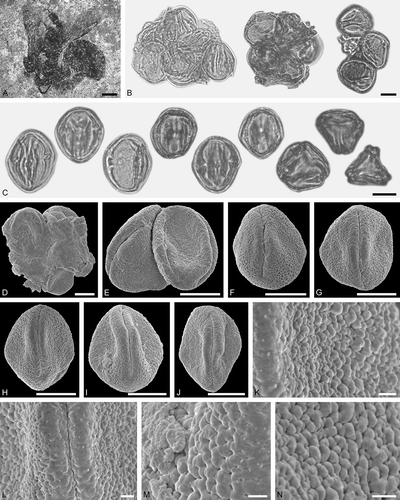
Figure 3. Protobombus messelensis Engel et Wappler from Messel and associated pollen grains. A. FIS MeI 6388, holotype, female. B‒C, K. LM micrographs. D‒J. SEM micrographs. B. Clump with Aralioideae gen. et sp. indet. 1 and Tilioideae gen. et sp. indet. pollen grains (left), Aralioideae gen. et sp. indet. 1 pollen in equatorial and polar view (right). C. Aralioideae gen. et sp. indet. 1 grains in equatorial view (left) and polar view (right). D, F, G. Aralioideae gen. et sp. indet. 1 grains in equatorial view. E. Aralioideae gen. et sp. indet. 1 grain in polar view. H‒J. Aralioideae gen. et sp. indet. 1, details of tectum surface. K. Tilioideae gen. et sp. indet. pollen grains. Scale bars – 1 mm (A), 10 µm (B‒C, E‒G, K), 1 µm (D, H‒J).
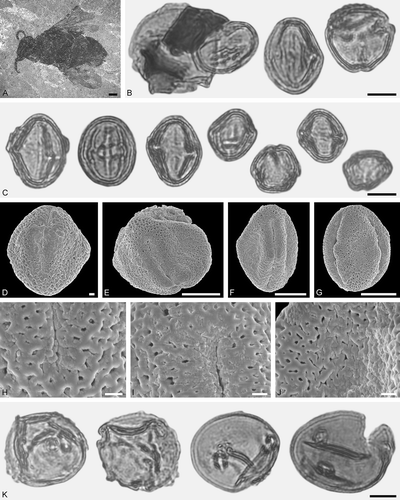
Figure 4. Associated pollen grains of Protobombus messelensis Engel et Wappler (FIS MeI 6388) from Messel. A. LM micrograph. B‒O. SEM micrographs. A. Tilioideae gen. et sp. indet. pollen in polar view. B‒D, F, H, J, L, N. Tilioideae gen. et sp. indet. pollen in polar view. E, G, I, K, M, O. Tilioideae gen. et sp. indet., details of tectum surface. H‒K. Same pollen grain rotated. H‒I. Proximal side. J‒K. Distal side. L‒O. Same pollen grain rotated. L‒M. Proximal side. N, O. Distal side. Scale bars – 10 µm (A‒D, F, H, J, L, N), 1 µm (E, G, I, K, M, O).
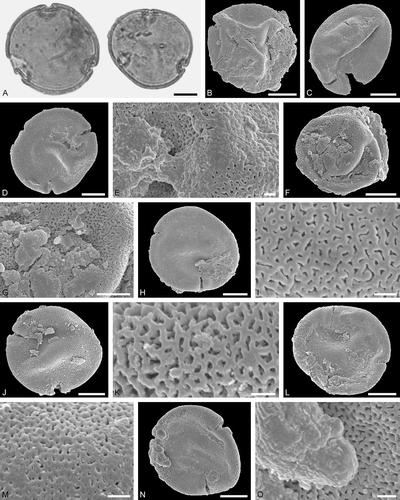
Figure 5. Electrapis sp. from Messel and associated pollen grains. A. Female (worker caste) FIS MeI 10890. B, K‒L. LM micrographs. C‒J, M‒Q. SEM micrographs. B. Clump with Castanopsis/Lithocarpus sp. pollen grains (left), grains in equatorial view (right). C‒G. Castanopsis/Lithocarpus sp. grains in equatorial view H‒J. Castanopsis/Lithocarpus sp., details of tectum surface. K. Clump with Mortoniodendron sp. pollen grains. L. Mortoniodendron sp. pollen in polar view. M, N. Mortoniodendron sp., same grain rotated. O‒Q. Mortoniodendron sp., details of tectum surface. Scale bars – 1 mm (A), 10 µm (B, K‒N), 1 µm (C‒J, O‒Q).
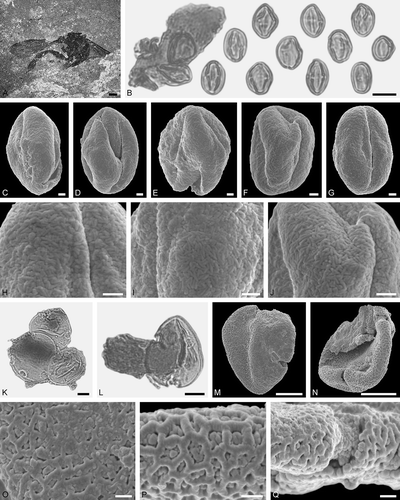
Figure 6. Electrapis sp. from Messel and Electrapis electrapoides Lutz from Eckfeld and associated pollen grains. A. Female (worker caste) FIS MeI 12151. B, C, H–J. LM micrographs. D–F, K–O. SEM micrographs. B. Clump with Aralioideae gen. et sp. indet. 2 pollen grains. C. Aralioideae gen. et sp. indet. 2 pollen in equatorial view. D. Aralioideae gen. et sp. indet. 2 pollen in equatorial view. E. Aralioideae gen. et sp. indet. 2, detail of tectum surface. F. Aralioideae gen. et sp. indet. 2, wall structure. G. Female (worker caste) PE 1997/20.LS. H, I. Large clump and smaller clumps with Olax sp. pollen grains. J. Olax sp. pollen in polar view (upper/lower) and equatorial view (middle). K‒M. Olax sp. pollen in polar view. N, O. Olax sp., detail of tectum surface. Scale bars – 1 mm (A, G), 10 µm (B‒D, H‒J), 1 µm (E, F, K‒O).
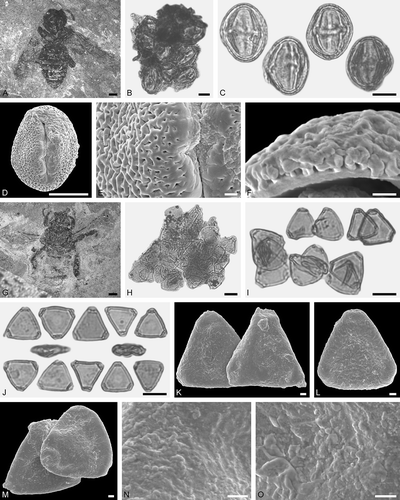
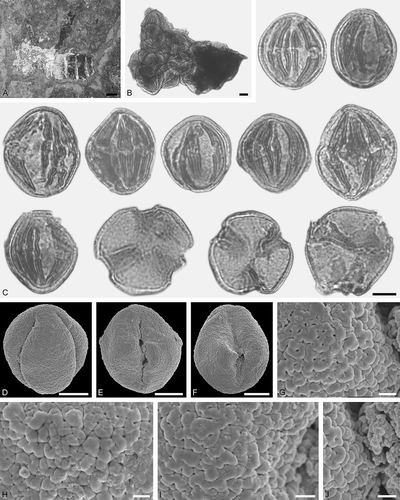
Figure 7. Electrapis sp. from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2000/846a,b.LS. B, C, I, O. LM micrographs. D, H, J‒N, P‒R. SEM micrographs. B. Clumps dominated by Elaeocarpus sp. 1 pollen, also with Eudicot ord., fam., gen. et sp. indet. 1, Euphorbiaceae gen. et sp. indet. 1, and Castanopsis/Lithocarpus sp. pollen grains. C. Elaeocarpus sp. 1 pollen grains in equatorial and polar view. D. Clump with Elaeocarpus sp. 1 pollen grains. E‒G. Elaeocarpus sp. 1 pollen in equatorial view. H. Elaeocarpus sp. 1, detail of tectum surface. I‒L. Eudicot ord., fam. gen. et sp. indet. 1 pollen grains (large) and Castanopsis/Lithocarpus sp. (small, attached). M. Eudicot ord., fam., gen. et sp. indet. 1, detail of tectum surface. N. Castanopsis/Lithocarpus sp., detail of tectum surface. O, P. Euphorbiaceae gen. et sp. indet. 1 in equatorial view. Q, R. Euphorbiaceae gen. et sp. indet. 1, detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B, C, I, O), 1 µm (D‒H, J‒N, P‒R).
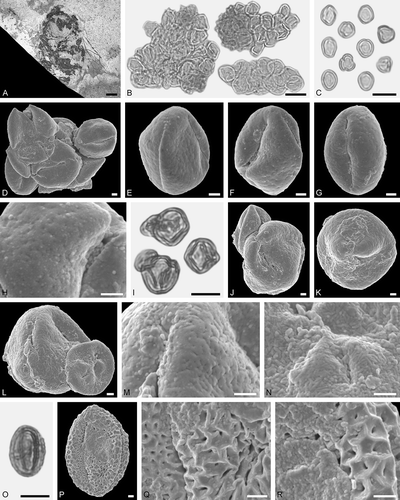
Figure 8. Electrapis prolata Wappler et Engel from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2000/847a,b.LS. B‒D, K. LM micrographs. E‒J, L‒Q. SEM micrographs. B‒D. Clumps dominated by Iridoideae gen. et sp. indet. and Eudicot ord., fam., gen. et sp. indet. 2 pollen grains, also with pollen of Eudicot ord., fam., gen. et sp. indet. 3, and rare Anacardiaceae gen. et sp. indet. pollen grains. D‒J. Same clump rotated. G. Eudicot ord., fam., gen. et sp. indet. 2 pollen in equatorial view. H. Iridoideae gen. et sp. indet., detail of tectum surface. I. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. J. Eudicot ord., fam., gen. et sp. indet. 3, detail of tectum surface. K‒Q. Same clump rotated. N. Iridoideae gen. et sp. indet., detail of tectum surface. O, P. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. Q. Iridoideae gen. et sp. indet., detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B‒F, K‒M), 1 µm (G‒J, N‒Q).
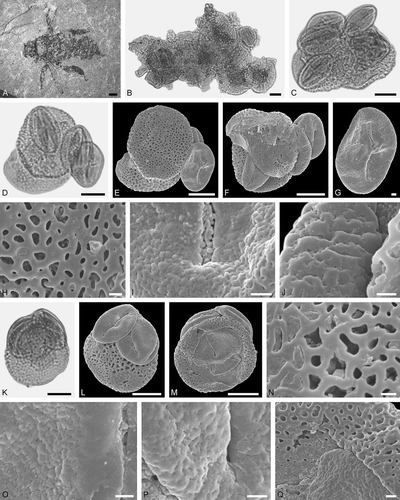
Figure 9. Pollen grains on Electrapis prolata Wappler et Engel (PE 2000/847a,b.LS) from Eckfeld. A, D, J, M, P. LM micrographs. B, C, E‒I, K, L, N, O, Q‒S. SEM micrographs. A‒C. Clump with Iridoideae gen. et sp. indet. and Eudicot ord., fam., gen. et sp. indet. 3 pollen grains. C. Iridaceae gen. et sp. indet., detail of tectum surface. D‒G. Clump with Iridaceae gen. et sp. indet. and Eudicot ord., fam., gen. et sp. indet. 3 pollen grains. F. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. G. Iridoideae, detail of tectum surface. H, I. Clump with Iridoideae gen. et sp. indet. and Anacardiaceae gen. et sp. indet. pollen grains. I. Iridoideae gen. et sp. indet. (right) and Anacardiaceae gen. et sp. indet. (left), detail of tectum surface. J‒L. Iridoideae gen. et sp. indet. L. Iridoideae gen. et sp. indet., detail of tectum surface. M‒O. Eudicot ord., fam., gen. et sp. indet. 2. O. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. P‒R. Eudicot ord., fam., gen. et sp. indet. 2. R. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. S. Iridoideae gen. et sp. indet. Scale bars – 10 µm (A, B, D, E, H, J, K, M, P, S), 1 µm (C, F, G, I, L, N, O, Q, R).
Description
Pollen, oblate, elliptic in polar and equatorial view; equatorial diameter 28‒34 µm in LM, 24‒30 µm in SEM; sulcate, sulcus long, extending between apices; exine 1.1‒1.8 µm thick (LM), nexine thinner than sexine; semitectate; sculpturing reticulate in LM, and SEM, columellae are narrow and high, muri pluricolumellate, muri varying in width, lumina varying in size and form, lumina decrease in size towards apices and towards sulcus, muri fused around sulcus (SEM).
Remarks
The LM and SEM pollen morphology of extant Iridoideae has been studied by Goldblatt and Le Thomas (Citation1992). The thin columellae and smooth and flattened muri of the fossil pollen grains suggest affiliation to Iridoideae.
Locality
Eckfeld.
Previous records
This type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld. This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen (and species)
PE 2000/847.LS (Electrapis prolata Wappler et Engel); found on leg.
Occurrence
In large groups along with Eudicot ord., fam., gen. et sp. indet. 2, Eudicot ord., fam., gen. et sp. indet. 3 and Anacardiaceae gen. et sp. indet. pollen types.
Life form and pollination
Herbaceous perennial, evergreen or deciduous; entomophilous (can also be zoophilous).
Clade Fabids
Order Oxalidales Bercht. et J.Presl.
Family Elaeocarpaceae Juss. ex DC.
Genus Elaeocarpus L.
Elaeocarpus sp. 1
()
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 8‒10 µm long in LM, 8‒9 µm long in SEM, equatorial diameter 6‒7 µm in LM, 6‒7 µm in SEM; tricolporate; exine 0.7‒0.8 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, psilate, perforate, granulate in SEM, colpus membrane microverrucate, sexine protruding in area of endoaperture forming a bridge (SEM).
Remarks
The pollen morphology of extant Elaeocarpaceae has been studied using LM and SEM by Tang and Wu (Citation1990). The small size vs. the wall thickness of these tricolporate grains in combination with the endoapertures forming a bridge and the psilate, perforate to granulate sculpturing clearly places these pollen grains in Elaeocarpus.
Locality
Eckfeld.
Previous records
This pollen type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld. This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2000/846a,b.LS (Electrapis sp.); found on thorax and leg.
Occurrence
In large pure groups.
Life form and pollination
Evergreen tree or shrub; entomophilous.
Elaeocarpus sp. 2
()
Figure 10. Electrapis sp. from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2000/849a,b.LS. B‒D, O. LM micrographs. E‒N, P, Q. SEM micrographs. B, C. Clumps dominated by Eudicot ord., fam., gen. et sp. indet. 2 pollen grains, also rare Decodon sp. pollen. E, F, J. Clumps with Eudicot ord., fam., gen. et sp. indet. 2 pollen grains. G‒I. Eudicot ord., fam., gen. et sp. indet. 2 pollen grains in equatorial view. K‒N. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. O‒Q. Decodon sp. pollen. Q. Decodon sp., detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B‒D, O), 1 µm (F‒N, P, Q).
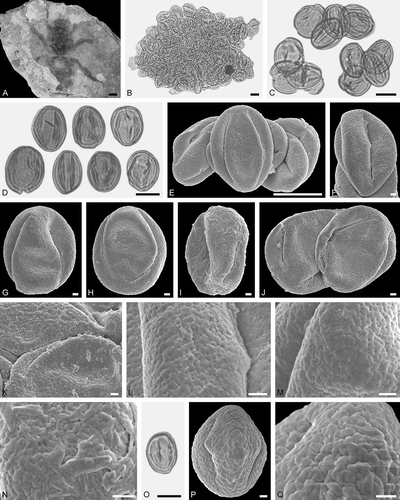
Figure 11. Pollen grains on Electrapis sp. (PE 2000/849a,b.LS) from Eckfeld. A‒C. LM micrographs. D‒O. SEM micrographs. A, B. Clumps with Eudicot ord., fam., gen. et sp. indet. 2. C. Eudicot ord., fam., gen. et sp. indet. 2 pollen grains in equatorial view (left) and polar view (right). D‒G. Clumps with Eudicot ord., fam., gen. et sp. indet. 2 pollen grains. H‒J. Eudicot ord., fam., gen. et sp. indet. 2 pollen grains in equatorial view. K. Eudicot ord., fam., gen. et sp. indet. 2 pollen grain in polar view. L‒O. Eudicot ord., fam., gen. et sp. indet. 2, detail of tectum surface. Scale bars – 10 µm (A‒E), 1 µm (F‒O).
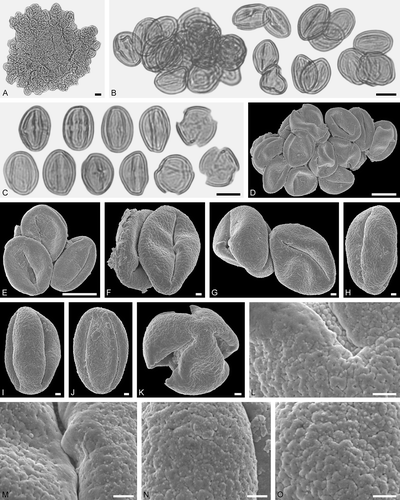
Figure 12. Electrapis micheneri Wappler et Engel from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2000/852a,b.LS. B. LM micrograph. C‒P. SEM micrographs. B. Groups with Eudicot ord., fam., gen. et sp. indet. 4 and Eudicot ord., fam., gen. et sp. indet. 5 pollen grains (left), and pollen of Eudicot ord., fam., gen. et sp. indet. 4 in equatorial and polar view (right). C‒E. Clumps with Eudicot ord., fam., gen. et sp. indet. 4 pollen grains. F‒H. Eudicot ord., fam., gen. et sp. indet. 4 pollen grains in equatorial view. I. Eudicot ord., fam., gen. et sp. indet. 4 pollen in polar view. J‒L. Eudicot ord., fam., gen. et sp. indet. 4, detail of tectum surface. M, N. Eudicot ord., fam., gen. et sp. indet. 5 pollen grains in equatorial view. O, P. Eudicot ord., fam., gen. et sp. indet. 5, detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B‒D), 1 µm (E‒P).
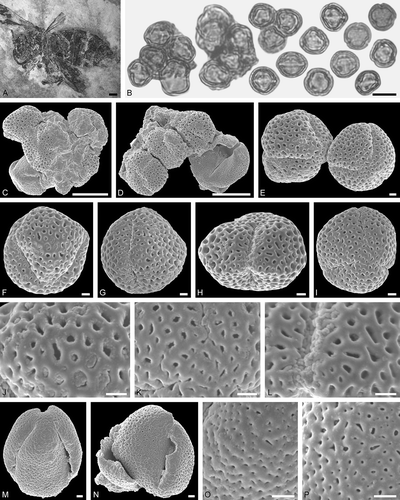
Figure 13. Pollen grains on Electrapis micheneri Wappler et Engel (PE 2000/852a,b.LS) from Eckfeld. A, D, L‒N. LM micrographs. B‒C, E‒K, O. SEM micrographs. A. Group with Pouteria sp. pollen grains and singe grains in equatorial view. B. Pouteria sp. pollen in equatorial view. C. Pouteria sp., detail of tectum surface. D. Pouteria sp. pollen grains in equatorial (left) and polar view (right). E‒H. Pouteria sp. pollen in equatorial view. I‒K. Pouteria sp., detail of tectum surface. L, M. Clumps with Euphorbiaceae gen. et sp. indet. 2 pollen grains. N. Euphorbiaceae gen. et sp. indet. 2 pollen grains in equatorial view. O. Euphorbiaceae gen. et sp. indet. 2 pollen in equatorial view. Scale bars – 10 µm (A, D, L‒N), 1 µm (B, C, E‒K, O).
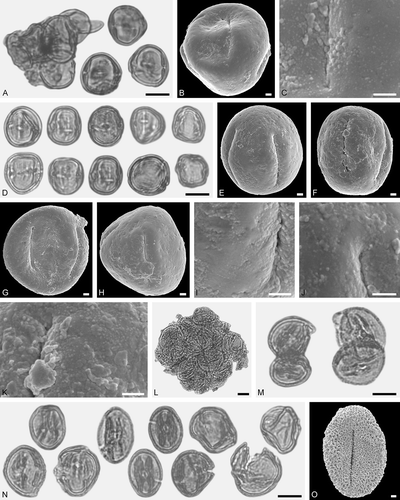
Figure 14. Pollen grains on Electrapis micheneri Wappler et Engel (PE 2000/852a,b.LS) from Eckfeld. A‒N, P, Q. SEM micrographs. O. LM micrograph. A, B. Clumps with Euphorbiaceae gen. et sp. indet. 2 pollen grains. C‒H. Euphorbiaceae gen. et sp. indet. 2 pollen grains in equatorial view. I‒N. Euphorbiaceae gen. et sp. indet. 2, detail of tectum surface. O, P. Engelhardioideae gen. et sp. indet. pollen grain in polar view. Q. Engelhardioideae gen. et sp. indet., detail of tectum surface. Scale bars – 10 µm (A, B, D, F, O), 1 µm (C, E, G, I‒N, P, Q).
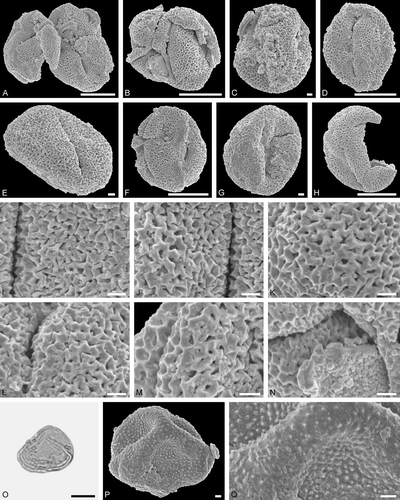
Figure 15. Protobombus pristinus Wappler et Engel from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2000/863a,b.LS. B, C, N. LM micrographs. D‒M, O, P. SEM micrographs. B. Clumps dominated by Elaeocarpus sp. 2 pollen grains, with rare Euphorbiaceae gen. et sp. indet. 3 pollen grains. C. Elaeocarpus sp. 2 pollen grains in equatorial view. D, F, G. Clumps of Elaeocarpus sp. 2 pollen grains. E, H, M. Elaeocarpus sp. 2 pollen in equatorial view. I. Elaeocarpus sp. 2 pollen in polar view. J‒L. Elaeocarpus sp. 2, detail of tectum surface. N, O. Euphorbiaceae gen. et sp. indet. 3 pollen in equatorial view. P. Euphorbiaceae gen. et sp. indet. 3, detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B-D, N-O), 1 µm (E‒M, P).
Description
Pollen, prolate to spheroidal, lobate in polar view, elliptic in equatorial view; polar axis 13‒16 µm long in LM, 12‒14 µm long in SEM, equatorial diameter 11‒14 µm in LM, 10‒12 µm in SEM; tricolporate, endoapertures short lalongate, sexine protruding over pori forming a bridge; exine 0.8‒1.1 µm thick in LM, nexine thinner than sexine (LM); tectate, sculpturing psilate in LM, slightly microverrucate, perforate, granulate in SEM, colpus membrane microechinate to microverrucate (SEM).
Locality
Eckfeld.
Previous records
This type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld.
Bee specimen and species
PE 2000/863a,b.LS (Electrapis sp.); found on thorax, abdomen.
Occurrence
In large pure groups, some groups with occasional grains of Euphorbiaceae gen. et sp. indet. 3 pollen type.
Life form and pollination
Evergreen tree or shrub; entomophilous.
Order Malpighiales Juss. ex Bercht. et J.Presl.
Family Euphorbiaceae Juss.
Euphorbiaceae gen. et sp. indet. 1
()
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 17‒19 µm long in LM, 18‒19 µm long in SEM, equatorial diameter 12‒13 µm in LM, 12‒13 µm in SEM; tricolporate, endoapertures lalongate rectangular, nexine thickened along endopori and colpi (LM); exine 1.2‒1.4 µm thick, nexine thinner than sexine (LM); semitectate, sculpturing reticulate in LM and SEM, lumina funnel shaped, lumina decrease close to colpus (SEM).
Remarks
The funnel shaped lumina of the reticulum is very typical for pollen of Euphorbiaceae. Several genera in the Euphorbiaceae share very similar pollen morphology. We therefore refrain from assigning this pollen type to a particular genus.
Locality
Eckfeld.
Previous records
This pollen type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld. This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2000/846a,b.LS (Electrapis sp.); found on leg.
Occurrence
Single grains.
Life form and pollination
From herb to succulent to shrub or tree; entomophilous (can also be zoophilous).
Euphorbiaceae gen. et sp. indet. 2
(; )
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 20‒25 µm long in LM, 19‒23 µm long in SEM, equatorial diameter 15‒19 µm in LM, 13‒18 µm in SEM; tricolporate, endoapertures circular, nexine thickened around endopori forming a costa, nexine thickened along colpi (LM); exine 1.2‒1.4 µm thick in SEM, 1.5‒1.7 µm thick in LM, nexine thinner than sexine (LM); semitectate, sculpturing scabrate in LM, microreticulate in SEM, muri broad, muri decrease in size and become more numerous in the area around apertures (SEM).
Locality
Eckfeld.
Previous records
This type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld.
Bee specimen and species
PE 2000/852a,b.LS (Electrapis micheneri Wappler et Engel); found on leg.
Occurrence
In large pure groups.
Life form and pollination
From herb to succulent to shrub or tree; entomophilous (can also be zoophilous).
Euphorbiaceae gen. et sp. indet. 3
()
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 21‒23 µm long in LM, 19‒21 µm long in SEM, equatorial diameter 18‒20 µm in LM, 14‒17 µm in SEM; tricolporate, endoapertures lalongate with a costa (LM); exine 1.7‒1.9 µm thick in LM, nexine thinner than sexine (LM); semitectate, sculpturing scabrate in LM, microreticulate in SEM, lumen funnel-shaped (SEM).
Locality
Eckfeld.
Previous records
This type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld.
Bee specimen and species
PE 2000/863a,b.LS (Protobombus pristinus Wappler et Engel); found on body.
Occurrence
Rare grains in clumps dominated by the Elaeocarpus sp. 2 pollen type.
Life form and pollination
From herb to succulent to shrub or tree; entomophilous (can also be zoophilous).
Order Fagales Engl.
Family Fagaceae Dumort.
Subfamily Castaneoideae Orsted.
Genus Castanopsis (D.Don) Spach vel Lithocarpus Blume sp.
(; , , , )
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 11‒16 µm long in LM, 10‒13 µm long in SEM, equatorial diameter 9‒11 µm in LM, 7‒9 µm in SEM; tricolporate, endoapertures lalongate slit-like; exine 0.8‒1.0 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, microrugulate, perforate in SEM.
Remarks
The pollen morphology of extant and extinct Fagaceae has been described in detail (e.g. Praglowski Citation1982, Citation1984; Solomon Citation1983a, Citation1983b; Denk & Grimm Citation2009; Denk et al. Citation2012; Grímsson et al. Citation2015b). The similarities between pollen of Castanopsis and Lithocarpus (Praglowski Citation1984) prevents assigning this pollen type to one of the two genera.
Locality
Messel and Eckfeld.
Previous records
This type corresponds to Tricolporopollenites cingulum ssp. oviformis (R.Potonié) Thomson et Pflug as figured by Thiele-Pfeiffer (Citation1988, plate 11, figures 28‒34) from the dispersed pollen flora of Messel, and by Nickel (Citation1996, plate 9, figures 37‒38) from the dispersed pollen flora of Eckfeld. Wilde (Citation1989) and Collinson et al. (Citation2012) did not report any Fagaceae fruits, seeds or leaves from Messel. This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
FIS MeI 10890 (Electrapis sp.; Messel); PE 2000/846a,b.LS (Electrapis sp.; Eckfeld); found on thorax (Messel specimen), leg (Eckfeld specimen).
Occurrence
In large pure groups and as single grains. Also found in small groups along with Eudicot ord., fam., gen. et sp. indet. 1 pollen type.
Life form and pollination
Evergreen tree, entomophilous.
Family Juglandaceae DC. ex Perleb
Subfamily Engelhardioideae Iljinskaya
Engelhardioideae gen. et sp. indet.
()
Description
Pollen, oblate, convex triangular in polar view, elliptic in equatorial view; equatorial diameter 23‒25 µm in LM, 19‒22 µm in SEM; triporate; exine 0.9‒1.2 µm thick, nexine thinner than sexine, nexine linearly thickened from pori to polar areas forming endoplicae; tectate; sculpturing psilate to scabrate in LM, microechinate in SEM, microechini at regular intervals (SEM).
Remarks
The LM and SEM pollen morphology of extant Juglandaceae genera have been studied by Stone and Broome (Citation1975). Based on the morphology of the fossil pollen it represents an extinct taxon of the Engelhardioideae.
Locality
Eckfeld.
Previous records
This type may correspond to Plicatopollis hungaricus as figured by Nickel (Citation1996, plate 6, ‒). Wilde and Frankenhäuser (Citation1998) reported several different Juglandaceae macro-fossils from Eckfeld. These include engelhardioid compound leaves and leaflets, engelhardioid fruits (Palaeocarya sp.), Hooleya-like pterocaryoid fruits, and fragments of platycaryoid male inflorescences.
Bee specimen and species
PE 2000/852a,b.LS (Electrapis micheneri); found on leg.
Occurrence
Single grain.
Life form and pollination
Deciduous or evergreen tree; anemophilous or possibly entomophilous. Among extant Juglandaceae, insect pollination has only been documented for Platycarya (Endress Citation1986). Since most Juglandaceae are wind-pollinated it cannot be ruled out that this pollen grain is a contamination from the sediment.
Clade Malvids
Order Myrtales Juss. ex Bercht. et J.Presl
Family Lythraceae J.St.-Hil.
Genus Decodon J.F.Gmel.
Decodon sp.
()
Description
Pollen, prolate, elliptic in equatorial view, circular in polar view, meridional ridges running down mid-mesocolpial areas, polar axis 18‒19 µm long in LM, 14‒16 µm in SEM, equatorial diameter 13‒15 µm in LM, 11‒13 µm in SEM; tricolporate, exine 0.9‒1.1 µm thick, nexine thinner than sexine, sexine slightly thickened on meridional ridges and in polar regions; tectate, sculpturing psilate to scabrate in LM, microrugulate to rugulate, perforate, fossulate in SEM, sexine protruding forming a bridge in area of endopori (SEM).
Remarks
The fossil record of Decodon and the pollen morphology of the genus has been described in detail by Grímsson et al. (Citation2012). The meridional ridges observed on the fossil pollen clearly place it in genus Decodon.
Locality
Eckfeld.
Previous records
This pollen type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld. This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2000/849a,b.LS (Electrapis sp.); found on thorax, abdomen.
Occurrence
Rare grains in large clumps dominated by Eudicot ord., fam., gen. et sp. indet. 2 pollen type.
Life form and pollination
Perennial shrub; entomophilous.
Order Malvales Juss. ex Bercht. et J.Presl
Family Malvaceae Juss.
Subfamily Tilioideae
Genus Mortoniodendron Standley et Steyerm.
Mortoniodendron sp.
()
Description
Pollen, oblate, circular to convex-triangular in polar view, elliptic in equatorial view; equatorial diameter 37‒44 µm in LM, 32‒40 µm in SEM; brevitricolporate, planaperturate, exine 1.3‒1.6 µm thick, nexine thinner than sexine, nexine slightly thickened around endopori, thickenings semi-circular in outline; semitectate, sculpturing microreticulate in LM and SEM, lumina on distal polar area larger than those occurring on the proximal side, lumina with densely packed freestanding columellae, lumina slightly smaller close to colpi, colpus membrane microrugulate (SEM).
Remarks
Extant pollen grains of various Malvaceae genera including Mortoniodendron have been described using LM and SEM by Perveen et al. (Citation2004). The most characteristic feature of these fossil pollen grains are the lumina showing densely packed freestanding columellae that are not observed in this form in Tilia or Craigia.
Locality
Messel.
Previous records
This pollen type is not present in the dispersed pollen flora described by Thiele-Pfeiffer (Citation1988). Wilde (Citation1989) and Collinson et al. (Citation2012) did not report any Malvaceae fruits, seeds or leaves from Messel.
Bee specimen and species
FIS MeI 10890; FIS MeI 12151 (Electrapis sp.); found on leg (FIS MeI 10890), head (FIS MeI 12151).
Occurrence
In pure clumps with few grains.
Life form and pollination
Shrub or tree, evergreen; entomophilous.
Tilioideae gen. et sp. indet.
(; )
Description
Pollen, oblate, circular in polar view, elliptic in equatorial view; equatorial diameter 37‒41 µm in LM, 34‒39 µm in SEM; brevitricolporate, planaperturate, endoaperture 6‒7 µm wide, colpi 9‒10 µm long (LM); exine 1.4‒1.7 µm thick, nexine thinner than sexine, nexine thickened around endopori, thickenings semi-circular in outline; semitectate, sculpturing microreticulate in LM and SEM, lumina on distal polar area larger than those occurring on the proximal side, muri are fused around apertures (SEM).
Remarks
Even though we are unable to affiliate this fossil pollen type to a particular extant genus it does resembles in situ grains of the fossil species Cragia bronnii (Unger) Z.Kvaček, Bůžek et Manchester (Kvaček et al. Citation2002), as well as extant pollen of Craigia and Tilia (cf. Perveen et al. Citation2004).
Locality
Messel.
Previous records
Reported as Intratriporopollenites cf. I. maxoides Krutzsch in Thiele-Pfeiffer (Citation1988, plate 8, figures 35–36).
Bee specimen and species
FIS MeI 6388 (Protobombus messelensis Engel et Wappler); found on leg, thorax, abdomen.
Occurrence
As single grains and in large groups along with Aralioideae gen. et sp. indet. pollen type.
Life form and pollination
Small to large tree, possibly deciduous; entomophilous (possibly also anemophilous).
Order Sapindales Juss. ex Bercht. et J.Presl
Family Anacardiaceae R.Br.
Anacardiaceae gen. et sp. indet.
()
Description
Pollen, spheroidal to prolate, circular to lobate in polar view, elliptic in equatorial view, tricolporate; exine 0.9‒1.0 µm thick, nexine thinner than sexine (LM); tectate, sculpturing striate in SEM, numerous perforations in grooves between striae, perforations in groups and/or rows, distal ends of colpi truncated to round, colpus membrane microverrucate to microechinate (SEM).
Remarks
The typical striate sculpturing including the grouped perforations and the way the colpi terminate suggest that this pollen type belongs in Anacardiaceae. This pollen is also superficially similar to pollen of Landeenia, an extinct Sapindales from the Eocene of Wyoming, USA (Manchester & Hermsen Citation2000). The Landeenia pollen is tricolpate, has much longer striae, and is considerable larger than the Anacardiaceae type pollen, and is therefore not comparable.
Locality
Eckfeld.
Previous records
This pollen type might be the same as reported by Nickel (Citation1996, plate 9, figures 31–32) as Aceripollenites striatus (Pflug) Thiele-Pfeiffer from the dispersed pollen flora of Eckfeld. Wilde and Frankenhäuser (Citation1998) reported a possible Anacardiaceae fruit, cf. Pentoperculum, from the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2000/847.LS (Electrapis prolata); found on leg.
Occurrence
In large groups along with Iridaceae gen. et sp. indet., Eudicot ord., fam., gen. et sp. indet. 2 and Eudicot ord., fam., gen. et sp. indet. 3 pollen types.
Life form and pollination
Leaves evergreen or deciduous; trees or shrubs; entomophilous.
Order Santalales R.Br. ex Bercht. et J.Presl
Family Olacaceae R.Br.
Genus Olax L.
Olax sp.
()
Description
Pollen, oblate, triangular in polar view, elliptic in equatorial view; polar axis 6‒7 µm long in LM, equatorial diameter 12‒15 µm in LM, 11‒13 µm wide in SEM; triporate, sexine protruding over pori forming an atrium; exine 0.8‒1.1 µm thick (LM), nexine thinner than sexine; tectate; sculpturing psilate in LM, psilate, irregularly granulate, slightly microrugulate in SEM.
Remarks
The pollen morphology of Olacaceae has been studied by Maguire et al. (Citation1974) from specimens in North America, and by the Institute of Botany and South China Institute of Botany (Citation1982), from south-eastern Asian material. The generally small, extremely oblate (almost flat), triangular and triporate pollen grains are characteristic for the genus Olax. The inconspicuous sculpturing seen under SEM on these small type of pollen grains is also typical for this genus.
Locality
Eckfeld.
Previous records
This type corresponds to Olaxipollis matthesi ssp. minor Krutzsch as figured by Nickel (Citation1996, plate 7, figure 34). This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
PE 1997/20.LS (Electrapis electrapoides Lutz); found on thorax.
Occurrence
In large pure clumps.
Life form and pollination
Evergreen shrub or small tree; entomophilous.
Clade Asterids
Order Cornales Link.
Family Cornaceae Bercht. et J.Presl
Subfamily Mastixioideae Harms
Mastixioideae gen. et sp. indet.
()
Figure 16. Protobombus sp. from Eckfeld and associated pollen grains. A. Female (worker caste) PE 2014/1a,b.LS. B, C. LM micrographs. D‒J. SEM micrographs. B. Clump with Mastixioideae gen. et sp. indet. pollen grains. C. Mastixioideae gen. et sp. indet. pollen grains in equatorial view (upper left) and polar view (lower right). D‒F. Mastixioideae gen. et sp. indet. pollen grains in equatorial view. G‒J. Mastixioideae gen. et sp. indet., detail of tectum surface. Scale bars – 1 mm (A), 10 µm (B‒F), 1 µm (G‒J).
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 34‒42 µm long in LM, 31‒37 µm long in SEM, equatorial diameter 29‒38 µm in LM, 28‒33 µm in SEM; tricolporate, nexine markedly thickened along colpi, sexine sometimes protruding over pori forming a bridge; exine 2.0‒2.2 µm thick in LM, nexine thinner than sexine (LM); tectate, sculpturing scabrate in LM, verrucate, rugulate, perforate, fossulate in SEM, verrucae often with central positioned perforation, colpus membrane verrucate to microverrucate, rugulate (SEM).
Remarks
The pollen morphology of Cornaceae has been studied using LM and SEM by Ferguson (Citation1997). The fossil pollen clearly belongs in the subfamily Mastixioideae in which there are two modern genera and several extinct ones known from fruits (Mai Citation1995). We are unable to assign this pollen type to a particular extant genus, but the morphology of the fossil pollen suggests it is close to Mastixia.
Locality
Eckfeld.
Previous records
This type was not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld. Wilde and Frankenhäuser (Citation1998) reported Mastixiaceae fruits from the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2014/1a,b.LS (Protobombus sp.); found on thorax, abdomen.
Occurrence
In large pure clumps.
Life form and pollination
Evergreen tree; entomophilous.
Subfamily Nyssoideae Arn.
Genus Nyssa L.
Nyssa sp.
()
Description
Pollen, prolate, triangular in polar view, elliptic in equatorial view; polar axis 25‒27 µm long in LM, 22‒24 µm long in SEM, equatorial diameter 21‒23 µm in LM, 19‒20 µm in SEM; tricolporate, nexine thickened along colpi and endopori (costa; LM); exine 1.8‒2.2 µm thick, nexine slightly thinner or as thick as sexine (LM); tectate, sculpturing scabrate in LM, microverrucate, microrugulate, fossulate, perforate in SEM, sculpture elements are more dense in area of mesocolpium, sculpture elements more fused along colpi forming a margo (SEM).
Remarks
Pollen of several extant species of Nyssa has been described using LM and SEM by Göschl (Citation2008). The fossil pollen grains described here clearly fall within the morphological range of extant Nyssa pollen grains. The arrangement and form of the apertures as well as the sculpturing type seen under SEM undoubtedly associates these pollen grains with Nyssa.
Locality
Messel.
Previous records
It is possible that this type corresponds to Nyssapollenites kruschi ssp. analepticus in Thiele-Pfeiffer (Citation1988, plate 15, figures 28–29). Collinson et al. (Citation2012) described Nyssa disseminata (R.Ludw.) Kirchheimer endocarps from Messel. Wilde (Citation1989) also reported Nyssa sp. leaves from this locality.
Bee specimen and species
FIS MeI 3300 (Electrapis sp.); found on leg.
Occurrence
Large clumps with numerous pollen grains. All grains in clumps of same type.
Life form and pollination
Small to large evergreen tree (possibly deciduous); entomophilous (perhaps also anemophilous).
Order Ericales Bercht. et J.Presl
Family Sapotaceae Juss.
Genus Pouteria Aubl.
Pouteria sp.
()
Description
Pollen, prolate, circular in polar view, elliptic in equatorial view; polar axis 18‒19 µm long in LM, 17‒18 µm long in SEM, equatorial diameter 15‒18 µm in LM, 13‒17 µm in SEM; stephano(4,5)colporate, endoapertures lalongate elliptic, endoapertures with costa, colpi often short, narrow with broad round to truncated distal ends, nexine in area of colpi thickened; exine 1.2‒1.4 µm thick, nexine thinner than sexine (LM); tectate, sculpturing psilate in LM, psilate, perforate in SEM, with irregularly distributed sporopollenin granules (SEM).
Remarks
Pollen of the Sapotaceae (including genus Pouteria) have been described in detail using LM and SEM by Harley (Citation1991). The shape and outline of the fossil pollen grains in combination with the number, arrangement and form of the apertures clearly associate these pollen grains with Sapotaceae. The size and sculpturing seen under SEM further suggests that they belong to genus Pouteria (cf. Harley Citation1991).
Locality
Eckfeld.
Previous records
This type corresponds to Tetracolporopollenites sp. 3 figured by Nickel (Citation1996, plate 13, figures 34–36). This family was not reported by Wilde and Frankenhäuser (Citation1998) in the macro-fossil record of Eckfeld.
Bee specimen and species
PE 2000/852a,b.LS (Electrapis micheneri); found on head, leg.
Occurrence
Large pure clumps.
Life form and pollination
Small to large evergreen tree; entomophilous.
Clade Campanulids
Order Apiales Nakai
Family Araliaceae Juss.
Subfamily Aralioideae Eaton
Aralioideae gen. et sp. indet. 1
()
Description
Pollen, prolate, convex-triangular in polar view, elliptic in equatorial view; polar axis 19‒27 µm long in LM, 15‒27 µm long in SEM, equatorial diameter 17‒21 µm in LM, 17‒21 µm in SEM; tricolporate, endopori lalongate rectangular, nexine thickened along polar sides of endoapertures, nexine slightly thickened along colpi; exine 1.6‒1.9 µm thick, nexine thinner than sexine (LM), sexine sometimes protruding in area of endoapertures forming a bridge; semitectate, sculpturing scabrate in LM, microreticulate to perforate in SEM, muri denser with slit-like lumen in central mesocolpium area, muri more fused along colpi (SEM).
Remarks
The morphology of this pollen type places it within the Aralioideae. Many genera of the Aralioideae have pollen of similar morphology. There are few detailed generic accounts on the pollen morphology of extant Aralioideae, and we therefore refrain from assigning this to a particular extinct/extant genus, but point out the similarities of this pollen to those from many of the so-called panax-type pollen (including Acanthopanax, Dendropanax, Heteropanax, Kalopanax, Oplopanax and Panax; e.g. IBSCIB-CAS Citation1982; Miyoshi et al. Citation2011).
Locality
Messel.
Previous records
It is possible that this type corresponds to Tricolporopollenites sp. 18 in Thiele-Pfeiffer (Citation1988, plate 14, figures 69–72), but difficult to confirm because the specimen was not investigated by SEM. Collinson et al. (Citation2012) did not report any Araliaceae fruits or seeds from Messel. Wilde (Citation1989) described two different types of Araliaceae leaves (Hedera sp., ?cf. Fatsia) from Messel.
Bee specimen and species
FIS MeI 6388 (Protobombus messelensis); found on leg, thorax, abdomen.
Occurrence
As single grains and in large clumps along with Tilioideae gen. et sp. indet. pollen type.
Life form and pollination
Most likely an evergreen tree or shrub; entomophilous.
Aralioideae gen. et sp. indet. 2
()
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 23‒25 µm long in LM, 21‒23 µm long in SEM, equatorial diameter 19‒20 µm in LM, 17‒19 µm in SEM; tricolporate, endoapertures lalongate rectangular, nexine thickened along endopori and colpi (LM); exine 1.3‒1.5 µm thick, nexine thinner than sexine (LM); semitectate, sculpturing scabrate in LM, microreticulate in SEM, lumina funnel shaped, muri varying in height, muri fused along colpus forming a margo, sexine protruding over endoaperture forming a bridge (SEM).
Locality
Messel.
Previous records
This pollen type was not found in the dispersed pollen flora described by Thiele-Pfeiffer (Citation1988).
Bee specimen and species
FIS MeI 12151 (Electrapis sp.); found on abdomen.
Occurrence
In large pure groups.
Life form and pollination
Most likely an evergreen tree or shrub; entomophilous.
Eudicots
Remarks
We are unable to affiliate the following five pollen types from Eckfeld to any extinct or extant Eudicot family. All five types were not reported by Nickel (Citation1994, Citation1996) in the dispersed pollen flora from Eckfeld.
Eudicot ord., fam., gen. et sp. indet. 1
()
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 13‒16 µm long in LM, 12‒14 µm long in SEM, equatorial diameter 12‒13 µm in LM, 11‒12 µm in SEM; tricolporate; exine 1.0‒1.3 µm thick (LM), nexine thinner than sexine; tectate; sculpturing scabrate in LM, microrugulate, perforate in SEM, sexine protruding markedly in area of endoaperture forming a conspicuous bridge (SEM).
Bee specimen and species
PE 2000/846a,b.LS (Electrapis sp.); found on leg.
Occurrence
As single grains or in small clumps along with the Castanopsis/Lithocarpus sp. pollen type.
Life form and pollination
Unknown; probably entomophilous.
Eudicot ord., fam., gen. et sp. indet. 2
(, , , , , , )
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 18‒21 µm long in LM, 18‒20 µm long in SEM, equatorial diameter 12‒17 µm in LM, 12‒16 µm in SEM; tricolporate, endoapertures lalongate slit-like; exine 0.7‒1.0 µm thick, nexine thinner than sexine (LM); tectate, sculpturing scabrate in LM, microrugulate, perforate in SEM, microrugulae fused around colpi forming a psilate broad margo, sexine often protruding around endoapertures forming a bridge, distal ends of colpi truncated to round, colpus membrane microverrucate to microechinate, surface often overlain by irregularly distributed granular sporopollenin particles (SEM).
Bee specimen and species
PE 2000/847.LS (Electrapis prolata); PE 2000/849a,b.LS (Electrapis sp.); found on leg (both specimens).
Occurrence
In large groups along with Iridaceae gen. et sp. indet., Eudicot ord., fam., gen. et sp. indet. 3 and Anacardiaceae gen. et sp. indet. pollen types.
Life form and pollination
Unknown; probably entomophilous.
Eudicot ord., fam., gen. et sp. indet. 3
(, ; , )
Description
Pollen, prolate, lobate in polar view, elliptic in equatorial view; polar axis 20‒23 µm long in LM, 18‒22 µm long in SEM, equatorial diameter 12‒14 µm in LM, 11‒12 µm in SEM; tricolporate; exine 0.9‒1.2 µm thick, nexine thinner than sexine (LM); tectate, sculpturing scabrate in LM, rugulate, perforate in SEM, numerous perforations in grooves between rugulae, perforations in groups and/or rows, distal ends of colpi truncated to round, colpus membrane microverrucate to microechinate (SEM).
Bee specimen and species
PE 2000/847.LS (Electrapis prolata); found on leg.
Occurrence
In large groups along with Iridaceae gen. et sp. indet., Eudicot ord., fam., gen. et sp. indet. 2 and Anacardiaceae gen. et sp. indet. pollen types.
Life form and pollination
Unknown; probably entomophilous.
Eudicot ord., fam., gen. et sp. indet. 4
()
Description
Pollen, oblate to spheroidal, circular to lobate in polar view, elliptic in equatorial view; polar axis 11‒13 µm long in LM, 9‒13 µm long in SEM, equatorial diameter 12‒14 µm in LM, 11‒14 µm in SEM; tricolporate, endoapertures long, lalongate rectangular to slit-like, forming an endocingulum; exine 1.8‒2.0 µm thick, nexine thinner than sexine (LM); semitectate, sculpturing scabrate in LM, microreticulate in SEM, lumina decreasing in polar areas, sexine slightly protruding over endoapertures forming a bridge, colpus membrane microverrucate to microrugulate (SEM).
Bee specimen and species
PE 2000/852a.b,LS (Electrapis micheneri); found on thorax, abdomen.
Occurrence
In large groups along with Eudicot ord., fam., gen. et sp. indet. 5 pollen type.
Life form and pollination
Unknown; probably entomophilous.
Eudicot ord., fam., gen. et sp. indet. 5
()
Description
Pollen, monad, prolate, outline lobate in polar view, elliptic in equatorial view; polar axis 15‒17 µm long in LM, 15‒17 µm long in SEM, equatorial diameter 13‒15 µm in LM, 13‒15 µm in SEM; tricolporate; exine 0.9‒1.1 µm thick, nexine thinner than sexine (LM); tectate, sculpturing psilate in LM, perforate to microreticulate in SEM, perforations decreasing in size towards colpi (SEM).
Bee specimen and species
PE 2000/852a.b,LS (Electrapis micheneri); found on thorax, abdomen.
Occurrence
In large groups along with the Eudicot ord., fam., gen. et sp. indet. 4 pollen type.
Life form and pollination
Unknown; probably entomophilous.
Discussion
Comparison of in situ bee pollen inventory with that of the sediments
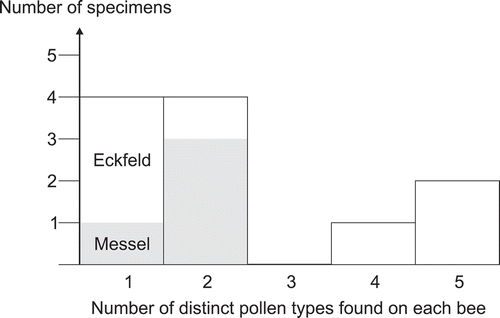
Despite the relatively low number of distinct pollen types (23 types from 12 families of 11 angiosperm orders), the in situ pollen cover a substantial taxonomic range of eudicots and one representative of the monocots (, ). Nine of the types described here may refer to dispersed pollen described earlier (Thiele-Pfeiffer Citation1988; Nickel Citation1994, Citation1996); the other 15 (including five eudicot pollen of unknown affinity) are new for the two localities Messel and Eckfeld (). The maximum number of pollen types found on a single bee is five (specimen PE 2000/846a,b.LS; Electrapis sp. from Eckfeld); the median amount is two (; ). The LM studies by Thiele-Pfeiffer (Citation1988) and Nickel (Citation1994, Citation1996) list in total 240 pollen types (including 125 of unknown or uncertain systematic affinity). The remaining 114 pollen types are assigned to 38 families of angiosperms from 25 orders (File S1). The total numbers of distinguished pollen types cannot be straightforwardly compared between our study and the earlier LM studies. Pollen types distinguished under LM may represent the same SEM-defined pollen taxon and vice versa. A one-to-one comparison would necessitate studying dispersed pollen obtained from sediment samples at both localities using SEM. Nevertheless, taxonomic control is typically poorer in LM than in SEM studies. As far as known, SEM studies of the same dispersed pollen flora reveal usually higher biodiversity and taxonomic resolution than LM studies (Grímsson & Zetter Citation2011; Grímsson et al. Citation2011, Citation2015a, Citation2016; Bouchal et al. Citation2016). Thus, it could be expected that a comprehensive SEM study of the dispersed pollen at Messel and Eckfeld would provide a large number of distinct taxa (genera, groups of genera).
Figure 17. Angiosperm pollen diversity at the Eocene Messel and Eckfeld localities mapped on currently accepted angiosperm phylogeny (Stevens 2001+; APG III Citation2009), deep relationships modified after (Goremykin et al. Citation2015). Grey filled boxes indicate orders present in the LM studied sedimentary pollen record of Messel and Eckfeld, black stars highlight angiosperm orders documented here from bee fossils originating from both localities.
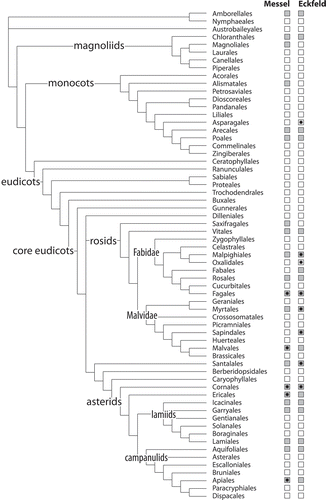
Figure 18. Bar chart visualising the number of pollen types found on individual bee specimens (cf. ).
It is clear that the in situ pollen on the bees only cover a relatively small fraction of the actual middle Eocene floral diversity in and around the Messel and Eckfeld localities. There are several explanations for the low diversity of pollen attached to the bee fossils. The most important elements at both localities – regarding the number of distinguished pollen taxa in the dispersed pollen record – are the fabids. Fabids at Messel and Eckfeld account for 43% and 32%, respectively, of pollen taxa listed with unambiguous systematic affinity, followed by asterids of the orders Cornales and Ericales (14% and 21%, respectively). Both angiosperm groups include many wind-pollinated modern genera and species, and, in the case of Fagales, which account for over 20% of the LM-diagnosed pollen taxa at both localities, are predominately or partly wind-pollinated. In addition, eusocial bees harvest pollen within a limited range of their dwellings, and the extinct species may have had collection preferences similar to some of their modern-day relatives (Wappler et al. Citation2015), almost all of whom are polylectic (e.g. Apini, Meliponini, Bombini). Furthermore, insect-pollinated taxa are typically underrepresented in the dispersed pollen record because of their much lower pollen yield, and many groups are easily overlooked, particularly when the dispersed pollen flora is studied using LM only. This is highlighted by our results.
Despite the fact that the pollen obtained in situ from the 12 bee fossils cover only one-third of the families found in the dispersed pollen inventories, we have discovered for the first time evidence of the monocot order Aspergales (Iridoideae gen. et sp. indet.), the fabid order Oxidales (Elaeocarpus sp. 1, sp. 2; , ) and the family Euphorbiaceae (three pollen types; a member of the Malpighiales) in the Eocene of Eckfeld. Previously, the Malpighiales were represented by only a single pollen taxon (Tricolpopollenites retiformis P.W.Thomson et Pfug) with affinity to Salix and reported from both localities (File S2; Thiele-Pfeiffer Citation1988; Nickel Citation1996). Also new is the discovery of Decodon (Lythraceae) at Eckfeld. Lythraceae pollen types with putative affinity to Ammannia, Lawsonia, Rotala and Woodfordia have been described so far only from Messel (File S2; Thiele-Pfeiffer Citation1988). However, the new type of Mastixioideae pollen confirms prior LM studies that distinguished one or two taxa at both localities (File S2; Thiele-Pfeiffer Citation1988; Nickel Citation1994, Citation1996). In the case of Messel, our Mortoniodendron pollen adds to the known Tilioideae diversity (two taxa described by Thiele-Pfeiffer Citation1988). In conclusion, our investigation of pollen adherent to bee fossils shows that these natural ‘pollen traps’ can provide new insights and complement our knowledge on the broader palaeo-palynological assemblages.
Pollen-collecting/-feeding insects as natural ‘pollen traps’
There are only a few studies, which diagnosed pollen in or on insect fossils. This is surprising given the considerable interest in insect–plant interactions and coevolution, and that such pollen is perhaps the best direct evidence for such associations. Probably the oldest record of insects feeding on pollen (with affinities to conifer, glossopterid and peltasperm pollen) is from the Cisuralian (Krassilov & Rasnitsyn Citation1996). In addition, the same authors described conifer pollen and pollen of unknown affinity from the intestines of Lower Cretaceous sawflies (Krassilov & Rasnitsyn Citation1982). Pollen with potential affinity to angiosperms (Afropollis sp.) has been cited to occur also in the intestines of mid-Cretaceous wasps (Caldas et al. Citation1989). A spectacular find was a taxonomically identifiable orchid pollinium attached to a worker of the fossil stingless bee Proplebeia dominicana (Wille et Chandler) in early Miocene (19–15 Ma) amber from the Dominican Republic (Ramírez et al. Citation2007). Prior to this, orchid fossils had not been found from the Dominican Republic, although their presence was predicted by the occurrence of orchid bees (Euglossini) in the same Miocene deposits (Engel Citation1999) and the same is true for Mexican amber (Engel Citation2014). Many fossil bees preserved in amber, not only corbiculate bees, have pollen preserved in their scopae or corbiculae (e.g. Engel Citation1995, Citation1998, Citation2006, Citation2009), but it is often not possible to observe sufficient details owing to an inability to prepare the material close to the individual grains without destroying the bee. Unfortunately, the oldest definitive bee, Cretotrigona prisca (Michener et Grimaldi) in Maastrichtian amber from New Jersey, is not preserved with its pollen (Engel Citation2000) and meaningful evidence from the Cretaceous record of bee–plant interactions is challenging to ascertain (Michez et al. Citation2012).
Naturally, bees are not the only pollinating insects and fossil pollen is also preserved on the bodies of other such insect groups (e.g. amber inclusions of fig wasps; Peñalver et al. Citation2006). Even though known to exist for quite a long time, at least for the Eckfeld locality (Lutz Citation1993), pollen clumps contained in the corbiculae of ancient bees had not previously been examined. Whereas the in situ pollen in flowers embedded in coeval Baltic amber has been studied to some degree (Weitschat & Wichard Citation2002), pollen on those bees reported from the same fossil resins (Engel Citation2001a) have yet to be explored.
The pollen documented here provides the oldest unambiguous (pollen) record of Elaeocarpus (Oxidales: Elaeocarpaceae), Mortoniodendron (Malvales: Malvaceae: Tilioideae), Olax (Santalales: Olacaceae) and Pouteria (Ericales: Sapotaceae). Today, Elaeocarpus and Olax are found mainly in the Old World Tropics (sub-Saharan Africa, Madagascar, south and southeast Asia, tropical Australasia; ), and add two more examples of early Northern Hemisphere occurrences of lineages today restricted to the tropics and extra-tropical Southern Hemisphere (Grímsson et al. Citation2014). These same records may provide new minimum age priors for calibrating molecular-only estimates of clade divergence times. For instance, in their dating of the Elaeocarpaceae, Crayn et al. (Citation2006) fixed the crown clade of Elaeocarpus to 30 Ma based on Oligocene mesocarps from Australia (Dettmann & Clifford Citation2001), which they considered the earliest attributable fossils for the genus and at the same time reflecting an initial radiation for the group. They estimated a late Eocene (c. 39 Ma) age for the divergence of Elaeocarpus from its sister group, and their value is about 5 Ma younger than the pollen carried by the ancient bees at Eckfeld Maar in the Northern Hemisphere. Infrafamilial relationships among Santalales have been investigated using molecular data (e.g. Malécot & Nickrent Citation2008), but the phylogenies were not dated due to a lack of suitable fossils. The same applies to the asterid family Sapotaceae (Manchester et al. Citation2015). Hence, our pollen of Olax and Pouteria may serve as the first available ingroup dating constraints for Olacaceae and Sapotaceae.
Table II. Pollen diversity (in number of distinguished types) at Messel and Eckfeld localities with respect to the bee specimen on which the pollen types were found. Number for dispersed pollen types refer to the studies of Thiele-Pfeiffer (Citation1988) and Nickel (Citation1996).
Table III. Further information for taxa found on Messel and Eckfeld bees compiled from various resources.
Mortoniodendron is a tropical-subtropical New World relative of the widespread lindens (Tilia), today found only in Central America. To our knowledge, there has been no effort to date the divergences among or within the three genera included in the subfamily Tilioideae (Craigia, Mortoniodendron and Tilia). The Eckfeld bees also carry the oldest European evidence of the genus Decodon; pollen coeval to the oldest pollen described from the Princeton Chert, British Columbia, Canada (Grímsson et al. Citation2012). Decodon is known from the Late Cretaceous and Paleogene of the Americas (Graham Citation2013), hence providing further proof that this New World taxon expanded to continental Europe by the middle Eocene (Grímsson et al. Citation2012). These discoveries highlight the importance of studying pollen found on fossil insects and that dispersed pollen from sediments should also be studied using SEM to more accurately reveal the total ‘true’ diversity of a given palaeo-flora, which is often underestimated by the LM based pollen/sedimentary record, as well as elucidate the interactions of the plants with their pollinators.
Ecological and palaeo-climatic framework of Messel and Eckfeld
Wilde and Frankenhäuser (Citation1998) refrained from an explicit climatic interpretation based on the palaeo-floristic assemblage (macro-, meso- and micro-fossils) found at Eckfeld. They noted that many of the macro- and meso-fossils cannot be assigned to modern taxa with any degree of confidence, and highlighted the special taphonomic composition around the maar lake. Major floristic elements around the palaeo-lake were Juglandaceae with affinities to Platycarya as well as Engelhardioideae; a high abundance of wind-pollinated Engelhardioideae explains the recovery of this pollen type (single grains) from one of the bees (). In addition, palms were present but may not have been abundant (Wilde & Frankenhäuser Citation1998). Palms, Platycarya and Engelhardioideae thrive in tropical as well as subtropical and temperate habitats without frost or snow in winter.
The pollen found on the bees indicate the presence of several more modern genera (Decodon, Elaeocarpus, Olax, Pouteria) and three additional groups (evergreen Castaneoideae, Iridoideae, Mastixioideae), and since the bees can be considered to have harvested pollen directly from flowers of plants within a limited area, they most likely originate from the immediate vicinity of the palaeo-lake. Hence, these records in association with the bees provide a limited but localised impression about the climate, but also evidence for Eocene distributional areas in contrast to modern ranges. In the case of Messel, Aralioideae coexisted with evergreen Castaneoideae, Tilioideae (including Mortoniodendron) and Nyssa. The geographical distribution of potential modern analogues of the plants producing the in situ bee pollen as well as their ‘Köppen signatures’, which indicate the general climate zones occupied by a certain taxon (cf. Denk et al. Citation2013; Grímsson et al. Citation2016), also suggest that a warm humid climate prevailed at Eckfeld and Messel during the middle Eocene () The distribution range of all taxa that coexisted at Messel cover subtropical, fully humid and winter-dry climates (Cfa-, Cw-climates according to the Köppen classification). Previous accounts on the Messel macro-flora have also suggested a very warm and humid, tropical to subtropical climate (Wilde Citation1989, p. 119–120; Collinson et al. Citation2012, p. 13).
Nearly all Eckfeld taxa are found today also in the Tropics except for Decodon. However, this genus is today only represented by a single species in eastern North America, but had a much wider distribution in the past encompassing low latitudes (Graham Citation2013). Analogously, the Mastixioideae, the only taxon in Eckfeld absent from today’s Cfa-climates, had a wider distribution in the past. Overall, a warm, humid, subtropical Cfa-climate transient to a tropical climate could have provided the environment for the Eckfeld locality (c. 2 Ma years younger than Messel), whereas a distinctly subtropical climate, either fully humid (Cfa) or with more summer than winter precipitation (Cwa), is favoured for Messel owing to the autochthonous pollen community collected by the bee populations.
Conclusion
Despite the intensive effort required and limited taxonomic coverage, the study of pollen actively or passively collected by insects can provide valuable new insights and contribute to a more holistic understanding of the floristic components of past ecosystems. One advantage in studying fossil pollen adhered to fossil insects is that insect-pollinated plant taxa that are rare or otherwise hard or even impossible to find in the dispersed pollen (sedimentary) record can be revealed. Such information is vital for neontological research that aims at correlating angiosperm and insect distribution patterns, exploring the evolution of plant–insect interactions and coevolution, or appropriately calibrating molecular estimates of divergence times. Another advantage is the autochthony of pollen found in situ on (or in) bees, reflecting a potentially informative fragment of a highly localised flora.
Acknowledgements
TW is supported by the German Research Foundation (WA 1496/6-1, Heisenberg grant WA 1496/8-1), and FG is supported by the Austrian Science Fund (FWF), grant (P24427-B25). This is Contribution 306 of the Evolution of Terrestrial Ecosystems Consortium at the National Museum of Natural History, in Washington, DC, and a contribution of the Division of Entomology, University of Kansas, Natural History Museum. Guido Grimm, University Vienna, Austria, is thanked for editorial service and preparation of in-text tables and online supplement files, and of figures 17 and 18.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. doi:10.1111/(ISSN)1095-8339.
- Bouchal JM, Zetter R, Denk T. 2016. Pollen and spores of the uppermost Eocene Florissant Formation, Colorado: A combined light and scanning electron microscopy study. Grana 55: xxx–xxx. doi:10.1080/00173134.2015.1108362.
- Caldas EB, Martins-Neto RG, Lima Filho FP. 1989. Afropollis sp. (pollen) no trato intestinal de vespa (Heminoptera: Apocrita: Xyelidae) no Cretaceo da Baccia do Araripe. Atas Simposio de Geologia do Nordeste, Fortalesa 13: 195.
- Collinson ME, Manchester SR, Wilde V. 2012. Fossil fruits and seeds of the middle Eocene Messel Biota, Germany. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 570: 1–251.
- Crayn DM, Rossetto M, Maynard DJ. 2006. Molecular phylogeny and dating reveals an Oligo-Miocene radiation of dry-adapted shrubs (former Tremandraceae) from rainforest tree progenitors (Elaeocarpaceae) in Australia. American Journal of Botany 93: 1328–1342. doi:10.3732/ajb.93.9.1328.
- Denk T, Grimm GW. 2009. Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus (Fagaceae). International Journal of Plant Sciences 170: 926–940. doi:10.1086/592012.
- Denk T, Grimm GW, Grímsson F, Zetter R. 2013. Evidence from “Köppen signatures” of fossil plant assemblages for effective heat transport of Gulf Stream to subarctic North Atlantic during Miocene cooling. Biogeosciences 10: 7927–7942. doi:10.5194/bg-10-7927-2013.
- Denk T, Grímsson F, Zetter R. 2012. Fagaceae from the Early Oligocene of central Europe: Persisting New World and emerging Old World biogeographic links. Review of Palaeobotany and Palynology 169: 7–20. doi:10.1016/j.revpalbo.2011.09.010.
- Dettmann ME, Clifford HT. 2001. The fossil record of Elaeocarpus L. fruits. Memoirs of the Queensland Museum 46: 461–497.
- eFloras. 2008. Flora of China and Flora of North America. http://www.efloras.org; accessed August 2015.
- Endress PK. 1986. An entomophily syndrome in Juglandaceae: Platycarya strobilacea. Veröffentlichungen des Geobotanischen Institutes der Eidgenössischen Technischen Hochschule, Stiftung Rübel, Zürich 87: 100–111.
- Engel MS. 1995. Neocorynura electra, a new fossil bee species in Dominican amber (Hymenoptera: Halictidae). Journal of the New York Entomological Society 103: 317–323.
- Engel MS. 1998. A new species of the Baltic amber bee genus Electrapis (Hymenoptera: Apidae). Journal of Hymenoptera Research 7: 94–101.
- Engel MS. 1999. The first fossil Euglossa and phylogeny of the orchid bees (Hymenoptera: Apidae; Euglossini). American Museum Novitates 3272: 1–14.
- Engel MS. 2000. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). American Museum Novitates 3296: 1–11. doi:10.1206/0003-0082(2000)3296<0001:ANIOTO>2.0.CO;2.
- Engel MS. 2001a. A monograph of the Baltic amber bees and evolution of the Apoidea (Hymenoptera). Bulletin of the American Museum of Natural History 259: 1–192. doi:10.1206/0003-0090(2001)259<0001:AMOTBA>2.0.CO;2.
- Engel MS. 2001b. Monophyly and extensive extinction of advanced eusocial bees: Insights from an unexpected Eocene diversity. Proceedings of the National Academy of Sciences, USA 98: 1661–1664. doi:10.1073/pnas.98.4.1661.
- Engel MS. 2006. A giant honey bee from the Middle Miocene of Japan (Hymenoptera: Apidae). American Museum Novitates 3504: 1–12. doi:10.1206/0003-0082(2006)504[0001:AGHBFT]2.0.CO;2.
- Engel MS. 2009. Two new halictine bees in Miocene amber from the Dominican Republic (Hymenoptera, Halictidae). ZooKeys 29: 1–12. doi:10.3897/zookeys.29.257.
- Engel MS. 2014. An orchid bee of the genus Eulaema in Early Miocene Mexican amber (Hymenoptera: Apidae). Novitates Paleoentomologicae 7: 1–15.
- Ferguson IK. 1997. Cornaceae Dum. In: Nilsson S, ed. World pollen and spore flora, Vol. 4 Stockholm: Almquist & Wiksell.
- Goldblatt P, Le Thomas A. 1992. Pollen apertures, exine sculpturing and phylogeny in Iridaceae subfamily Iridoideae. Review of Palaeobotany and Palynology 75: 301–315. doi:10.1016/0034-6667(92)90022-9.
- Goremykin VV, Nikiforova SV, Cavalieri D, Pindo M, Lockhart PJ. 2015. The root of flowering plants and total evidence. Systematic Biology 64: 879–891. doi:10.1093/sysbio/syv028.
- Göschl W. 2008. Beiträge zur Pollenmorphologie ausgewählter rezenter und fossiler Vertreter der Davidiaceae und Nyssaceae. PhD Thesis, Universität Wien, Vienna.
- Graham SA. 2013. Fossil records in the Lythraceae. The Botanical Review 79: 48–145. doi:10.1007/s12229-012-9116-1.
- Grímsson F, Ferguson DK, Zetter R. 2012. Morphological trends in the fossil pollen of Decodon and the paleobiogeographic history of the genus. International Journal of Plant Sciences 173: 297–317. doi:10.1086/663968.
- Grímsson F, Grimm GW, Meller B, Bouchal JM, Zetter R. 2016. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part IV. Magnoliophyta 2 – Fagales to Rosales. Grana 55: xxx–xxx. doi:10.1080/00173134.2015.1096566.
- Grímsson F, Meller B, Bouchal JM, Zetter R. 2015a. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part III. Magnoliophyta 1 – Magnoliales to Fabales. Grana 54: 85–128. doi:10.1080/00173134.2015.1007081.
- Grímsson F, Zetter R. 2011. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part II. Pinophyta (Cupressaceae, Pinaceae and Sciadopityaceae). Grana 50: 262–310. doi:10.1080/00173134.2011.641450.
- Grímsson F, Zetter R, Baal C. 2011. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part I. Bryophyta, Lycopodiophyta, Pteridophyta, Ginkgophyta, and Gnetophyta. Grana 50: 102–128. doi:10.1080/00173134.2011.585804.
- Grímsson F, Zetter R, Grimm GW, Krarup Pedersen G, Pedersen AK, Denk T. 2015b. Fagaceae pollen from the early Cenozoic of West Greenland: Revisiting Engler’s and Chaney’s Arcto-Tertiary hypotheses. Plant Systematics and Evolution 301: 809–832. doi:10.1007/s00606-014-1118-5.
- Grímsson F, Zetter R, Halbritter H, Grimm GW. 2014. Aponogeton pollen from the Cretaceous and Paleogene of North America and West Greenland: Implications for the origin and palaeobiogeography of the genus. Review of Palaeobotany and Palynology 200: 161–187. doi:10.1016/j.revpalbo.2013.09.005.
- Harley M. 1991. The pollen morphology of the Sapotaceae. Kew Bulletin 46: 379–491. doi:10.2307/4110538.
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. 2009. Pollen terminology: An illustrated handbook. Wien, New York: Springer.
- IBSCIB-CAS (Institute of Botany and South China Institute of Botany, Chinese Academy of Sciences). 1982. Angiosperm pollen flora of tropical and subtropical China. Beijing: Science Press.
- Krassilov VA, Rasnitsyn AP. 1982. Unikal’naya nakhodka: pyl’tsa v zheludke nizhnemelovykh ksielotomid [A unique find: Pollen in the intestines of early Cretaceous sawflies]. Palaeontologicheskij Zhurnal 4: 83–96. [in Russian].
- Krassilov VA, Rasnitsyn AP. 1996. Pollen in the guts of Permian insects: First evidence of pollinivory and its evolutionary significance. Lethaia 29: 369–372. doi:10.1111/let.1996.29.issue-4.
- Kvaček Z, Manchester SR, Zetter R, Pingen M. 2002. Fruits and seeds of Craigia bronnii (Malvaceae, Tilioideae) and associated flower buds from the late Miocene Inden Formation, Lower Rhine Basin, Germany. Review of Palaeobotany and Palynology 119: 311–324. doi:10.1016/S0034-6667(01)00135-X.
- Lutz H. 1993. Eckfeldapis electrapoides gen. nov., sp. nov., eine „Honigbiene“ aus dem Mittel-Eozän des „Eckfelder Maares“ bei Manderscheid/Eifel, Deutschland (Hymenoptera: Apidae, Apinae). Mainzer Naturwissenschaftliches Archiv 31: 177–199.
- Lutz H, Neuffer FO, Harms F-J, Schaal S, Micklich N, Gruber G, Schweigert G, Lorenz V. 2000. Tertiary maars as fossil deposits: Eckfeld, Messel, Randeck, Höwenegg, Öhnigen. Mainzer Naturwissenschaftliches Archiv, Beiheft 24: 125–160.
- Maguire B, Wurdack JJ, Huang Y-C. 1974. Pollen grains of some American Olacaceae. Grana 14: 26–38. doi:10.1080/00173137409434771.
- Mai DH. 1995. Tertiäre Vegetationsgeschichte Europas. Jena: Gustav Fischer.
- Malécot V, Nickrent DL. 2008. Molecular phylogenetic relationships of Olacaceae and related Santalales. Systematic Botany 33: 97–106. doi:10.1600/036364408783887384.
- Manchester SR, Grímsson F, Zetter R. 2015. Assessing the fossil record of asterids in the context of our current phylogenetic framework. Annals of the Missouri Botanical Garden 100: 329–363. doi:10.3417/2014033.
- Manchester SR, Hermsen EJ. 2000. Flowers, fruits, seeds, and pollen of Landeenia gen. nov., an extinct sapindalean genus from the Eocene of Wyoming. American Journal of Botany 87: 1909–1914. doi:10.2307/2656842.
- Mertz DF, Renne PR. 2005. A numerical age for the Messel fossil deposit (UNESCO World Heritage Site) derived from 40Ar/39Ar dating on a basaltic rock fragment. Courier Forschungsinstitut Senckenberg 255: 67–75.
- Mertz DF, Swisher CC, Franzen JL, Neuffer FO, Lutz H. 2000. Numerical dating of the Eckfeld maar fossil site, Eifel, Germany: A calibration mark for the Eocene time scale. Naturwissenschaften 87: 270–274. doi:10.1007/s001140050719.
- Michez D, Vanderplanck M, Engel MS. 2012. Fossil bees and their plant associates. In: Patiny S, ed. Evolution of plant-pollinator relationships, 103‒164. Cambridge: Cambridge University Press.
- Miyoshi N, Fujiki T, Kimura H. 2011. Pollen flora of Japan. Sapporo: Hokkaido University Press.
- Nickel B. 1994. Neue palynologische Untersuchungen am mitteleozänen Ölschiefer von Eckfeld bei Manderschei/Eifel. Erste Ergebnisse. Mainzer Naturwissenschaftliches Archiv 32: 7–25.
- Nickel B. 1996. Die mitteleozäne Mikroflora von Eckfeld bei Manderscheid/Eifel. Mainzer Naturwissenschaftliches Archiv, Beiheft 18: 1–147.
- Peñalver E, Engel MS, Grimaldi DA. 2006. Fig wasps in Dominican amber (Hymenoptera: Agaonidae). American Museum Novitates 3541: 1–16. doi:10.1206/0003-0082(2006)3541[1:FWIDAH]2.0.CO;2.
- Perveen A, Grafström E, El-Ghazaly G. 2004. World Pollen and Spore Flora 23. Malvaceae Adams. Subfamilies Grewioideae, Tilioideae, Brownlowioideae. Grana 43: 129–155. doi:10.1080/00173130410000730.
- Praglowski J. 1982. Fagaceae L. Fagoideae. In: Nilsson S, ed. World pollen and spore flora, Vol. 11. Stockholm: Almquist & Wiksell.
- Praglowski J. 1984. Fagaceae Dumort. Castaneoideae Oerst. In: Nilsson S, ed. World pollen and spore flora, Vol. 13. Stockholm: Almquist & Wiksell.
- Punt W, Hoen P, Blackmore S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. doi:10.1016/j.revpalbo.2006.06.008.
- Ramírez SR, Gravendeel B, Singer RB, Marshall CR, Pierce NE. 2007. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature 448: 1042–1045. doi:10.1038/nature06039.
- Schaal S, Ziegler W. 1992. Messel. An insight into the history of life and of the Earth. Oxford: Clarendon Press.
- Schultz TR, Engel MS, Ascher JS. 2001. Evidence for the origin of eusociality in the corbiculate bees (Hymenoptera: Apidae). Journal of the Kansas Entomological Society 74: 10–16.
- Solomon AM. 1983a. Pollen morphology and plant taxonomy of red oaks in eastern North America. American Journal of Botany 70: 495–507. doi:10.2307/2443160.
- Solomon AM. 1983b. Pollen morphology and plant taxonomy of white oaks in eastern North America. American Journal of Botany 70: 481–492. doi:10.2307/2443159.
- Stevens PF. (2001+). Angiosperm Phylogeny Website. Version 12. http://www.mobot.org/MOBOT/research/APweb/; accessed August 2015.
- Stone DE, Broome R. 1975. Juglandaceae A. Rich. ex Kunth. In: Nilsson S, ed. World pollen and spore flora, Vol. 4. Stockholm: Almquist & Wiksell.
- Tang Y, Wu Z. 1990. Study on the pollen morphology of Chinese Elaeocarpaceae. Acta Botanica Yunnanica 12: 397–403.
- Thiele-Pfeiffer H. 1988. Die Mikroflora aus dem mitteleozänen Ölschiefer von Messel bei Darmstadt. Palaeontographica. Abteilung B 211: 1–86.
- Wappler T, Engel MS. 2003. The Middle Eocene bee faunas of the Eckfeld Maar and Messel, Germany (Hymenoptera: Apoidea). Journal of Paleontology 77: 908–921. doi:10.1666/0022-3360(2003)077<0908:TMEBFO>2.0.CO;2.
- Wappler T, Labandeira CC, Engel MS, Zetter R, Grímsson F. 2015. Specialist pollen-collection strategies in an ancient bee lineage. Current Biology 25: 1–7.
- Wedmann S, Wappler T, Engel MS. 2009. Direct and indirect fossil records of megachilid bees from the Paleogene of central Europe (Hymenoptera: Megachilidae). Naturwissenschaften 96: 703–712. doi:10.1007/s00114-009-0525-x.
- Weitschat W, WichardWeitschat W. 2002. Atlas of plants and animals in Baltic amber. München: Friedrich Pfeil.
- Wilde V. 1989. Untersuchungen zur Systematik der Blattreste aus dem Mitteleozän der Grube Messel bei Darmstadt (Hessen, Bundesrepublik Deutschland). Courier Forschungsinstitut Senckenberg 115: 1–113.
- Wilde V, Frankenhäuser H. 1998. The Middle Eocene plant taphocoenosis from Eckfeld (Eifel, Germany). Review of Palaeobotany and Palynology 101: 7–28. doi:10.1016/S0034-6667(97)00067-5.