Abstract
The palm family, Arecaceae, is notoriously depauperate in Africa today, and its evolutionary, paleobiogeographic, and extinction history there are not well documented by fossils. In this article we report the pollen of two new extinct species of the small genus, Sclerosperma (Arecoideae), from a late Oligocene (27–28 Ma) stratum exposed along the Guang River in Chilga Wereda of north-western Ethiopia. The pollen are triporate, and the two taxa can be distinguished from each other and from modern species using a combination of light and scanning electron microscopy, which reveals variations in the finer details of their reticulate to perforate exine sculpture. We also report a palm leaf fragment from a stratum higher in the same section that is in the Arecoideae subfamily, and most likely belongs to Sclerosperma. The implications of these discoveries for the evolutionary history of this clade of African arecoid palms is that their diversification was well underway by the middle to late Oligocene, and they were much more widespread in Africa at that time than they are now, limited to West and Central Africa. Sclerosperma exhibits ecological conservatism, as today it occurs primarily in swamps and flooded forests, and the sedimentology of the Guang River deposits at Chilga indicate a heterogeneous landscape with a high water table. The matrix containing the fossil pollen is lignite, which itself indicates standing water, and a variety of plant macrofossils from higher in the section have been interpreted as representing moist tropical forest or seasonally inundated forest communities.
Sclerosperma G. Mann et H. Wendl. is a small genus in the palm family, Arecaceae (Arecoideae, Sclerospermeae; Dransfield et al. Citation2005), found in tropical West and Central Africa where three species are known; Sclerosperma mannii H. Wendl., S. profizianum Valk. et Sunderl. and S. walkeri A. Chev. (van Valkenburg et al. Citation2008). The genus was first described by Gustav Mann and Hermann Wendland in 1864 based on material belonging to S. mannii collected by Mann in an inundated forest near the Gaboon (now the Ogooué) River upstream from Point Clara (Mann & Wendland Citation1864). But, Sclerosperma remained rather enigmatic over time, and although new species were described, they were so rarely collected that the circumscription of these taxa was clarified only recently (van Valkenburg et al. Citation2008). All three species of Sclerosperma are small, clustering understorey palms, ranging in height from 2 to 6 (to 12) m. They are vulnerable, pleonanthic, monoecious palms that grow in tropical lowland rainforests (from sea level to c. 1400 m), on swampy sites as well as, less commonly, on terra firma. Sclerosperma is present in old secondary forests dominated by okoumé (Aucoumea klaineana Pierre) and persists in secondary growth near human habitations where the leaves are often collected for roof thatch. The stem in Sclerosperma is usually absent above-ground, is stout with closely-spaced ringed leaf scars, and if above-ground may extend horizontally and produce suckers. Occasionally, S. profiziana can produce a stout stem up to 9 cm in diameter and 2 m in height. The crown consists of numerous leaves, broadly radiating, pointing upwards, that accumulate debris, and thus obscure the base of the plant. The leaves are reduplicate, undivided or irregularly pinnate, the apex deeply bifid with the rachis continued in a fibre. All Sclerosperma species have intrafoliar, solitary, spike-like inflorescences, which are protogynous. The inflorescence is enclosed by a peduncular bract which becomes web-like in the median part at anthesis and with a distal opening. This peduncular bract is somewhat persistent but usually disintegrates when fruits are fully developed. The inflorescence is often obscured by debris, can be overlooked, and thus rarely collected. The fruits are readily sought by animals (e.g. gorillas), so in faunal-rich regions intact infructescences are rarely found (van Valkenburg et al. Citation2008). The unique pollen morphology of Sclerosperma (triangular, triporate, reticulate) within the Arecaceae was first noted by Erdtman and Sing (Citation1957) and often discussed by M.M. Harley and collaborators in the years 1991 to 2008 during their comprehensive work on the pollen morphology of this family (Harley & Hall Citation1991; Harley Citation1996, Citation1999, Citation2004; Harley & Baker Citation2001; Harley & Dransfield Citation2003; Dransfield et al. Citation2008). The pollen morphology of all three extant Sclerosperma species was recently revised by Grímsson et al. (Citation2018), showing that they produce four different pollen morphologies. S. mannii and S. walkeri share the same distinct reticulate pollen, but S.profizianum appears to produce three different pollen types (microreticulate, fossulate, and perforate).
The fossil record of Sclerosperma is meagre, and is only represented by leaf specimens from a site called ‘Mero’, near Kamituga, in eastern Democratic Republic of the Congo (DRC), South Kivu District. Lakhanpal (Citation1966) described and figured S. saffiannikoffii from this locality, which was referred to as middle Miocene in age. The fossil pollen record, so far, has mostly been questioned or rejected by Harley and Baker (Citation2001) and Harley (Citation2004, Citation2006). Nevertheless, the fossil record of Arecaceae in Africa can help to understand its depauperate representation today relative to other tropical regions of the world (e.g. 65 species in Africa versus 437 in South America), and to assess the relative importance of Cainozoic extinctions versus moderate speciation rates compared with other, richer, regions (Richards Citation1973; Morley Citation2000; Pan et al. Citation2006; Couvreur et al. Citation2011; Kissling et al. Citation2012; Couvreur & Baker Citation2013; Faye et al. Citation2016).
Here we describe fossil Sclerosperma pollen, assigned to two new species, from the late Oligocene of Chilga, north-western Ethiopia. The fossil pollen is compared to that of extant Sclerosperma taxa in light of recent descriptions presented by Grímsson et al. (Citation2018), and the evolution of Sclerosperma pollen morphology is discussed. The fossil pollen record is summarised, and conclusions are drawn regarding the palaeophytogeographic history of the genus, African palms in general, and the significance of Sclerosperma for palaeoenvironmental interpretations of the Chilga site. We also report an unidentified palm leaf fragment from the same site that can be assigned to Arecoideae, and although it was found at a different stratigraphic level could belong to a Sclerosperma taxon described herein.
Material and methods
Geologic background of sedimentary sample
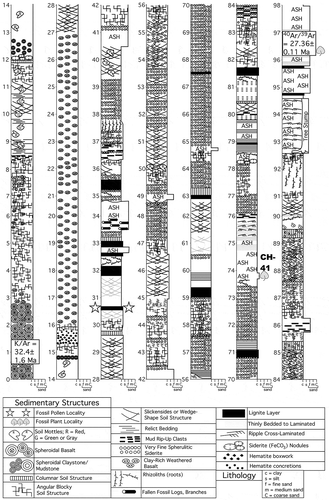
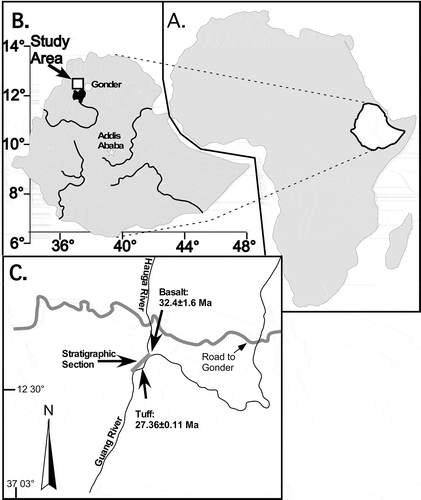
The Sclerosperma fossils, both pollen and leaves, were found in sedimentary rocks from the late Oligocene of Chilga deposits of north-western Ethiopia (Figure 1). Fossil rich sedimentary rocks outcrop in an area of about 100 km2, approximately 60 km to the west-southwest (WSW) of Gonder, the historic capital of Ethiopia. Physiographically, this region constitutes a part of the Ethiopian Highlands to the west of the main Ethiopian Rift, and lies at elevations of 1800 to 2000 m. The Chilga sedimentary sequence, >100 m thick, occurs in a structural basin underlain by basalts of early Oligocene age. A volcanic ash layer near the top of the Chilga section provided a 40Ar–39Ar age of 27.36 ± 0.11 Ma, and the underlying basalts associated with the origin of the Ethiopian Plateau is dated by whole-rock K-Ar at 32.4 ± 0.11 Ma (; Kappelman et al. Citation2003). The sedimentary rocks are also correlated with the paleomagnetic time scale, constraining the age of the fossiliferous strata to 28–27 Ma, within the limits of Chron C9n (Kappelman et al. Citation2003). Fossil vertebrates and plants were collected widely across the Chilga outcrop area, but plant macrofossils and microfossils were studied most intensively from localities exposed along the Guang River and its smaller tributary, the Hauga River (; e.g. Yemane et al. Citation1985, Citation1987; Kappelman et al. Citation2003; Sanders et al. Citation2004; Jacobs et al. Citation2005; Pan & Jacobs Citation2009; Engel et al. Citation2013; Pan et al. Citation2014). The sedimentary sample that produced the pollen reported here, is a lignite from 30.5 m above the base of the measured section along the Guang River (), and the fossil palm leaf fragments were found in an ash-rich siderite approximately 43 m higher in the section (74 m, ).
Figure 1. Locality map. A. Location of Ethiopia in Africa. B. The study area, Gonder, and the current capital city, Addis Ababa. Major rivers are shown, including the Blue Nile and its source, Lake Tana, to the immediate south of the study area. C. Location of the Guang and Hauga Rivers, and measured section shown in .
Preparation of fossil pollen
The sedimentary rock sample was treated with hydrochloric acid (HCl) and hydrofluoric acid (HF) to remove any carbonates and silicates, followed by an oxidising solution, according to the protocol outlined in Grímsson et al. (Citation2008). The fossil Sclerosperma pollen grains were investigated both by light microscopy (LM) and scanning electron microscopy (SEM) using an isolated single grain as described in Zetter (Citation1989) and Halbritter et al. (Citation2018). Plant macrofossils were collected in blocks of matrix from four closely-spaced sublocalities along a single, organic rich stratum, mostly lacking bedding planes or laminations. The mode of deposition is interpreted as overbank associated with a flood event.
Conservation of fossil material
SEM stubs with the fossil Sclerosperma pollen produced during this study are stored in the collection of the Department of Palaeontology, University of Vienna, Austria, under the accession numbers IPUW 7513/223 to IPUW 7513/229. The fossil leaves are housed permanently in the Chilga paleobotany collections of the National Museum of Ethiopia, Addis Ababa, under accession numbers CH41-9A and CH41-9B (A and B are part and counterpart).
Systematic and descriptive palaeobotany
Pollen descriptions include diagnostic features observed both in LM and SEM. The pollen terminology follows Punt et al. (Citation2007; LM) and Halbritter et al. (Citation2018; SEM). Classification follows Dransfield et al. (Citation2005) and APG IV (Citation2016). The pollen grains are described first, followed by the leaf remains. The fossil pollen types are compared to pollen of extant taxa in . Terminology for palm leaf morphology follows Hickey (Citation1973), Ellis et al. (Citation2009), and especially Dransfield et al. (Citation2008).
Table I. Pollen morphology of extant and fossil Sclerosperma.
Family Arecaceae Bercht. et J. Presl
Genus Sclerosperma G. Mann et H. Wendl.
Species Sclerosperma protomannii Grímsson et R. Zetter, sp. nov.
()
Diagnosis
Pollen with 10 to 20 lumina per 100 µm2 at central distal face, elliptic to polygonal lumina; nanogemmae free-standing columellae extremely rare and singular; opercula with a reticulate supra-layer. All other pollen features that can be observed under LM and SEM comparable to that observed in two of the modern species: Sclerosperma mannii and S. walkeri.
Holotype
IPUW 7513/223 ().
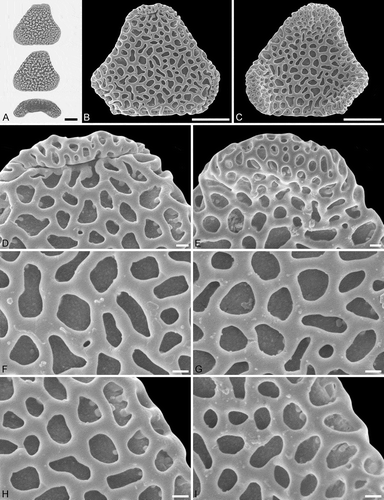
Figure 3. Light microscopy (LM) (A) and scanning electron microscopy (SEM) (B–I) micrographs of Sclerosperma protomannii sp. nov. (holotype: IPUW 7513/223). A. Pollen grain in polar view (upper in high focus and middle in optical cross-section) and equatorial view (lower). B. Pollen grain in polar view, distal side. C. Pollen grain in polar view, proximal side. D. Close-up of apex with aperture, distal side. E. Close-up of apex, proximal side. F. Close-up of central polar area, distal side. G. Close-up of central polar area, proximal side. H. Close-up of interapertural area, distal side. G. Close-up of interapertural area, proximal side. Scale bars – 10 µm (A–C), 1 µm (D–I).
Paratypes
PUW 7513/224 to IPUW 7513/226 (, ).
Figure 4. Light microscopy (LM) (A) and scanning electron microscopy (SEM) (B–E) micrographs of Sclerosperma protomannii sp. nov. (paratype: IPUW 7513/224). A. Pollen grain in polar view (optical cross-section). B. Pollen grain in polar view, distal side. C. Pollen grain in polar view, proximal side. D. Close-up of central polar area, distal side. E. Close-up of interapertural area, proximal side. LM (F) and SEM (G–J) micrographs of S. protomannii sp. nov. (paratype: IPUW 7513/225). F. Pollen grain in polar view (high focus). G. Pollen grain in polar view, distal side. H. Pollen grain in polar view, proximal side. I. Close-up of apex with aperture, distal side. J. Close-up of apex, proximal side. Scale bars – 10 µm (A–C, F–H), 1 µm (D, E, I, J).
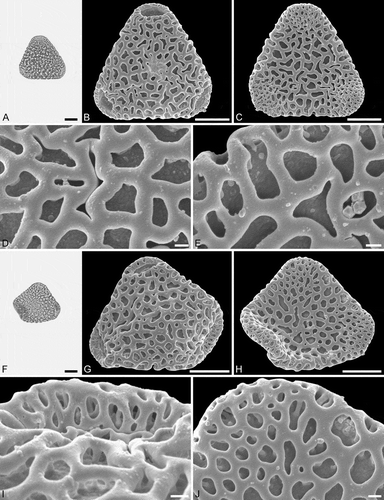
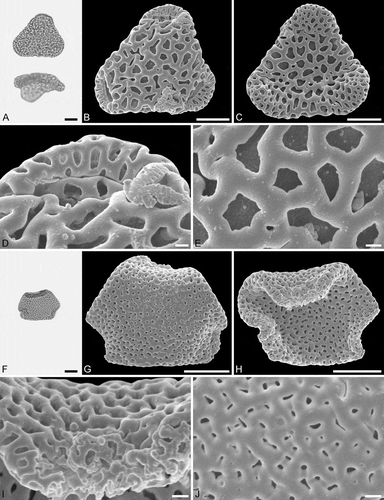
Figure 5. Light microscopy (LM) (A) and scanning electron microscopy (SEM) (B–E) micrographs of Sclerosperma protomannii sp. nov. (paratype: IPUW 7513/226). A. Pollen grain in polar view (upper, high focus) and equatorial view (lower). B. Pollen grain in polar view, distal side. C. Pollen grain in polar view, proximal side. D. Close-up of apex with aperture, distal side. E. Close-up of central polar area, distal side. LM (F) and SEM (G–J) micrographs of S. protoprofizianum sp. nov. (holotype: IPUW 7513/227). F. Pollen grain in polar view (high focus). G. Pollen grain in polar view, distal side. H. Pollen grain in polar view, proximal side. I. Close-up of apex with aperture, distal side. J. Close-up of central polar area, distal side. Scale bars – 10 µm (A–C, F–H), 1 µm (D, E, I, J).
Type locality
Guang River, Chilga, North Gondar Zone, Amhara Region, north-western Ethiopia; c. 12° 30′ N, 37° 7′ E.
Stratigraphy
Chilga strata ().
Age
Late Oligocene, 28–27 Ma (Kappelman et al. Citation2003).
Species epithet
After the extant species, Sclerosperma mannii, that also shows a clear reticulate sculpture in SEM.
Description
Pollen, monad, heteropolar, polar/equatorial (P/E) ratio oblate, outline straight-triangular to slightly concave-triangular in polar view, bean-shaped in equatorial view (convex distal face versus concave proximal face); equatorial diameter 27–38 µm in LM, 24–35 µm in SEM, polar axis 9–12.5 µm in LM; triporate, pori positioned sub-apically on the distal polar face, pori elliptic, 5.5–8.0 µm in diameter, pori equipped with opercula; exine 1.7–2.5 µm thick in LM, nexine thinner than sexine; pollen wall semitectate; sculpture reticulate in LM, reticulate to perforate in SEM; distal face reticulate with broad muri and elliptic to polygonal lumina, 10–20 lumina per 100 µm2 at central distal face, nanogemmae free-standing columellae extremely rare and singular when present (SEM); proximal face reticulate to perforate, lumina/perforations elliptic to polygonal, nanogemmae free-standing columellae extremely rare and singular when present, central polar area and mesoporium reticulate, sculpture becoming microreticulate to perforate towards apices; opercula with a distinct reticulate supra-layer (SEM).
Remarks
The outline of this fossil pollen type in both polar and equatorial view, along with its reticulate sculpture, distally placed apertures and reticulate opercula, places it firmly within the extant genus Sclerosperma (). Of the four pollen morphologies produced by extant Sclerosperma (see Grímsson et al. Citation2018), the S. protomannii sp. nov. pollen is most similar to that of S. mannii and S. walkeri. Both these taxa produce distinctly reticulate pollen with broad muri and elliptic to polygonal lumina. Still, the S. protomannii sp. nov. pollen is much smaller than the S. walkeri pollen and equal in size only to the smallest of the S. mannii pollen (). The S. protomannii pollen has up to 20 lumina per 100 µm2 at the central distal pole, but both S. mannii and S. walkeri can have up to 25 lumina per 100 µm2. Also, S. mannii and S. walkeri pollen has up to six nanogemmae free-standing columellae per lumina, but the lumina in S. protomannii sp. nov. rarely have any free-standing columellae, and when present they are tiny and singular. Additionally, the operculum of S. protomannii sp. nov. has a reticulate supra-layer versus microreticulate in both S. mannii and S. walkeri.
Since this is the first fossil Sclerosperma pollen ever described using SEM, and the fossil pollen record for this genus is nearly non-existent (see Discussion; ), comparisons are limited. The only illustrated fossil Sclerosperma pollen grains, published by Medus (Medus Citation1975, plate 4, figures 2 and 3), are badly preserved, and in the one focus level provided it is hard to distinguish if the two LM micrographs show reticulate or rugulate sculpture.
Table II. Fossil record of Sclerosperma
Species Sclerosperma protoprofizianumGrímsson et R. Zetter, sp. nov.
(, )
Diagnosis
Pollen straight-triangular in outline; semitectate to tectate pollen wall; microreticulate to perforate sculpture at distal face; 50–65 lumina per 100 µm2 at central distal face; elliptic to circular to polygonal or slit-like lumina/perforations; opercula with microreticulate supra-layer. Most other pollen features that can be observed under LM and SEM comparable to that observed in two pollen types (A and C) of the modern species: Sclerospermaprofizianum.
Holotype
IPUW 7513/227 ().
Paratypes
IPUW 7513/228 and IPUW 7513/229 ().
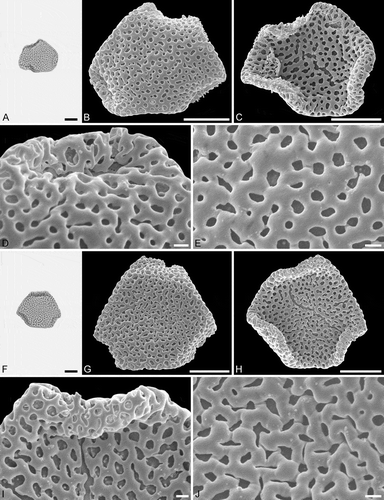
Figure 6. Light microscopy (LM) (A) and scanning electron microscopy (SEM) (B–E) micrographs of Sclerosperma protoprofizianum sp. nov. (paratype: IPUW 7513/228). A. Pollen grain in polar view (high focus). B. Pollen grain in polar view, distal side. C. Pollen grain in polar view, proximal side. D. Close-up of apex with aperture, distal side. E. Close-up of central polar area, distal side. LM (F) and SEM (G–J) micrographs of S. protoprofizianum sp. nov. (paratype: IPUW 7513/229). F. Pollen grain in polar view (high focus). G. Pollen grain in polar view, distal side. H. Pollen grain in polar view, proximal side. I. Close-up of apex, proximal side. J. Close-up of central polar area, distal side. Scale bars – 10 µm (A–C, F–H), 1 µm (D, E, I, J).
Type locality
Guang River, Chilga, North Gondar Zone, Amhara Region, north-western Ethiopia; c. 12° 30′ N, 37° 7′ E.
Stratigraphy
Chilga strata ().
Age
Late Oligocene, 28–27 Ma (Kappelman et al. Citation2003).
Species epithet
After the extant species, Sclerosperma profizianum, that also shows a clear microreticulate to perforate sculpture in SEM.
Description
Pollen, monad, heteropolar, P/E ratio oblate, outline straight-triangular in polar view, bean-shaped in equatorial view (convex distal face versus concave proximal face); equatorial diameter 23–35 µm in LM, 21–29 µm in SEM, polar axis 9–12 µm in LM; triporate, pori positioned sub-apically on the distal polar face, pori elliptic, 6.0–8.0 µm in diameter, pori equipped with opercula; exine 1.7–2.5 µm thick in LM; pollen wall semitectate to tectate; sculpture reticulate in LM, microreticulate to perforate in SEM; distal face microreticulate to perforate with broad muri and elliptic to polygonal lumina or circular to slit-like perforations, 50–65 lumina/perforations per 100 µm2 at central distal face, free-standing columellae rare, up to four nanogemmae free-standing columellae per lumina when present (SEM); proximal face microreticulate to perforate, lumina/perforations elliptic to circular to polygonal or slit-like, free-standing columellae rare, up to four nanogemmae free-standing columellae per lumina when present, central polar area and mesoporium microreticulate to perforate, sculpture becoming nanoreticulate to perforate towards apices; opercula with nanoverrucate to granulate sublayer and a microreticulate supra-layer (SEM).
Remarks
The morphology of this fossil pollen type also clearly places it within Sclerosperma (). Of the four extant pollen types it is most similar to S.profizianum Type A and S.profizianum Type C (see Grímsson et al. Citation2018), and could be considered an intermediate form between those two extant pollen types. The S. protoprofizianum sp. nov. differs from the S.profizianum Type A and Type C pollen in both outline and size. The S.protoprofizianum sp. nov. pollen has only been recovered showing a straight-triangular outline in polar view, but both S.profizianum Type A and Type C pollen is also concave-triangular or convex-triangular. The S. protoprofiziana sp. nov. pollen is smaller than both the S.profizianum Type A and Type C pollen. The sculpture at the central distal face of S.protoprofizianum sp. nov. ranges from microreticulate to perforate and the pollen wall is therefore considered semitectate to tectate versus microreticulate and semitectate in S.profizianum Type A versus perforate and tectate in S. profizianum Type C. The S.protoprofizianum sp. nov. pollen has 50–65 lumina/perforations per 100 µm2 at the central distal face, but S.profizianum Type C has 45–55 per 100 µm2 and S.profizianum Type A has only 35–45 per 100 µm2. Also, outline of the lumina in S.protoprofizianum sp. nov. is varying and overlapping with that observed in both S.profizianum Type A and Type C. The supra-layer of the operculum is clearly microreticulate in S.protoprofizianum sp. nov., but reticulate in S.profizianum Type A and perforate in S.profizianum Type C.
As mentioned above, the scant fossil record (see Discussion) makes it impossible to compare these fossil pollen grains to fossil material other than the Sclerosperma protomannii sp. nov. described herein. The two new fossil taxa are easily distinguished using either LM or SEM. The S. protomannii sp. nov. pollen appears coarsely reticulate in LM, but the S. protoprofiziana sp. nov. pollen is very finely reticulate and usually smaller. Also, the S.protoprofizianum sp. nov. pollen is straight-triangular in outline, but often with the apices infolded towards the proximal side, giving the pollen a hexagonal appearance. In SEM, the S. protomannii sp. nov. pollen is mostly reticulate with 10–20 lumina per 100 µm2 at central distal face, but the S.protoprofizianum sp. Nov. pollen is microreticulate to perforate with 50–65 lumina/perforations per 100 µm2 at central distal face.
Subfamily Arecoideae Burnett
()
Diagnosis
The unarmed leaflets and reduplicate plication indicates that the fossil belongs to either the subfamily Arecoideae or Ceroxyloideae.
Specimen numbers
CH41-9A and CH41-9B (part and counterpart).
Locality
Sublocality CH41 of the Guang River flora. Guang River, Chilga, North Gondar Zone, Amhara Region, northwestern Ethiopia; c. 12° 30′ N, 37° 7′ E.
Stratigraphy
Chilga strata ().
Age
Late Oligocene, 28–27 Ma (Kappelman et al. Citation2003).
Description
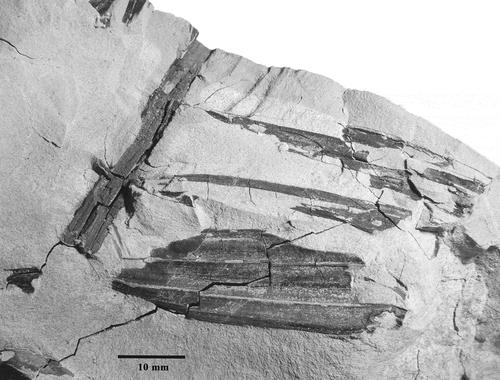
A compound palm leaf fragment preserved as a compression, with one leaflet attached to a rachis, and two additional leaflets on the same side that appear to have been attached to the same rachis. Leaflets and rachis are exposed. Venation indicates the leaflets are reduplicate, with splitting apparently having been along the minor abaxial ribs. There are three, more prominent, adaxial ribs preserved on the largest of the leaflets, but the specimen is incomplete and this is a minimum number. In addition, the dimensions of the original leaf, including its rachis and leaflets are unknown. The fragmentary, largest leaflet on the specimen measures approximately 40 mm long by 16 mm wide.
Remarks
The unprotected leaflets and reduplicate plication indicates the fossil belongs to either the subfamily Arecoideae or Ceroxyloideae. Extant Ceroxyloideae palms are absent from the African continent, but the genus Ravenea is endemic to Madagascar and the Comoros (Dransfield & Beentje Citation1995), and the monotypic Oraniopsis is endemic to Queeensland, Australia (Dransfield et al. Citation2008). Both Ravenea and Oraniopsis differ from the fossil by having a prominent midrib on the leaflets that is thicker and wider than exmedial primary veins. Among living Arecoideae, Africa has very few genera and species: those found on the mainland are Elaeis, Podococcus, and Jubaeopsis, all of which are monotypic, and Sclerosperma with three species as discussed herein. The monotypic genus, Dypsis, occurs on the island of Pemba (Dransfield Citation1988; Govaerts & Dransfield Citation2005). The fossil resembles Elaeis, Podococcus, and Dypsis in having pinnately-compound leaves with unarmed leaflets, but differs from Elaeis and Dypsis leaflets which have prominent midribs, and from Podococcus leaflets which are distinctively wedge-shaped (Dransfield et al. Citation2008). Jubaeopsis leaflets possess a prominent single reduplicate fold, also unlike the fossil. If the fossil accurately represents leaflets and rachises occurring on the whole plant, then Sclerosperma is the only extant African palm taxon that resembles the fossil leaflet material.
Discussion
Fossil record of Sclerosperma
The macrofossil and pollen records of palms (Arecaceae) have been summarised in detail by e.g. Muller (Citation1981), Harley (Citation1996, Citation2004, Citation2006), Harley and Baker (Citation2001), Pan et al. (Citation2006), Dransfield et al. (Citation2008) and Martínez et al. (Citation2016). These summaries show that there have been several fossil pollen taxa (e.g. Constantinisporis Belsky, Dorreenipites Biswas, Retitrilatiporites Misra, Singh et Ramanujam, Trilatiporites Ramanujam, Victorisporis Belsky, Boltenhagen & Potonie; Misra et al. Citation1996), from the Upper Cretaceous to late Cainozoic (Miocene), affiliated with extant Sclerosperma. Most of these affiliations were rejected by Harley and Baker (Citation2001) and Harley (Citation2004, Citation2006). The LM and SEM based pollen morphology of extant Sclerosperma presented by Grímsson et al. (Citation2018) also supports the rejection of any affiliation of these fossil pollen grains with extant Sclerosperma, and excludes all previous fossil pollen accounts except for a single record. The only convincing fossil pollen record of Sclerosperma, prior to this study (), is that from the late Miocene of Senegal (West Africa) by Medus (Citation1975, plate 4, figures 2 and 3). The macrofossil record is also scant (), with only a single find including leaves with cuticles presumed to be of Miocene age from the DRC (Lakhanpal Citation1966, plate 1, figure 1; plate 2, figure 1; plate 3, figures 1 and 2). This provides little information on which to base palaeophytogeographic interpretations, but the Chilga fossils demonstrate that Sclerosperma can be traced back to the late Oligocene of East Africa, its earliest fossil record, and that it most likely had a tropical transcontinental distribution across Africa during the late Oligocene/early Miocene.
Origin and evolution of Sclerosperma pollen
Molecular phylogenetic studies estimate that the Arecaceae evolved (stem node/lineage) and started to diverge (crown node age) around 120 to 100 Ma (e.g. Janssen & Bremer Citation2004; Couvreur et al. Citation2011; Baker & Couvreur Citation2013a, Citation2013b; Hertweck et al. Citation2015). This scenario seems to fit quite well with the age of the earliest palm fossils summarised in Harley (Citation2006) and Dransfield et al. (Citation2008), that date back to the Aptian (125–113 Ma; ICS Citation2017) and/or Albian (113–100 Ma; ICS Citation2017). According to these authors palm fossils apparently become more frequent and diverse in Coniacian (c. 90–86 Ma; ICS Citation2017) sediments, and especially in strata of Maastrichtian age (c. 72–66 Ma; ICS Citation2017). Molecular dating analyses also suggest the crown node age of subfamily Arecoideae to be 75–70 Ma (Baker & Couvreur Citation2013b), and that Sclerosperma diverged from Orania, its potential sister genus in the early Oligocene, 35–30 Ma (see figure 2 in Roncal et al. Citation2010, and figure 4 in Baker & Couvreur Citation2013b), at the latest. This allows for a potential intrageneric divergence period in Sclerosperma of about 20 million years prior to the deposition of the Chilga fossils. It is interesting that the two genera, Podococcus G. Mann et H. Wendl. (tribe Podococceae) and Orania Zipp. (tribe Oranieae), believed to be genetically closest to Sclerosperma, both produce only monosulcate pollen (Dransfield et al. Citation2008). A sulcus is the basic aperture type in Arecaceae, and suggests that the last common ancestor of Orania, Podococcus and Sclerosperma also produced sulcate pollen. Erdtman and Singh (Citation1957) suggested an evolutionary transition in apertures from sulcate to trichotomosulcate, to triporate in Sclerosperma, showing drawings of non-mature Sclerosperma pollen with an apparent ‘crypto-trichotomosulcus’. No such features were observed by Grímsson et al. (Citation2018) in their study on extant Sclerosperma pollen, but this kind of evolutionary trend seems quite plausible and was also recognised by Harley (Citation1999) and Harley and Baker (Citation2001, figure 109). Still, the morphology of the earliest Sclerosperma pollen, and documentation of early morphological evolutionary trends in the pollen of this genus, will only be possible through the study of Eocene to Oligocene fossils from (palaeo) tropical Africa. Based on the fossil material presented here, and the LM and SEM pollen morphology of extant Sclerosperma by Grímsson et al. (Citation2018), it is clear that most sculpture features observed in the extant taxa were already present during the late Oligocene. The fossil material from the late Oligocene, 28–27 Ma (Kappelman et al. Citation2003), of Chilga shows that African Sclerosperma had already diverged into at least two clearly defined morphological lineages: a coarsely reticulate lineage leading to both S. mannii and S. walkeri, and a microreticulate/perforate lineage leading to the S. profizianum (pollen Type A, B and C) species complex.
Sclerosperma and the palaeovegetation – a match or mismatch
There are several reports of macrofossils, and a few about fossil pollen and spores, recorded from the Chilga sediments exposed along the Guang River. The macrofossil record is derived from two main strata shown in : one is the dated ash bed (actually a series of repeated ashfalls) at 96–97 m above the base of the section, and the other is the stratum that produced the unknown Arecoid palm leaf fragment discussed above (at 30.5 m above the base of the measured section; ). The ash fall (paleo) surfaces were apparently quickly colonised by ferns and allies including Blechnum (Blechnaceae), Marsilea (Marsileaceae), Acrostichum (Pteridaceae), Salvinia (Salviniaceae), Cyclosorus (Thelypteridaceae) and Equisetum (Equisetaceae) (García Massini et al. Citation2006, Citation2010; García Massini Citation2009). Monocots found in some horizons among the ash deposits included Pandanites (Pandanaceae), Typha (Typhaceae), and palms including Hyphaene (García Massini Citation2009; García Massini et al. Citation2010), along with rare dicots. The richly fossiliferous horizon at 30.5 m above the base of the section (), also referred to as the Guang River Flora, includes a variety of palm (Arecaceae) macrofossils including Eremospatha chilgaensis (subfamily Calamoideae; tribe Calameae; subtribe Ancistrophyllinae), Hyphaene kappelmannii (subfamily Coryphoideae; tribe Borasseae; subtribe Hyphaeninae), along with a few other monocots, including Scadoxus (Amaryllidaceae), and Dioscorea wilkinii (Dioscoreaceae; Pan et al. Citation2006, Citation2014; Pan Citation2007). The dicot record is richer, comprising among others Sorindeia (Anacardiaceae), Alstonia (Apocynaceae), Tetracera (Dilleniaceae), Macaranga (Euphorbiaceae), Afzelia afro-arabica and Cynometra chaka (Fabaceae), Ocotea (Lauraceae), Strychnos (Loganiaceae), Cola amharaensis (Malvaceae), Eugenia (Myrtaceae), Vepris and Clausena (Rutaceae), Cardiospermum (Sapindaceae), Pouzolzia (Urticaceae), and Sapotaceae (Pan Citation2007, Citation2010; García Massini Citation2009; Pan & Jacobs Citation2009; García Massini et al. Citation2010; Pan et al. Citation2010, Citation2014; Currano et al. Citation2011). Many of the macrofossils, all of which are compressions, are well preserved and with cuticles. Detailed analyses of some of these taxa show that their potential modern relatives are typical of tropical to subtropical African riparian forest or flooded forest communities of West, Central, and East Africa (e.g. Pan et al. Citation2014).
The pollen identified so far has mostly been analysed using LM only. The majority of the taxa mentioned by Yemane et al. (Citation1985, Citation1987), as well as all the taxa listed by García Massini (Citation2009) and García Massini and Jacobs (Citation2011), are not accompanied by micrographs and therefore excluding the possibility of validation or revision. The micrographs provided by Yemane et al. (Citation1987) and Danehy (Citation2010) suggest that some of the pollen are assigned to incorrect families and genera, or show that the features observed in LM do not allow for a particular species/genus/family affiliation. Still, the palynflora contains many thermophilous components that are typical of tropical to subtropical climates (Yemane et al. Citation1985, Citation1987; García Massini Citation2009; Danehy Citation2010; García Massini & Jacobs Citation2011).
Stratigraphic and sedimentological studies of the Chilga basin suggest a heterogeneous late Oligocene landscape, determined primarily by lateral variations in the water table, with shallow gradient rivers affecting vegetation units via erosion, flooding and deposition (Jacobs et al. Citation2005). Both plant macrofossils and fossil pollen/spores, along with sedimentological and insect damage analysis from different sublocalites within the Chilga basin, reflect varying wetland and mesic forest habitats (floodplain, stream margins, ponds, swamps, bogs, as well as various successional stages of a tropical palaeoforest (Jacobs et al. Citation2005; Currano et al. Citation2011; García Massini & Jacobs Citation2011).
All three extant Sclerosperma species have a restricted distribution confined to the Guineo-Congolian Region of West and Central Africa. Sclerosperma mannii has a disjunct distribution with a population in Liberia, and from southeast Nigeria southward to the DRC, and as far east as the border area of the DRC and Rwanda (van Valkenburg et al. Citation2008). This species has also been reported from the island of Bioko (Guinea López Citation1946). It is found in shrub layers of lowland evergreen rainforests, ranging from vegetation occurring just behind the mangroves, swamp forests, periodically flooded forests to valley bottom forests at higher elevations, and persisting in secondary growth up to 1400 m elevation (van Valkenburg et al. Citation2008). Sclerosperma profizianum has a clear disjunct distribution with a population in southwest Ghana and another in the larger tributary of the Congo River, including the extreme southwest of Gabon (van Valkenburg et al. Citation2008; Bourobou Bourobou et al. Citation2016). It is found on relatively dry patches in swampy areas, valley bottom forests or forests that are waterlogged and/or along streams. Sclerosperma walkeri occurs in the interior of Gabon and along the lower reaches of the Congo River. It grows in lowland evergreen rainforests, ranging from swamp forests, periodically flooded forests to lower slopes on terra firma, and persisting in secondary growth at 300–400 m elevation. This species appears to prefer terra firma forests as opposed to S. mannii (van Valkenburg et al. Citation2008).
It is interesting that the current ecology of Sclerosperma seems to fit perfectly with the visualised palaeoenvironment, habitats and vegetation units during the late Oligocene at Chilga, as suggested by previous studies based on various geological and palaeontological proxies (Jacobs et al. Citation2005; Currano et al. Citation2011; García Massini & Jacobs Citation2011; Pan et al. Citation2014). During the late Oligocene, Sclerosperma was most likely an important component of the paleovegetation, growing along streams, in swampy areas and on most available periodically flooded substrates.
Conclusion and outlook
The discovery of pollen representing two extinct species of Sclerosperma in strata of late Oligocene age in north-western Ethiopia, demonstrates a much wider distribution for this genus in the past, and provides a minimum age for its evolutionary diversification, which led to its unusual triporate morphology. Today, Sclerosperma is restricted to the Guineo-Congolian forested regions of West and Central Africa, and is known to prefer edaphically wet habitats. Thus, the genus shows ecological conservatism, as the pollen was found in an organic-rich, lignitic, matrix, and plant macrofossils from other deposits in the Guang River section were interpreted as representing moist and/or seasonally inundated forests similar structurally to those of modern West, Central, and eastern Africa. The primary difference between modern communities that may serve as living analogues, and the Chilga forests is the common presence of palms during the late Oligocene, even in terra firma sediments. Further resolution and the evolution (and extinctions) of African Arecaceae, and their ecological role during the Paleogene, must be resolved through additional palaeobotanical research.
Acknowledgements
This study was funded by the Austrian Science Fund (FWF) with a grant to FG, project number P29501-B25. Field research was funded by the National Science Foundation (NSF) grant EAR0617306 to BFJ and NT, and done in cooperation with the National Museum of Ethiopia and the Authority for Research and Conservation of Cultural Heritage, Addis Ababa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- APG IV 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20.
- Baker WJ, Couvreur TLP 2013a. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography 40: 274–285. doi:10.1111/j.1365-2699.2012.02795.x.
- Baker WJ, Couvreur TLP 2013b. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. Diversification history and origin of regional assemblages. Journal of Biogeography 40: 286–298. doi:10.1111/j.1365-2699.2012.02794.x.
- Bourobou Bourobou PH, Niangadouma R, Issembe Y, Couvreur TLP 2016. Two new records of palm species for Gabon: Sclerosperma profizianum Valk. & Sunder. and Eremospatha quiquecostulata Becc. Biodiversity Data Journal 4: e10187. doi:10.3897/BDJ.4.e10187.
- Couvreur TLP, Baker WJ 2013. Tropical rain forest evolution: Palms as a model group. BMC Biology 11: 48. doi:10.1186/1741-7007-11-88.
- Couvreur TLP, Forest F, Baker WJ 2011. Origin and global diversification patterns of tropical rain forests: Inferences from a complete genus-level phylogeny of palms. BMC Biology 9: 44. doi:10.1186/1741-7007-9-44.
- Currano ED, Jacobs BF, Pan AD, Tabor NJ 2011. Inferring ecological disturbance in the fossil record: A case study from the late Oligocene of Ethiopia. Palaeogeography, Palaeoclimatology, Palaeoecology 309: 242–252. doi:10.1016/j.palaeo.2011.06.007.
- Danehy DR 2010. Terrestrial vegetation reconstructions spanning the Paleogene – Neogene boundary in the Ethiopian highlands. PhD Thesis, Southern Methodist University, Dallas, TX.
- Dransfield J. 1988. The palms of Africa and their relationships. In: Goldblatt P, Lowry PP, eds. Systematic studies in African botany, 95–103. St Louis, MO: Missouri Botanical Garden Press.
- Dransfield J, Beentje HJ. 1995. The palms of Madagascar. Kew, UK: Royal Botanic Gardens Press.
- Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. 2005. A new phylogenetic classification of the palm family, Arecaceae. Kew Bulletin 60: 559–569.
- Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. 2008. Genera Palmarum. The evolution and classification of palms. Kew, UK: Kew Publishing.
- Ellis B, Daly DC, Hickey LJ, Johnson KR, Mitchell JD, Wilf P, Wing SL. 2009. Manual of leaf architecture. New York: Cornell University Press.
- Engel MS, Pan AD, Jacobs BF. 2013. A termite from the Late Oligocene of northern Ethiopia. Acta Palaeontologica Polonica 58: 331–334.
- Erdtman G, Sing G 1957. On the pollen morphology in Sclerosperma mannii. Bulletin du Jardin botanique de l’État a Bruxelles 27: 217–220. doi:10.2307/3666958.
- Faye A, Deblauwe V, Mariac C, Richard D, Sonké B, Vigouroux Y, Couvreur TLP 2016. Phylogeography of the genus Podococcus (Palmae/Arecaceae) in Central African rain forests: Climate stability predicts unique genetic diversity. Molecular Phylogenetics and Evolution 105: 126–138. doi:10.1016/j.ympev.2016.08.005.
- García Massini JL 2009. Paleoenvironmental reconstruction of late Oligocene deposits from the northwestern Ethiopian Plateau. PhD Thesis, Southern Methodist University, Dallas, TX.
- García Massini JL, Jacobs BF 2011. The effects of volcanism on Oligocene-age plant communities from the Ethiopian Plateau, and implications for vegetational resilience in a heterogeneous landscape. Review of Palaeobotany and Palynology 164: 211–222. doi:10.1016/j.revpalbo.2010.12.003.
- García Massini JL, Jacobs BF, Pan A, Tabor NJ, Kappelman J 2006. The occurrence of the fern Acrostichum in Oligocene volcanic strata of the northwestern Ethiopian Plateau. International Journal of Plant Sciences 167: 909–918. doi:10.1086/504390.
- García Massini JL, Jacobs BF, Tabor NJ. 2010. Paleobotany and sedimentology of late Oligocene terrestrial strata from the northwestern Ethiopian Plateau. Palaeontologia Electronica 13.1: 6A.
- Govaerts R, Dransfield J. 2005. World checklist of palms. Kew, UK: Royal Botanic Gardens Press.
- Grímsson F, Denk T, Zetter R 2008. Pollen, fruits, and leaves of Tetracentron (Trochodendraceae) from the Cainozoic of Iceland and western North America and their palaeobiogeographic implications. Grana 47: 1–14. doi:10.1080/00173130701873081.
- Grímsson F, van Valkenburg JLCH, Wieringa JJ, Xafis A, Jacobs BF, Zetter R. 2018. Pollen morphology of the African Sclerosperma (Arecaceae). Grana. doi:10.1080/00173134.2018.1519033.
- Guinea López E. 1946. Ensayo Geobotánica de la Guinea Continental Española. Madrid, Spain: Dirección de agricultura de los Territorios Españoles del Golfo de Guinea.
- Halbritter H, Ulrich S, Grímsson F, Weber M, Zetter R, Hesse M, Buchner R, Svojtka M, Frosch-Radivo A. 2018. Illustrated pollen terminology, 2nd Edn. Vienna, Austria: Springer.
- Harley MM 1996. Palm pollen and the fossil record. PhD Thesis, University of East London, London, UK.
- Harley MM. 1999. Tetrad variation: Its influence on pollen form and systematics in the Palmae. In: Kurmann MH, Hemsley AR, eds. Evolution of plants architecture, 289–304. Kew, UK: Kew Publishing.
- Harley MM 2004. Triaperturate pollen in the monocotyledons: Configurations and conjectures. Plant Systematics and Evolution 247: 75–122. doi:10.1007/s00606-003-0107-x.
- Harley MM 2006. A summary of fossil records for Arecaceae. Botanical Journal of the Linnean Society 151: 39–67. doi:10.1111/boj.2006.151.issue-1
- Harley MM, Baker WJ 2001. Pollen aperture morphology in Arecaceae: Application within phylogenetic analyses, and a summary of the fossil record of palm-like pollen. Grana 40: 45–77. doi:10.1080/00173130152591877.
- Harley MM, Dransfield J 2003. Triporate pollen in the Arecaceae. Grana 42: 3–19. doi:10.1080/00173130310008535.
- Harley MM, Hall DH. 1991. Pollen morphology of the African palms. Palaeoecology of Africa and the surrounding islands 22: 11–25.
- Hertweck KL, Kinney MS, Stuart SA, Maurin O, Mathews S, Chase MW, Gandolfo MA, Pires JC 2015. Phylogenetics, divergence times and diversification from three genomic partitions in monocots. Botanical Journal of the Linnean Society 178: 375–393. doi:10.1111/boj.2015.178.issue-3.
- Hickey LJ 1973. Classification of the architecture of dicotyledonous leaves. American Journal of Botany 60: 17–33. doi:10.1002/j.1537-2197.1973.tb10192.x.
- ICS 2017. International Chronostratigraphic Chart v2017/02. http://www.stratigraphy.org/ICSchart/ChronostratChart2017-02.pdf
- Jacobs BF, Tabor N, Feseha M, Pan A, Kappelman J, Rasmussen T, Sanders W, Wiemann M, Crabaugh J, Massini JLG. 2005. Oligocene terrestrial strata of northwestern Ethiopia: A preliminary report on paleoenvironments and paleontology. Palaeontologia Electronica 8(1): 25A.
- Janssen T, Bremer K 2004. The age of major monocot groups inferred from 800+ rbcL sequences. Botanical Journal of the Linnean Society 146: 385–398. doi:10.1111/j.1095-8339.2004.00345.x.
- Kappelman J, Rasmussen DT, Sanders WJ, Feseha M, Brown T, Copeland P, Crabaugh J, Fleagle J, Glantz M, Gordon A, Jacobs B, Maga M, Muldoon M, Pan A, Pyne L, Richmond B, Ryan T, Seiffert ER, Sen S, Todd L, Wiemann MC, Winkler A 2003. Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature 426: 549–552. doi:10.1038/nature02192.
- Kissling WD, Wolf LE, Baker WJ, Borchseniusa F, Couvreur TLP, Balsleva H, Svenninga J-C 2012. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. PNAS 109: 7379–7384. doi:10.1073/pnas.1120467109.
- Lakhanpal RN. 1966. Some middle Tertiary plant remains from South Kivu, Congo. Annales du Musée Royal de l’Afrique Centrale Serie 8(52): 21–30.
- Mann G, Wendland HA 1864. On the Palms of Western Tropical Africa. Transactions of the Linnean Society of London 24: 421–440. doi:10.1111/j.1096-3642.1863.tb00165.x.
- Martínez LCA, Archangelsky S, Prámparo MB, Archangelsky A 2016. Early Cretaceous palm pollen tetrads from Patagonia, Argentina. Cretaceous Research 59: 129–139. doi:10.1016/j.cretres.2015.10.023.
- Medus J. 1975. Palynologie de sédiments Tertiaires du Sénégal méridional. Pollen et Spores 17: 545–601.
- Misra BK, Singh A, Ramanujam CGK 1996. Trilatiporate pollen from Indian Palaeogene and Neogene sequences: Evolution, migration and continental drift. Review of Palaeobotany and Palynology 91: 331–352. doi:10.1016/0034-6667(95)00070-4.
- Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester, UK: John Wiley and Sons.
- Muller J. 1981. Fossil pollen records of extant angiosperms. The Botanical Review 47: 1–142.
- Pan AD 2007. The Late Oligocene (28–27 Ma) Guang River flora from the northwestern Plateau of Ethiopia. PhD Thesis, Southern Methodist University, Dallas, TX.
- Pan AD 2010. Rutaceae leaf fossils from the Late Oligocene (27.23 Ma) Guang River flora of northwestern Ethiopia. Review of Palaeobotany and Palynology 159: 188–194. doi:10.1016/j.revpalbo.2009.12.005.
- Pan AD, Jacobs BF 2009. The earliest record of the genus Cola (Malvaceae sensu lato: Sterculioideae) from the Late Oligocene (28–27 Ma) of Ethiopia and leaf characteristics within the genus. Plant Systematics and Evolution 283: 247–262. doi:10.1007/s00606-009-0225-1.
- Pan AD, Jacobs BF, Currano ED. 2014. Dioscoreaceae fossils from the Late Oligocene and early Miocene of Ethiopia. Botanical Journal of the Linnean Society 175: 17–28. doi:10.1111/boj.12150.
- Pan AD, Jacobs BF, Dransfield J, Baker WJ 2006. The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Botanical Journal of the Linnean Society 151: 69–81. doi:10.1111/j.1095-8339.2006.00523.x.
- Pan AD, Jacobs BF, Herendeen PS 2010. Detarieae sensu lato (Fabaceae) from the Late Oligocene (27.23 Ma) Guang River flora of north-western Ethiopia. Botanical Journal of the Linnean Society 163: 44–54. doi:10.1111/boj.2010.163.issue-1.
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. doi:10.1016/j.revpalbo.2006.06.008.
- Richards PW. 1973. Africa, the ‘Odd mand out’. In: Meggers BJ, Ayensu ES, Duckworth WD, eds. Tropical forest ecosystems of Africa and South America: A comparative review, 21–26. Washington, DC: Smithsonian Institution Press.
- Roncal J, Borchsenius F, Asmussen-Lanf CB, Balslev H. 2010. Divergence times in the tribe Geonomateae (Arecaceae) coincide with Tertiary geological events. In: Seberg O, Petersen G, Barford AS, Davis JI, eds. Diversity, phylogeny, and evolution in the monocotyledons, 245–265. Aarhus, Denmark: Aarhus University Press.
- Sanders WJ, Kappelmann J, Rasmussen DT. 2004. New large-bodied mammals from the late Oligocene site of Chilga, Ethiopia. Acta Palaeontologica Polonica 49: 365–392.
- van Valkenburg JLCH, Sunderland TCH, Couvreur TLP 2008. A revision of the genus Sclerosperma (Arecaceae). Kew Bulletin 63: 75–86. doi:10.1007/s12225-007-9002-x.
- Yemane K, Bonnefille R, Faure H 1985. Palaeoclimate and tectonic implications of Neogene microflora from the Northwestern Ethiopian highlands. Nature 318: 653–656. doi:10.1038/318653a0.
- Yemane K, Robert C, Bonnefille R 1987. Pollen and clay mineral assemblages of a late Miocene lacustrine sequence from the nortwestern Ethiopian highlands. Palaeogeography, Palaeoclimatology, Palaeoecology 60: 123–141. doi:10.1016/0031-0182(87)90028-9.
- Zetter R. 1989. Methodik und Bedeutung einer routinemäßig kombinierten lichtmikroskopischen und rasterelektonenmikroskopischen Untersuchung fossiler Mikrofloren. Courier Forschungsinstitut Senckenberg 109: 41–50.