Abstract
The palm honey (Sabal yapa C. Wright ex Becc.) (Sabal honey henceforth) is one of 22 unifloral honeys recognised in the Yucatan Peninsula. This honey is harvested in February and March when Sabal yapa bloom but encompasses other important melliferous plants during the harvest season. A melissopalynological study of 24 honey samples from Tizimín, Yucatan (Mexico) was used to determine if Sabal honey is monofloral or multifloral, and to investigate the pollen contribution of other plants. Consequently, we determined the plant resources foraged by Apis mellifera. After analysis, 54 different pollen types were identified with the number of pollen types per honey sample ranging between nine and 21, with a mean of 14.75. Asteraceae and Fabaceae were the most diverse families and represented the highest percentage of contribution in pollen spectra. Sabal yapa pollen had a mean content of 56.9% (with a range of 45.4% to 84%). Bursera simaruba, Haematoxylum campechianum, Piscidia piscipula and Viguiera dentata were categorised as secondary pollen. Important minor pollen corresponded to Caesalpinia gaumeri, Ceiba pentandra, Pisonia aculeata, Thouinia paucidentata, and Trixis inula. Pollen composition revealed details of not only the rich native flora that accompanies Sabal yapa, but traditional human activities that occur around the apiaries. As expected, pollen of nectariferous species predominate in the samples, but also a number of nectarless species were found. Honeybees foraged mostly in trees present in remnants of primary vegetation, or at its different succession stages. Sabal honey could be labelled as monofloral.
The Yucatan Peninsula is the most productive beekeeping region in Mexico (SADER Citation2020). Each year it contributes approximately 35% of the total volume of honey produced in the country (SADER Citation2020). Of the honey produced in this region, between 5% and 20% is destined for domestic consumption, and the rest is exported mainly to European countries (Cruz-Zamudio Citation2017). The high production volumes in the region is due to the great diversity of melliferous species in the area (Villanueva-Gutiérrez Citation2002; Flores Citation2010) and the establishment of sedentary beekeeping (Castañón-Chavarría Citation2009).
Honey harvest season in the Yucatan Peninsula is according to plant flowering time. The season begins in January with Viguiera dentata (Cav.) Spreng., Gymnopodium floribundum Rolfe, Piscidia piscipula (L.) Sarg., Bursera simaruba (L.) Sarg., finishing in June with Lysiloma latisiliquum (L.) Benth. These species are native and have a wide geographical distribution, so honey production volumes are the highest (Villanueva-Gutiérrez et al. Citation2009; Alfaro-Bates et al. Citation2010), however there are approximately 20 more melliferous plants including Sabal yapa C. Wright ex Becc. The restricted populations of Sabal yapa implies that honey volumes are lower but significant for commercial purposes. Sabal yapa is mainly distributed in eastern Yucatan where it flowers mainly from February to March (Zona Citation1990; Alfaro-Bates et al. Citation2010; Ramos-Diaz et al. Citation2015) together with many other species in the region.
Sabal (Arecaceae: Coryphoideae) comprises 16 species (Zona Citation1990), ranging from the Caribbean Islands and south-eastern United States, through Mexico and Central America, to Venezuela (Zona Citation1990). In the Yucatan Peninsula there are four species: Sabal gretherae H.J. Quero, Sabal mauritiformis (H. Karst.) Griseb. et H. Wendl., Sabal mexicana Mart., and Sabal yapa, but only two Sabal mexicana and Sabal yapa are important from an apicultural point of view since they are melliferous and produce a honey labelled as huano honey in two areas, one in Champoton, Campeche and other in Tizimín, Yucatan. Sabal yapa is a native species inhabiting primary and secondary forest in eastern Yucatan (Zona Citation1990; Schultz Citation2005), and it is also a tolerated species in rangelands (Caballero Citation1993; Alfaro-Bates et al. Citation2010). It is known as huano, huano bon, xa’an or bon xa’an in Yucatecan Maya and is a valuable resource for local people to make handcrafts and for roofing traditional houses (Quero Citation1992; Caballero Citation1993).
Sabal yapa can reach up to 25 m tall; their inflorescences are highly branched, densely floriferous and can reach up to 2 m long (). Sabal inflorescences are short lived and function during the daylight hours when honeybees are active, converting Sabal yapa a natural source to feed honey bees. Due to those specialisations S. yapa is classified as a nectariferous and polliniferous species in melissopalynological studies (Porter-Bolland Citation2003; Alfaro-Bates et al. Citation2010; Flores Citation2010). A monofloral honey is usually considered to be produced mainly from one plant if the pollen frequency of that species is ≥ 45% (Louveaux et al. Citation1978; Rodopoulou et al. Citation2018), otherwise the honey is considered multifloral.
Figure 1. Sabal yapa. A. Habit. B. Inflorescence. C. Fruits. D. Petioles detached. Photographs: (A) J. Cuxim; (B-D) A. Suárez-Mariño.

Monofloral honeys have an important commercial value because they are regarded as more valuable, often offered for sale at higher prices than the multifloral honeys (Persano-Oddo & Bogdanov Citation2004). In Yucatan Peninsula there are over 20 monofloral honeys (Villanueva-Gutiérrez et al. Citation2009; Alfaro-Bates et al. Citation2010) that could be marketed under different names, however in international trade they are sold as multifloral due to lack of melissopalynological, physichochemical and organoleptic data. Honey collected in Tizimín and labelled as Sabal honey could be commercialised by beekeepers of Yucatan as long if it follows international melissopalynological standards.
The first recognition of a unifloral honey is made by beekeepers through the careful observation of plant species that are flowering extensively in the field. However, it has been demonstrated that errors in labelling may occur (Luz Citation2001; Luz et al. Citation2008, Citation2020) because many plant species flower simultaneously. Nevertheless, melissopalynological analyses are used to determine the botanical and geographical origin of honeys (Persano-Oddo & Piro Citation2004). We studied the melissopalynological content of commercial honey samples from eastern Yucatan and labelled as Sabal honey to determine if they correspond to the botanical origin declared by beekeepers. Moreover, we analyse the pollen spectra to determine the contribution of other plant species in the honey composition and the floral sources foraged by Apis mellifera L.
Material and methods
Study area
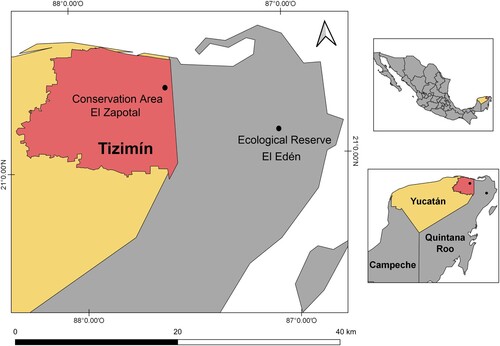
Tizimín is located in northeast of Yucatan () between 20°56ʹand 21°26ʹN and 87°32ʹand 88°32ʹW (INEGI Citation2010). The climate type is Aw2 (x’) i’) g (Orellana et al. Citation2010), warm sub-humid with a rainfall of 1200 mm annually, and percentage of winter rainfall is greater than 10.2, which means that during the cold period of the year, the collision of the westerly winds with the circumpolar winds generates large amounts of humidity and rain (Orellana et al. Citation2010). The mean annual temperature is 26 °C, with rainfall from June to October. The predominant vegetation types are primary and secondary seasonal dry forest, temporarily flooded forest and pastures of introduced grasses (García-Gil & Sosa-Escalante Citation2013).
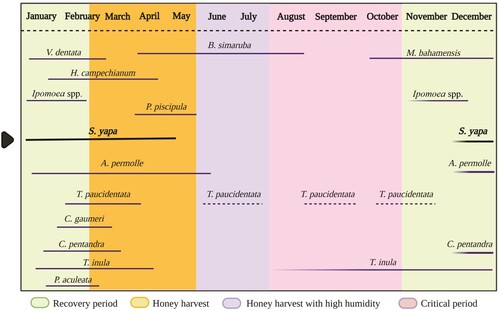
Schultz (Citation2005) listed the most important tree species in the primary forest as Manilkara zapota (L.) P. Royen, Metopium brownei Urb., Lysiloma latisiliquum (L.) Benth., Thrinax radiata Mart., Bursera simaruba, Sebastiania adenophora Pax et K. Hoffm, and Sabal yapa. Secondary seasonal dry forest are characterised by Swartzia cubensis (Britton et P. Wilson) Standl., Lysiloma latisiiquum, Bursera simaruba, Vitex gaumeri Greenm., Lonchocarpus rugosus Benth., Lonchocarpus xuul Lundell, Nectandra coriacea Griseb., Jatropha gaumeri Greenm., and Piscidia piscipula. Shrubs of Pisonia aculeata L., and numerous species of vines of Convolvulaceae and Cucurbitaceae are also found. In the temporarily flooded or tintal forest Haematoxylum campechianum L., Eugenia winzerlingii Standl., Erythroxylum confusum Britton and Byrsonima bucidaefolia Standl. are the most common trees. In the rangelands it is common to find the grass genera Urochloa P. Beauv. and Cynodon Rich. (García-Gil & Sosa-Escalante Citation2013) as well as Sabal yapa, left standing by farmers as a result of management practices (Caballero Citation1993). In this complex landscape there are abundant nectariferous and polliniferous resources (Flores Citation2010; Porter-Bolland Citation2003; Villanueva-Gutiérrez et al. Citation2009) that bloom before, during and after the flowering period of Sabal yapa ().
Figure 3. Flowering periods for some species whose pollen grains were identified in the samples analysed. Based on and modified from Porter-Bolland (Citation2003).
Honey sampling
Twenty-four samples of commercial honey from Tizimín were provided by Bio Mieles del Sureste, a honey collection centre located in Merida, Yucatan in March of 2019. The samples came from beekeeper's packed drums of 300 kg each. During honey collection, the responsible beekeeper was asked about the date of collection and source of the honey according to their knowledge. Although beekeepers themselves recognised them as Sabal honey, colour, aroma and taste were determined in the laboratory to corroborate the similarity parameters with the technical data sheet for Sabal yapa honey elaborated by Alfaro-Bates et al. (Citation2010).
Physicochemical and organoleptical analyses
Moisture and Brix degrees were measured using a portable Refracto-Polarimeter Atago® RePo-4 for honey following the instrument instructions for use. This parameter can affect the taste and aroma of the honeys if it is outside the normal established ranges (Primandasari et al. Citation2021). Colour (Pfund) was determined through a Honey Color photometer Hanna® HI 96785 according to the described procedure for honey (NOM-004-SAG/GAN-2018) as soon as the samples arrived at the laboratory. Monofloral honeys have characteristic colours depending on the floral sources from which they originate and this is one of the most important parameters in price determination. Colour is one of the first parameters to be determined because it can change during storage and exposure to heat (Pereyra et al. Citation1999). The procedure for sensorial evaluation is described in NOM-004-SAG/GAN-2018. Four olfactive and six gustative descriptors were registered.
Melissopalynological analysis
There are different methods for the recovery of pollen present in the honey but we opted for the method of Sawyer (Citation1988), up to step seven because we consider that method allows us to recover more pollen sediment when decanting. It uses the same quantity of honey (10 g) and water (20–30 ml) as Von der Ohe et al. (Citation2004) but instead of using a pointed glass centrifuge tube, the Sawyer method uses two 15 ml tubes. After centrifugation, the liquid from both tubes is decanted. One tube is filled halfway with water and shaken. The mixture is transferred to the other tube and centrifuged again. In order to reduce agglutination of the pollen grains for counting, after the recovery of the sediment, it was transferred to a 1.5 ml Eppendorf tube with five drops of 10% potassium hydroxide (KOH) as described by Caccavari and Cilla (Citation2010). Tubes were incubated in a water bath (90°C for 10 min) and then were vigorously stirred and centrifuged at 978 × g for 5 min. After decanting, the sediment was rinsed with 1.5 ml of distilled water and again centrifuged following the same conditions. Pollen grains were stained with three drops of fuchsin-stained glycerine water and sediment was homogeneously spread over an area equal to that of the coverslip. We also acetolysed samples using the method of Erdtman (Citation1969) modified by Alfaro-Bates et al. (Citation2010) in subsamples to corroborate pollen types present in honey and to confirm the botanical origin.
Pollen grain examination and counts were made using an Olympus BX41 light microscope under ×400 and photographed using an Olympus C5050 digital camera at ×1000. We counted at least 500 pollen grains per sample (Von der Ohe et al. Citation2004). Pollen identification was made by comparison to pollen reference collection of the Universidad Autónoma de Yucatán (UADY-PAL) and by using pollen atlases (Palacios-Chávez et al. Citation1991; Roubik & Moreno Citation1991), pollen guides of the Yucatan peninsula (Sánchez-Dzib Citation2006; Alfaro-Bates et al. Citation2010; Ramos-Diaz et al. Citation2015; Santiago-Briceño Citation2018) and information from websites (http://www.paldat.org/; Weber & Bucchner Citation2021). When it was possible, pollen grains were determined to species, genus or family level. Palynomorphs included pollen types that could not be determined occurred because we did not have enough information to determine even a more precise category, particularly in very numerous and complex botanical families such as the Asteraceae or Convolvulaceae. Therefore, if even a genus was determined, the palynomorphs will be numbered from 1, 2, … n within this genus. The three undetermined palynomorphs were not considered in the analyses.
The percentage of representation of each pollen type was calculated and classified according to the frequency classes proposed by Louveaux et al. (Citation1978): predominant pollen (D = ≥ 45%), secondary pollen (S = 15–45%), important minor pollen (M = 3–15%) and minor pollen (T = 1–3%). We also decided to use the term sporadic pollen (‘+’ = < 1%) for pollen grains that were present < 1% as Fagúndez and Caccavari (Citation2003) used it, because some grains were present in very low quantities. Unifloral honeys were considered to be those samples with clearly defined dominant pollen (≥ 45%) and multifloral honeys where no pollen dominance was observed.
We considered main nectar and pollen resources used by Apis mellifera as well as growth forms tree, shrub and herb to complement the analyses. For this, we follow Porter-Bolland (Citation2003), Villanueva-Gutiérrez et al. (Citation2009), Ramírez-Arriaga et al. (Citation2011), Coh-Martínez et al. (Citation2019) and the website flora digital of the Yucatan peninsula (Centro de Investigación Científica de Yucatán Citation2021). We only included pollen types identifications to species and genus to perform these analyses.
Results
Sabal honey samples were characterised in order to obtain information about their moisture, colour, aroma and taste (Supplementary material I). Colour ranged from 26 mm to 50 mm with an average of 34.95 mm in Pfund scale; moisture content varied from 18% to 20.4% with an average of 19.1%, Brix (°Bx) ranged 78–80.2 with an average of 79.34. The aroma of samples ranged from vegetal, floral and fresh-fruit to warm and woody and the taste ranged from warm, woody, and vegetal to chemical, floral, fresh-fruit and astringent.
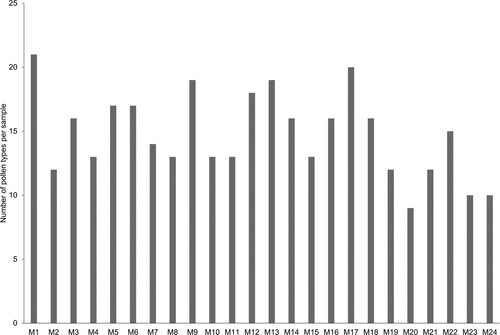
The melissopalynological analysis confirmed the identity of the honey sources indicated by the beekeepers. A total of 54 pollen types were identified (Supplementary material II). From these, 51 were determined to species, genus or family. Three types remained unidentified. The best represented pollen types were 27 and correspond to 14 families (, ). The number of pollen types per honey sample ranged from nine (sample 20) to 21 (sample 1), with an average of 14.75 (). Thirteen samples had more than 15 pollen types, whereas nine had between ten to 14. The remaining three samples contained ten or less pollen types. Sabal yapa and Viguiera dentata were present in all honey samples. An association formed by Trixis inula Crantz, Bursera simaruba, Ipomoea sp. 1, Jacquemontia pentantha (Jacq.) G. Don, Caesalpinia gaumeri Greenm., Piscidia piscipula, Abutilon permolle (Willd.) Sweet, Pisonia aculeata, Thouinia paucidentata Radlk., Amaranthus sp., and Mimosa bahamensis Benth. were also present in at least of 50% of samples.
Figure 4. Some pollen types commonly present in Sabal yapa honey samples. A. Sabal yapa. B. Viguiera dentata. C. Trixis inula. D. Caesalpinia gaumeri. E. Haematoxylum campechianum. F. Piscidia piscipula. G. Bursera simaruba. H. Pisonia aculeata. I. Thouinia paucidentata. J. Mimosa bahamensis. K. Asteraceae 1. L. Ceiba pentandra. Scale bars ‒ 10 µm.
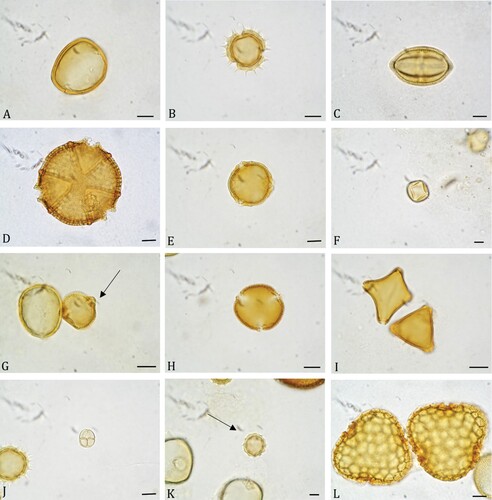
Table I. Main pollen types and their importance in samples of Sabal honey.
The results obtained showed the predominance of Sabal yapa in the pollen spectra ranged from 45.4% in sample 12 to 84% in sample 23, with an average of 56.9%. The pollen of Fabaceae and Asteraceae were present in all samples, while pollen of Convolvulaceae was present in 91.6% of samples. Malvaceae, Burseraceae (Bursera simaruba), Nyctaginaceae (Pisonia aculeata), Sapindaceae (Thouinia paucidentata) and the Amaranthaceae (Amaranthus sp.) were present in more than 50% of the samples.
The most diverse families in pollen types were Fabaceae and Colvulvulaceae with eight species, respectively, and Asteraceae and Malvaceae with five and four, respectively. The pollen from Fabaceae in Sabal honey was very common mainly of Piscidia piscipula, Caesalpinia gaumeri, and Mimosa bahamensis being present in 95.8%, 70.8%, and 58.3% of the samples, respectively. Haematoxylum campechianum and Mimosa pudica were also frequent in samples. In contrast, Convolvulaceae pollen types were always present below 3% in the spectra as minor or sporadic. Although Ipomoea sp.1 and Jacquemontia pentantha were present in 50% of the honey samples, both were categorised as minor. Within Asteraceae, Viguiera dentata and Trixis inula stand out over Vernonia sp. and Asteraceae sp. 1. The malvaceous Abutilon permolle, was present in 50% of samples and was categorised as minor. Pollen grains of Zea mays L. were present indicating apiaries are near cultivated lands. It is noteworthy that the great diversity of the present pollen types is in accordance with their low representation. Twenty-seven pollen types were categorised as sporadic, complementing the pollen spectrum of Sabal honey. Among these, pollen types of the hyperdiverse Ipomoea (4 spp.) stand out.
Nectar and polliniferous plants are valuable sources from an apicultural point of view. Most pollen types found in the honey come from 25 nectariferous plants and only two are polliniferous (Amaranthus sp. and Zea mays), representing 7.40%. Amaranthus sp. was present in 15 samples but only in three it was categorised as minor. Pollen of the crop Zea mays was found in four samples and categorised as important minor only in sample 18. The different stages of succession of the vegetation surrounding the apiaries, include plants growing from several life forms and flowering stages. This allows us to determine through the melissopalynological analysis the availability of feeding resources for honey bees (Apis mellifera) before and during the harvest time of Sabal honey. According to flowering times the main plant resources used by Apis mellifera come from trees, shrubs and herbs of primary and secondary forests. These bloom at the beginning of the year except for Jacquemontia pentantha which bloom well before, from November to January.
Discussion
Sabal yapa is one of the most important wild species in nectar production encompassing other important melliferous plants in Yucatan at the beginning of the honey harvest season. Its flowering time coincides with the flowering of a great diversity of herbaceous, shrub and trees which are present as secondary, important minor, minor or sporadic pollen in samples. The maximum number of pollen types found per sample in this study is slightly higher than those found by Villanueva-Gutiérrez et al. (Citation2009) in the Yucatan peninsula for other unifloral honeys (six to 17 taxa). The greater number of taxa in the Sabal honey samples during the short season may be due to the degree of conservation of the sites where the apiaries are located. Some apiaries where this honey is harvested are located close to a remnant of original vegetation known as ‘El Zapotal’ conservation area. (López-Jiménez et al. Citation2019). According to Villanueva-Gutiérrez (Citation1994) the bee diet is more diversified in areas with little disturbance.
The most diverse families were Asteraceae, Fabaceae, and Convolvulaceae. This agreed with Villanueva-Gutiérrez (Citation2002) and Villanueva-Gutiérrez et al. (Citation2009) in Yucatan Peninsula. Asteraceae and Fabaceae are important as nectar and pollen resources for honey bees and they bloom at different times along the harvest season (Porter-Bolland Citation2003; Flores Citation2010; Coh-Martínez et al. Citation2019), so its high pollen contribution in Sabal honey was expected. It is noteworthy that Fabaceae and Asteraceae are two of the most diverse families in Yucatan flora (Duno-de Stefano et al. Citation2018) and are found in different succession stages within the complex vegetation matrix of eastern Yucatan (López-Jiménez et al. Citation2019). The species diversity of Convolvulaceae contrast with low contribution to honey. At least six of the eight species of Convolvulaceae found in the pollen spectra belong to Ipomoea, a highly diverse genus with approximately 159 and 36 species in Mexico and the Yucatan Peninsula area respectively (Romero-Soler Citation2021). Ipomoea species like many other Convolvulaceae are well adapted to tropical and subtropical regions inhabiting open, sunny areas and their growth is favoured by natural or anthropogenic disturbances (Romero-Soler Citation2021; Schultz Citation2005). They are important plants as nectariferous and polliniferous sources during autumn (October and November) and what is known as the pre-harvest period (December and January, prior to Viguiera dentata bloom) in the Yucatan Peninsula when Ipomoea species and other Convolvulaceae bloom massively (Alfaro-Bates et al. Citation2010; Echazarreta et al. Citation1997; Tapia-Muñoz Citation2011) and therefore pollen grains of these species are present in Sabal honey. Nevertheless, pollen grains of this family are almost under-represented in honey due to their large size (Zavala-Olalde et al. Citation2013; Zerrouk et al. Citation2020) and this is the case for pollen grains of Convolvulaceae in our samples.
Viguiera dentata, Piscidia piscipula and Thouinia paucidentata, were the best represented species in samples from 100% to 83.4%. They are abundant plants in the tropical forest of Yucatan (Schultz Citation2005) and bloom before, during and after Sabal yapa so they are expected to occur in Sabal honey to some extent. Viguiera dentata is a common herb inhabiting open and sunny areas, meanwhile Piscidia piscipula and Thouinia paucidentata are trees of primary and secondary tropical forests. Their presence in three samples (samples 24, 23 and 20, respectively), indicate they provide nectar and pollen constantly in the diet of bees together with Sabal yapa.
Pollen from anemophilous and nectarless plants were identified in honey samples. Amaranthus sp. and Zea mays appeared in important percentages in honey samples and categorised as minor pollen. The presence of pollen from Zea mays and Amaranthus L. reflects agricultural practices near the apiaries (Sánchez-Blanco & Guevara-Féter Citation2013; Vázquez-Fuentes et al. Citation2019). Pollen from maize may have been blown into the honey by the wind accidentally or mixed with nectar collected by the bees (Castellanos-Potenciano et al. Citation2012) but some authors suggest that in periods of scarcity, bees may visit anemophilous plants such as maize to supplement their nutrition (Piedras-Gutiérrez & Quiroz-García Citation2007).
Melissopalynological analysis of honey samples confirms Sabal honey as monofloral since its pollen predominated in all samples. The percentage of occurrence of Sabal yapa pollen grains in the honey samples analysed indicates that this species shows a tendency to over-representation. The over-representation of pollen is observed in plants that produce large quantities of pollen and nectar and are abundant in a region, making them the main resource for bees (Valencia-Cardona & Velázquez-Ruíz Citation2014). Sabal yapa is abundant in primary and secondary tropical forest of eastern Yucatan (Schultz Citation2005) and massively blooming during February and March providing constant flow of pollen and nectar for bees. Sabal yapa has profusely floriferous long (80‒150 cm long) inflorescences (Zona Citation1990), which may cause its pollen to be over-represented in honey, but other human factors also play a role, such as the management of forests and woodlands by Mayan farmers. This palm is tolerated and kept standing together with other tree species that are useful when clearing the land for planting maize, which is why, over time, it has become an abundant element in the area (Pulido & Caballero Citation2006). This trend has also been observed in the monofloral honey of Bursera simaruba and with similar ranges (45‒85%) (Alfaro-Bates et al. Citation2010). This species is one of the most abundant trees and widely distributed in forest of the Yucatan Peninsula, and is frequently used as a living fence for paddock delimitation (Carvajal-Azcorra Citation2004).
The presence of pollen grains of species that do not coincide with the flowering and honey harvesting period of Sabal yapa may be due to several factors, for example the beekeeper's way of harvesting (Herrero et al. Citation2012) and management practices as some beekeepers harvest honey combs with bee bread (Zerrouk et al. Citation2020). In this respect, beekeepers in Yucatan do not usually harvest pollen which can remain in the cells for several months.
In this work, according to the most abundant life forms identified in the melissopalynological analyses, the best represented layer was herbaceous (25), followed by tree (15) and shrub layer (six). This is possibly so, because the herbaceous layer is fast growing and produce flowers faster compared to trees and shrubs. In the Yucatan Peninsula, the pattern of rainfall and drought is strongly delimited, with December to May being the driest period of the year and June to November, the rainy season (Villanueva-Gutiérrez et al. Citation2009). However, the geographical location of the Yucatan Peninsula means that it is influenced by cold fronts (also called ‘nortes’) due to the collision of circumpolar winds with westerly winds from mid-latitude anticyclones. These cold fronts sometimes cause an abrupt drop in temperature with strong winds and winter rains (Orellana et al. Citation2010). These rains during the cold period may be sufficient to allow annual herbaceous plants (as Convolvulaceae species) to bloom and be used by bees as floral resources to feed prior to the period of mass flowering. For this reason, in the Yucatan peninsula, the herbaceous and shrub layer are at their peak in the honey harvested during the month of January and tree layer together with the vines, in the month of February (Villanueva-Gutiérrez et al. Citation2009).
Despite the high number of herbaceous species identified, pollen from herbaceous plants contributed very little to the pollen spectrum of Sabal honey, mostly as sporadic or minor pollen compared to pollen from arboreal species (secondary and important minor pollen). Bourreria pulchra, Bursera simaruba, Caesalpinia gaumeri, Ceiba sp., Coccoloba sp., Cochlospermum vitifolium, Lonchocarpus sp, Mimosa bahamensis, Piscidia piscipula and Thouinia paucidentata are tree species present in secondary vegetation between 10 and 60 years old and some of these species (Coccoloba, Lonchocarpus and species such as Bursera simaruba and Piscidia piscipula) are part of the typical vegetation of mature or primary tropical forest in the Yucatan Peninsula (López-Jiménez et al. Citation2019). Haematoxylum campechianum was also found in some honey samples. This tree species predominate in temporarily flooded rainforest also known as tintal in the Yucatan Peninsula (Chablé-Vega et al. Citation2019; Plasencia-Vázquez et al. Citation2017), so this plant community is also present in the heterogeneous landscape of Tizimín (García-Gil & Sosa-Escalante Citation2013). The fact that pollen from tree species have a higher percentage contribution in the honey samples than pollen from herbaceous plants, can be interpreted as the apiaries are in preserved vegetation or in vegetation with more advanced stages of succession.
Conclusions
The melissopalynological analysis of the commercial samples of Sabal honey revealed that high diversity of pollen types come from nectariferous species of primary and secondary forests. Polliniferous species recorded are associated mainly to agriculture. As it was expected, the main pollen types found come from plants characteristic of tropical forest of Tizimín, thus the botanical and geographical origin of Sabal honey is confirmed. Sabal yapa is one of the most important native species in nectar production and blooms from February to March and significatively contributes to honey production in Yucatan. Efforts should be directed towards the recognition of this honey produced in Tizimín as a quality product of differentiated botanical origin and the conservation of the Sabal yapa individuals for the sustained production of monofloral honey. It is hoped that the palynological findings will serve to establish conservation strategies by strengthening beekeeping in the region and maintaining native plants, now known to be of beekeeping importance.
Supplementary_material_II.docx
Download MS Word (37.6 KB)Supplementary_material_I.docx
Download MS Word (17.1 KB)Acknowledgements
The authors would like to thank the local beekeepers Arturo Canul, Maximiliano Cupul, Manuela Hernández, Rafael Tun, José Cen, Paola Valladares, Jaime Cuxim and specially, Javier Cuxim for their support and for sharing their traditional knowledge about the floral origins of their honeys. To Alexander Suárez-Mariño for the photographs and assistance with the editing of the figures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00173134.2023.2178264.
Additional information
Funding
References
- Alfaro-Bates R, González-Acereto J, Ortiz-Díaz J, Viera-Castro F, Burgos-Pérez A, Martínez Hernández E, Ramírez-Arriaga E. 2010. Caracterización palinológica de las mieles de la Península de Yucatán. Mérida: Universidad Autónoma de Yucatán-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
- Caballero J. 1993. El caso del uso y manejo de la palma de huano (Sabal spp.) entre los mayas de Yucatán, México. In: Leff E, Carabias J, eds. Cultura y manejo sustentable de los recursos naturales, 203–248. Mexico: CII UNAM: Miguel Ángel Porrúa.
- Caccavari M, Cilla G. 2010. Removal new alternative to chemical and mechanical removal for the study of pollen carried on the wild bee scopae. Revista Del Museo Argentino de Ciencias Naturales 12: 255–262. doi:10.22179/REVMACN.12.244.
- Carvajal-Azcorra J. 2004. Establecimiento de postes de Chacah (Bursera simaruba, L. Sarg.) como cerco vivo. Livestock Research for Rural Development 17: http://www.lrrd.org/lrrd17/2/carv17022.htm.
- Castañón-Chavarría E. 2009. Mieles diferenciadas de la Península de Yucatán y su mercado. Mérida: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México, CONABIO.
- Castellanos-Potenciano BP, Ramírez-Arriaga E, Zaldivar-Cruz JM. 2012. Análisis del contenido polínico de mieles producidas por Apis mellifera L. (Hymenoptera: Apidae) en el estado de Tabasco, México. Acta Zoológica Mexicana 28: 13–36. doi:10.21829/azm.2012.281813
- Centro de Investigación Científica de Yucatán. 2021. Flora de la Península de Yucatán. https://www.cicy.mx; accessed 26 September 2021.
- Chablé-Vega MA, Plasencia-Vázquez AH, García-González A, Ferrer-Sánchez Y, Riverón-Giró FB, Zamora-Crescencio P. 2019. Distribución, densidad y estructura dasométrica de Haematoxylum campechianum y Haematoxylum calakmulense en Campeche, México. Ecosistemas y Recursos Agropecuarios 6: 65–77. doi:10.19136/era.a6n16.1784.
- Coh-Martínez ME, Cetzal-Ix W, Martínez-Puc JF, Kumar S, Noguera-Savelli E, Cuevas MJ. 2019. Perceptions of the local beekeepers on the diversity and flowering phenology of the melliferous flora in the community of Xmabén, Hopelchén, Campeche, Mexico. Journal of Ethnobiology and Ethnomedicine 15: 1–16. doi:10.1186/s13002-019-0296-1.
- Cruz-Zamudio A. 2017. Producción de miel convencional y orgánica en la Península de Yucatán. Master’s Thesis, El Colegio de la Frontera Sur, Université de Sherbrooke, Quintana Roo, Mexico.
- Duno-de Stefano R, Ramírez Morillo IM, Tapia-Muñoz JL, Hernández-Aguilar S, Can L, Cetzal-Ix W, Méndez-Jiménez N, Zamora-Crescencio P, Gutiérrez-Báez C, Carnevali-Fernández-Concha G. 2018. Aspectos generales de la flora vascular de la Península de Yucatán, México. Botanical Sciences 96(3): 515–532. doi:10.17129/botsci.1868.
- Echazarreta CM, Quezada-Euán JG, Medina LM, Pasteur KL. 1997. Beekeeping in the Yucatan Peninsula: development and current status. Bee World 78: 115–127. doi:10.1080/0005772X.1997.11099346.
- Erdtman G. 1969. Handbook of palynology. An introduction to the study of pollen grains and spores. Copenhagen: Munksguard.
- Fagúndez GA, Caccavari MA. 2003. Caracterización polínica y organoléptica de algunas mieles monoflorales del centro de la provincia de Entre Ríos, Argentina. Polen 12: 77–95.
- Flores J. 2010. Flora melífera. In: Durán R, Méndez M, eds. Biodiversidad y desarrollo humano en Yucatán. 345 Mérida: CICY: PPD-FMAM: CONABIO: SEDUMA.
- García-Gil G, Sosa-Escalante J. 2013. Ordenamiento Territorial del Estado de Yucatán: Visión 2030. Mérida: Universidad Autónoma de Yucatán, Gobierno del Estado de Yucatán.
- Herrero B, Valencia-Barrera RM, San-Martín R, Pando V. 2002. Characterization of honeys by melissopalynology and statistical analysis. Canadian Journal of Plant Science 82: 75–82. doi:10.4141/P00-187.
- INEGI. 2010. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos municipal 2010 Tizimín. Yucatán: Instituto Nacional de Estadística y Geografía. https://xdoc.mx/preview/prontuario-de-informacion-geografica-municipal-5ebdade63d0d8
- López-Jiménez LN, Durán-García R, Dupuy-Rada JM. 2019. Recuperación de la estructura, diversidad y composición en una selva mediana subperennifolia en Yucatán, México. Madera y Bosques 25(1–17): e2511587. doi:10.21829/myb.2019.2511587.
- Louveaux J, Maurizio A, Vorwohl G. 1978. Methods of Melissopalynology. Bee World 59: 139–157. doi:10.1080/0005772X.1978.11097714.
- Luz CFP. 2001. Determinação da origem geográfica e botânica do mel usando a análise palinológica. O apiário. Revista Do Apiário 160: 14–17.
- Luz CFP, Chaves SAM, Cano CB. 2020. Botanical and geographical origins of honey samples from Pantanal (Mato Grosso and Mato Grosso do Sul states, Brazil) certificated by melissopalynology. Grana 60: 189–216. doi: 10.1080/00173134.2020.1815831.
- Luz CFP, Cano CB, Felsner ML, Mendes JC. 2008. Caracterização botânica e físico-química do mel de Apis mellifera L. do Pantanal Norte (MT). Brasil. In: XII Simpósio de Paleobotânicos e Palinólogos. Florianópolis, SC, Brasil, 2–5 November 2008.
- Orellana R, Espadas C, Nava F. 2010. Climas. In: Durán R, Méndez M, eds. Biodiversidad y desarrollo humano en Yucatán, 10–11. Mérida: CICY: PPD-FMAM: CONABIO: SEDUMA.
- Palacios-Chávez R, Ludlow-Wiechers B, Villanueva-Gutiérrez R. 1991. Flora palinológica de la Reserva de la Biósfera de Sian Ka’an, Quintana Roo, México. Chetumal. México: Centro de Investigaciones de Quintana Roo.
- Pereyra González A, Burin L, Buera MP. 1999. Color changes during storage of honeys in relation to their composition and initial color. Food Research International 32: 185–191. doi: 10.1016/S0963-9969(99)00075-7.
- Persano-Oddo L, Bogdanov S. 2004. Determination of honey botanical origin: problems and issues. Apidologie 35(Suppl. 1): S2–S3. doi:10.1051/apido:2004044.
- Persano-Oddo L, Piro R. 2004. Main European unifloral honeys: descriptive sheets. Apidologie 35: S38–S81. doi:10.1051/apido:2004049.
- Piedras-Gutiérrez B, Quiroz-García DL. 2007. Estudio melisopalinológico de dos mieles de la porción sur del Valle de México. Polibotánica 23: 57–75.
- Plasencia-Vázquez AH, Villegas P, Ferrer-Sánchez Y, Zamora-Crescencio P. 2017. Distribución histórica de las especies del género Haematoxylum (Leguminosae) en la Península de Yucatán, México, basada en ejemplares de herbario. Acta Botánica Mexicana 119: 51–68. doi:10.21829/abm119.2017.1231.
- Porter-Bolland L. 2003. La apicultura y el paisaje maya. Estudio sobre la fenología de floración de las especies melíferas y su relación con el ciclo apícola en La Montaña, Campeche, México. Mexican Studies/Estudios Mexicanos 19: 303–330. doi:10.1525/msem.2003.19.2.303.
- Primandasari EP, Susilo A, Masythoh D. 2021. The effect of moisture content in Nusa Tenggara Timur Forest honey on viscosity, pH and total dissolved solids. IOP Conference Series: Earth and Environmental Science 788: 012108. doi:10.1088/1755-1315/788/1/012108.
- Pulido MT, Caballero J. 2006. The impact of shifting agriculture on the availability of non-timber forest products: the example of Sabal yapa in the Maya lowlands of Mexico. Forest Ecology and Management 222(1): 399–409. doi:10.1016/j.foreco.2005.10.043.
- Quero HJ. 1992. Las palmas silvestres de la Península de Yucatán. Instituto de Biología. In: Universidad Nacional Autónoma de México, Publicaciones especiales 10. Instituto de Biología, 1–63. Mexico: Universidad Nacional Autónoma de México.
- Ramírez-Arriaga E, Navarro-Calvo LA, Díaz-Carbajal E. 2011. Botanical characterisation of Mexican honeys from a subtropical region (Oaxaca) based on pollen analysis. Grana 50: 40–54. doi:10.1080/00173134.2010.537767.
- Ramos-Diaz A, San Román-Ávila D, Noriega-Trejo R, Góngora-Chin R, Sánchez-Contreras A, Rodríguez-Buenfil I. 2015. Catálogo de los principales tipos polínicos encontrados en las mieles producidas en la Península de Yucatán. Mérida: SIIES-CIATEJ-CEDESU.
- Rodopoulou MA, Tananaki C, Dimou M, Liolios V, Kanelis D, Goras G, Thrasyvoulou A. 2018. The determination of the botanical origin in honeys with over-represented pollen: combination of melissopalynological, sensory and physicochemical analysis. Journal of the Science of Food and Agriculture 98: 2705–2712. doi:10.1002/jsfa.8764.
- Romero-Soler KJ. 2021. Entre campanas y colores: sobre las especies de Ipomoea (Convolvulaceae) en la Península de Yucatán, México. Desde el Herbario CICY 13: 12–18. https://www.cicy.mx/Documentos/CICY/Desde_Herbario/2021/2021-01-21-Romero-Soler-Entre-campanas-y-colores.pdf.
- Roubik D, Moreno J. 1991. Pollen and spores of Barro Colorado Island. St Louis, MO: Missouri Botanical Garden.
- SADER. 2020. Norma Oficial Mexicana NOM-004-SAG/GAN-2018, Producción de miel y especificaciones. DOF - Diario Oficial de La Federación, 8. https://www.dof.gob.mx/nota_detalle.php?codigo=5592435&fecha=29/04/2020#gsc.tab=0.
- SADER. 2020. Base de datos estadísticos en relación con la producción por municipio 2019. Atlas Nacional de Las Abejas y Derivados Apícolas, Mexico, https://atlasnacionaldelasabejasmx.github.io/atlas/cap5.html; accessed 18 September 2021.
- Sánchez-Blanco J, Guevara-Féter F. 2013. Plantas arvenses asociadas a cultivos de maíz de temporal en suelos salinos de la ribera del Lago de Cuitzeo, Michoacán, México. Acta Botánica Mexicana 105: 107–129. doi:10.21829/abm105.2013.227
- Sánchez-Dzib Y. 2006. Morfología polínica de especies indicadoras de cambio de la selva mediana subperennifolia en la cuenca del río Candelaria, Campeche. Undergraduate Thesis, Universidad Autónoma de Campeche. Campeche, Mexico.
- Santiago-Briceño CI. 2018. Identificación de flora melífera con potencial ornamental y medicinal en Yucatán. Master’s Thesis, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A, C. Merida, Mexico.
- Sawyer R. 1988. Honey identification. Cardiff: Cardiff Academic Press.
- Schultz GP. 2005. Vascular flora of the El Edén Ecological Reserve, Quintana Roo, Mexico. The Journal of the Torrey Botanical Society 132: 311–322. doi:10.3159/1095-5674(2005)132[311:VFOTEE]2.0.CO;2.
- Tapia-Muñoz JL. 2011. La familia Convolvulaceae en la Península de Yucatán. Desde el Herbario CICY 3: 54–55. https://www.cicy.mx/Documentos/CICY/Desde_Herbario/2011/2011-06-30-Tapia-Convolvulaceae.pdf.
- Valencia-Cardona LO, Velázquez-Ruíz CA. 2014. Caracterización palinológica de mieles del apiario del laboratorio de investigaciones melitológicas y apícolas de la Universidad Nacional de Colombia, sede Medellín. Revista de la Facultad de Ciencias 3: 19–40.
- Vázquez-Fuentes YG, Quiroz-García DL, Acosta-Castellanos JS, Fernández-Nava R. 2019. Análisis palinológico de mieles de Apis mellifera L. (Apidae), Estado de Morelos, México. Polibotánica 48: 87–98. doi:10.18387/polibotanica.48.7.
- Villanueva-Gutiérrez R. 1994. Nectar sources of European and Africanized honey bees (Apis mellifera L.) in the Yucatán Peninsula, Mexico. Journal of Apicultural Research 33: 44–58. doi:10.1080/00218839.1994.11100848.
- Villanueva-Gutiérrez R. 2002. Polliniferous plant and foraging strategies of Apis mellifera (Hymenoptera:Apidae) in the Yucatan Peninsula. Revista de Biología Tropical 50: 1035–1044. https://revistas.ucr.ac.cr/index.php/rbt/article/view/16623/16128.
- Villanueva-Gutiérrez R, Moguel-Ordóñez Y, Echazarreta-González C, Arana-López G. 2009. Monofloral honeys in the Yucatán Peninsula, Mexico. Grana 48: 214–223. doi:10.1080/00173130902929203.
- Von der Ohe W, Oddo LP, Piana ML, Morlot M, Martin P. 2004. Harmonized methods of melissopalynology. Apidologie 35: S18–S25. doi:10.1051/apido:2004050.
- Weber M, Bucchner R. 2021. PalDat – Palynological Database. http://www.paldat.org; accessed 15 March 2021.
- Zavala-Olalde A, Colomo-González I, Matalí-Pérez N, Piana L, Olivier B, Méndez-Villarreal A, Vandame R. 2013. Characterization of four typical honeys from highly diverse tropical ecosystems. Journal of Apicultural Research 52: 24–34. doi: 10.3896/IBRA.1.52.2.05.
- Zerrouk S, Escuredo O, Rodríguez-Flores MS, Seijo MC. 2020. Palynological characterisation of sedra honeys (Ziziphus lotus) produced in Algeria. Grana 60: 69–80. doi:10.1080/00173134.2020.1770853.
- Zona S. 1990. A monograph of Sabal (Arecaceae: Coryphoideae). Aliso 12: 593–666. https://scholarship.claremont.edu/aliso/vol12/iss4/2/. doi:10.5642/aliso.19901204.02