?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The antibiogram signatures of E. coli O157:H7 (n=46) recovered from irrigation water and agricultural soil samples were assessed using the standard disc diffusion method against a panel of 16 antibiotics and molecular methods. The prevalent antibiotic resistance patterns follow the order: cefuroxime (95.7%), nitrofurantoin (93.5%), ampicillin (73.9%), chloramphenicol (71.7%), cefotaxime (60.9%) and trimethoprim/sulfamethoxazole (58.7%). The antibiotic resistance genes detected in the phenotypically resistant isolates include: tetA, tetB, tetC, catII, sulI, sulII, FOX-, CIT-, MOX-, EBC-type plasmid-mediated AmpC, and blaSHV extended-spectrum beta-lactamase. We conclude that irrigation water and agricultural soil are important reservoirs of multidrug-resistant E. coli O157:H7, hence a potential risk to South Africa’s one-health policy.
Introduction
Antimicrobial resistance (AMR) threatens global health because multidrug-resistant bacteria have emerged and spread through human beings and animals. Factors such as the misuse of antibiotics for animals and human beings, selling of antibiotics over the counter, poor hygiene and sanitation, inadequate prevention and control of infections in health-care settings, and the release of antimicrobial residues into the environment via manure and faeces have been recognised as some plausible causes of AMR [Citation1].
The environment can favour the emergence and spread of antibiotic-resistant bacteria as well as resistance genes [Citation2,Citation3]. This is increasingly attracting attention; the focus has shifted from hospital-acquired resistance. The environmental bacteria are forced to acquire resistance determinants, which are selectively linked to the antimicrobial residues resulting from anthropogenic activities [Citation4].
E. coli O157:H7 is an important environmental, clinical and veterinary pathogen because it can harbour virulence and antibiotic resistance genes [Citation5]. The use of antibiotics in infections caused by this pathogen is controversial because of the potential to promote the production of Shiga toxins. Nevertheless, the combination of right sets of antibiotics and supportive therapies like rehydration can be used to attack the infection caused by this pathogen, minimise the severity of the condition and improve the general outcome [Citation6,Citation7].
Studies have shown that E. coli O157:H7 retrieved from the environment, animals and human beings exhibits multidrug resistance (MDR) to commonly used antibiotics [Citation8,Citation9]. This is likely because livestock – the main reservoir – are constantly fed with antibiotics to improve yield. In the long run, this causes the selective emergence and spread of AMR strains and their determinants in the environment [Citation10].
E. coli O157:H7 can be used as indicators of antimicrobial resistance. They are invaluable for monitoring and surveillance of AMR in the environment, livestock, human beings and the food chain [Citation11]. There is a paucity of data on the occurrence of multidrug-resistant E. coli O157:H7 in irrigation water and agricultural soil, which aid the transfer of enteric bacteria from the farm to fresh produce. This paper reports the antibiogram imprints of E. coli O157:H7 isolated from irrigation water and agricultural soil samples collected from Amathole and Chris Hani District Municipalities, Eastern Cape Province, South Africa.
Materials and methods
Study sites
This study was carried out in Amathole and Chris Hani District Municipalities (DMs), Eastern Cape Province, South Africa. These Municipalities are well known for their agricultural activities including fresh produce production. Irrigation water and agricultural soil samples were retrieved from 19 study sites located within these two DMs (). Soil samples were not collected from S1, S4, S6, S10, S15 and S19 because the farms were not accessible.
Table 1. Description of sampling points
Sample collection, processing and isolation of E. coli O157:H7
A total of 19 irrigation water samples (approximately 1 litre each) and 13 agricultural soil samples (approximately 30 g each) were collected in triplicates from the study sites using 1 L sterile sample bottles and sterile plastic bags respectively. This was done after the research team obtained permission from farm owners. All the samples were placed on ice and transported to the laboratory for processing.
Ten millilitres of each water sample and 10 g of each soil sample were transferred to 90 ml of Trypticase soy broth (TSB) (Merck, SA) for enrichment purposes, and incubated at 37°C for 24 hours. A loop full of the broth was streaked on Sorbitol-MacConkey agar (SMA) (Merck, SA) augmented with cefixime (50 μg/L) and tellurite (25 mg/L) and incubated for 24 hours at 37°C. After incubation, 202 presumptive pure isolates were stored in 25% glycerol at −80 °C for subsequent investigations.
Molecular identification of E. coli O157:H7
The identification of E. coli O157:H7 was started off with DNA extraction of the presumptive isolates using the boiling technique described by Maugeri et al. [Citation12] and Lopez-Saucedo et al. [Citation13]. The isolates were then identified using the polymerase chain reaction (PCR) as described by Wang et al. [Citation14]. The primer sequence and conditions used for the identification of the target genes (rfbEO157 and fliCH7) are detailed in Supplementary Table 1. ‘E. coli O157:H7 ATCC 35,150’ was included in the reaction as a control strain. PCR amplicons (5.0 µL aliquot each) were electrophoresed in 2% (w/v) agarose gel (Merck, SA) as described by Wang et al. [Citation14] and photographed using a UV transilluminator. A 50-bp DNA ladder (Inqaba Biotec, SA) was included in each reaction to serve as a DNA size marker.
Antibiotic susceptibility testing
Following the recommendation of the ‘Clinical Laboratory Standard Institute’ (CLSI) [Citation15], the antibiotic susceptibility profiles of the confirmed isolates were evaluated using the disk diffusion method. Pure colonies of the confirmed isolates were picked and mixed with saline solution to match 0.5 McFarland standard. The mixture was evenly swabbed on Mueller-Hinton agar (Merck, SA) and the test antibiotics dispensed on the agar. All the plates were then incubated at 37°C for 16 to 24 hours. The cleared zones were read in mm and the values were interpreted as ‘Resistant (R)’, ‘Intermediate (I)’ or ‘Susceptible (S)’ as recommended by CLSI [Citation15]. A total of 16 antibiotics belonging to 11 classes which are of human and veterinary importance were used to carry out the antibiotic susceptibility testing and they include: Aminoglycosides; amikacin (AK-30 µg), gentamycin (GM-10 µg), beta-lactams; amoxycillin-clavulanic acid (AUG-30 µg), ampicillin (AP-10 µg), Carbapenems; imipenem (IMI-10 µg), meropenem (MEM-10 µg), Cephems; cefotaxime (CTX-30 µg), cefuroxime (CMX-), Fluoroquinolones; norfloxacin (NOR-30 µg), ciprofloxacin (CIP- 5 µg), Nitrofurans; nitrofurantoin (NI-300 µg), Phenicols; chloramphenicol (C-30 µg), Quinolones; nalidixic acid (NA-30 µg), Tetracyclines; tetracycline (T-30 µg), doxycycline (DXT-30 µg) and Sulphonamides; trimethoprim/sulphamethoxazole (TS-25 µg/25 µg) (Mast Diagnostics, UK). Ampicillin and tetracycline are used as growth promoters in livestock farms in the Eastern Cape Province. Amoxycillin-clavulanic acid, cefotaxime, imipenem, norfloxacin, ciprofloxacin, amikacin, chloramphenicol, gentamicin and sulfamethoxazole/trimethoprim are used for the treatment of infections caused by E. coli [Citation16].
Multiple antibiotic resistance phenotypes (MARPs) and multiple antibiotic resistance index (MARI)
The MARPs of the multidrug-resistant isolates were assessed as described by Krumperman [Citation17]. The MARPs patterns, number of resisted antibiotics and the number of observations of the patterns were accessed.
The MAR indices of MDR isolates were evaluated using the following mathematical equation [Citation17]:
Where ‘x-equals the number of antibiotics against which the isolates were resistant and y-represents the total number of antibiotics against which each isolate was tested’. MARI < 0.2 was set as the benchmark. Values equal to or greater than the benchmark indicates high-risk environments where antibiotics are indiscriminately used [Citation17].
Screening for resistance genes
Isolates that showed phenotypic resistance were screened for related antibiotic resistance genes (ARGs) using PCR assays. A total of 14 ARGs that codes for tetracycline, sulphonamide and phenicol resistance were screened as described in our previous report [Citation18]. Supplementary Table 1 details the amplification conditions, expected amplicon sizes and primer sequences of these genes. Twenty genes that code for plasmid-mediated AmpC (pAmpc), various extended-spectrum β-lactamases (ESBLs) and carbapenemases were also investigated as described by Dallenne et al. [Citation19]. Supplementary Table 2 shows the amplification conditions, expected amplicon sizes and primer sequences of these genes. Gel electrophoresis of the amplicons was done as described by Dallenne et al. [Citation19].
Multiple antibiotic resistance genotypes (MARGs)
The MARGs patterns in isolates from agricultural soil and irrigation water samples harbouring multiple resistance genes (ARGs ≥ 2) were evaluated. The number of non beta-lactamases, beta-lactamases including pAmpC and MARGs observations were accessed in isolates harbouring multiple ARGs.
Data analysis
Data were captured in Microsoft Excel (Version 16) and exported to IBM Statistical Package for Social Sciences (SPSS version 21), where descriptive analysis was carried out. A significant level of 0.05 was applied.
Results and discussion
Antibiotic susceptibility of confirmed isolates
Of the 202 presumptive isolates, 46 (23%) were confirmed as E. coli O157:H7. The antibiotic susceptibility profiles of the confirmed isolates are summarised in . According to the result, all the isolates were susceptible to amikacin. High frequency of susceptibility to gentamycin (97.8%), meropenem (95.7%), imipenem (93.5%), norfloxacin (91.3%), ciprofloxacin (89.1%), and nalidixic acid (60.9%) was also observed. Varied frequency of susceptibility to tetracycline (43.5%), doxycycline (43.5%) and amoxycillin-clavulanic acid (34.8%) was also reported. This corroborates previous studies [Citation20].
Figure 1. Antibiotic susceptibility profile of E. coli O157:H7. GM-gentamycin, AK-amikacin, AUG-amoxycillin clavulanic acid, AP-ampicillin, IMI-imipenem, MEM-meropenem, CTX-cefotaxime, CXM-cefuroxime, CIP-ciprofloxacin, NOR-norfloxacin, NI-nitrofurantoin, C-chloramphenicol, NA-nalidixic acid, TS-trimethoprim/sulphamethoxazole, T-tetracycline, DXT-doxycycline
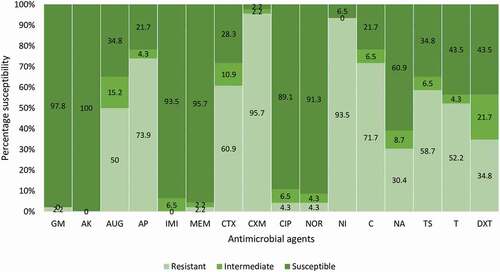
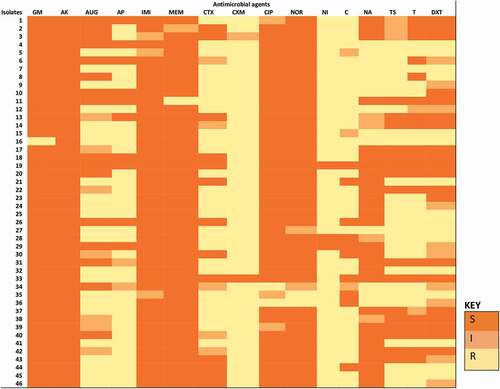
Figure 2. The phenotypic AMR pattern of each E. coli O157:H7 isolate. Isolates 1 to 18 were recovered from irrigation water samples. Isolates 19 to 46 were recovered from agricultural soil samples. S-sensitive, I-intermediate, R-resistance, GM-gentamycin, AK-amikacin, AUG-amoxycillin clavulanic acid, AP-ampicillin, IMI-imipenem, MEM meropenem, CTX-cefotaxime, CXM-cefuroxime, CIP-ciprofloxacin, NOR-norfloxacin, NI-nitrofurantoin, C-chloramphenicol, NA-nalidixic acid, TS-trimethoprim/sulphamethoxazole, T-tetracycline, DXT-doxycycline
Alternatively, high frequency of resistance against cefuroxime (95.7%), nitrofurantoin (93.5%), chloramphenicol (71.7%), ampicillin (73.9%), cefotaxime (60.9%) and trimethoprim/sulfamethoxazole (58.7%) was observed. This is also in agreement with a previous report [Citation20], suggesting that this group of antibiotics should not be prescribed during the remediation of uncomplicated E. coli O157:H7 induced infections. The high frequency of resistance to ampicillin is not surprising as they are used as livestock growth promoters in the Eastern Cape Province of South Africa [Citation16]. There has been increasing concern at the constant use of antibiotics to increase the yield of farm animals. This practice encourages the emergence of AMR against commonly used antibiotics, which is detrimental to one-health.
The phenotypic AMR pattern of the confirmed E. coli O157:H7
shows the phenotypic AMR patterns of the confirmed E. coli O157:H7 isolates to test antibiotics. The matrix describes how related the isolates are with respect to their responses to the test antibiotics. All the isolates varied in their responses to the test antimicrobials, suggesting non-relatedness in terms of susceptibility or resistance to the test antimicrobials. This probably implies that each isolate is uniquely exposed to the selective pressure caused by unique sets of antibiotics. It also suggests that over time, the isolates could have adapted unique resistance mechanisms that are not related genetically. The consequence is that the resistant strains soon dominate the bacterial population in the environment, transferring these dynamic resistance determinants to their progeny and other unrelated bacteria which could be commensals, saprophytes or pathogens.
The multiple antibiotic resistance phenotypes (MARPs) and multiple antibiotic resistance indices (MARI) of the isolates
show the various MARPs patterns and MAR indices of the isolates retrieved from the irrigation water and agricultural soil samples respectively. The isolates from the irrigation water samples portrayed 16 MARPs patterns to antimicrobials ranging from 3 to 11, many of which were unique; only 2 patterns appeared double. Aside from two isolates, the MAR indices of all the isolates from the irrigation water samples were all greater than 0.2, which is the standard for MARI [Citation17]. Isolates recovered from agricultural soil samples exhibited 19 patterns of MARPs to antimicrobials ranging from 3 to 10, most of which were unique and the rest of the patterns either appeared in duplicate, triplicate or quadruplet. Aside from one isolate, the MAR indices of all the isolates from the soil samples were greater than 0.2 which is the standard for MARI [Citation17].
Table 2. MARPs patterns and MAR indices of E. coli O157:H7 isolated from irrigation water samples
Table 3. MARPs patterns and MAR indices of E. coli O157:H7 isolated from agricultural soil samples
Table 4. Patterns of MAR genotypes of E. coli O157:H7 isolated from irrigation water samples
MDR E. coli O157:H7 isolates recovered from the irrigation water and agricultural soil samples in the present study exhibited very interesting MAR phenotypes. This probably suggests that the agro-ecosystem of the Eastern Cape Province, South Africa is saturated with sub-lethal levels of different classes of antibiotics. Studies have indicated that antibiotic use in health clinics, farm animals, and aquaculture has encouraged the extension of AMR reservoirs to other sections of the agro-ecosystem and eventually to human beings through the release of wastewater treatment plant (WWTP) effluents to receiving watersheds, spreading of bio-solids and manure to the soil, and irrigation of farm produce using greywater [Citation21–23]. This is evident in the present study as the MAR indices of most of the MDR isolates were greater than 0.2, suggesting that the isolates are from high-risk environments with a high level of AMR selective pressure.
Detection of antibiotic resistance genes
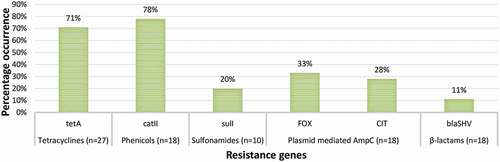
respectively show the prevalence of ARGs in confirmed E. coli O157:H7 isolates from irrigation water and agricultural soil samples. In irrigation water samples, 71% of the 27 phenotypically tetracycline-resistant isolates harboured tetA; 78% of the 18 phenotypically phenicol resistant isolates harboured catII; and 20% of the 10 phenotypically sulphonamide resistant isolates harboured sulI. Besides, 33% and 28% of the isolates (n = 18) recovered from irrigation water samples harboured the FOX- and CIT-type plasmid-mediated AmpC respectively, and 11% of the isolates (n = 18) harboured the blaSHV (SHV variants including SHV-1) ESBL encoding genes. In agricultural soil samples, 88%, 41% and 18% of 17 phenotypically tetracycline-resistant isolates harboured tetA, tetB and tetC genes respectively; 94% of the 17 phenotypically phenicol resistant isolates harboured catII; and 88% and 59% of the 17 phenotypically sulphonamide resistant isolates harboured sulI and sulII genes, respectively. Further, 7%, 4%, 7% and 43% of the isolates (n = 28) harboured the FOX-, MOX-, CIT- and EBC-type plasmid-mediated AmpC respectively and 4% of the isolates (n = 28) harboured the blaSHV (SHV variants including SHV-1) ESBL encoding genes.
Figure 4. The prevalence of ARGs in confirmed E. coli O157:H7 isolated from agricultural soil samples. n = number of phenotypically resistant isolates screened for ARGs
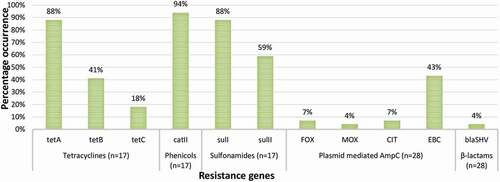
A very high prevalence of tetA was observed in isolates from the irrigation water and agricultural soil samples in the present study. It was observed to be the most prevalent tet gene. A lower prevalence of tetB and tetC was only observed in isolates from agricultural soil samples. This varies with a previous study [Citation24] where tetB was more prevalent at 60%. Generally, tetracycline resistance genes are known to be widespread in the environment owing to their constant use in dairy and feedlot cattle [Citation25]. The prevalence of catII in isolates from the irrigation water and agricultural soil samples was very high. This agrees with the findings of Ng et al. [Citation26] as they observed a high prevalence of catI in E. coli isolated from aquaculture and other environments. Just like the tet genes, cat genes are widely dispersed in the environment owing to the indiscriminate use of phenicols in agriculture. Most cat genes are borne by small multicopy or large transferable plasmids [Citation27], and so can be easily transferred to related and unrelated bacterial strains in the environment and beyond. In this study, a low prevalence of sulI was observed in isolates from irrigation water. A much higher prevalence of both sulI and sulII was observed in isolates from agricultural soil samples. This probably indicates that the origin of these genes is in the faeces of ruminants, used as manure. It also suggests that sulphonamides are more used for animal production than anything else in the Eastern Cape Province, South Africa.
Because of the high efficacy and low toxicity of beta-lactams, they are considered to be the most widely used antimicrobials in the remediation of a broad range of infections. Consequently, there is now an increasing prevalence of beta-lactam resistance genes including variants of plasmid-mediated AmpC and ESBLs in the environment. In this study, EBC-type pAmpC in isolates from agricultural soil samples was the most prevalent; followed by the FOX-type and CIT-type in isolates from irrigation water samples. The MOX-type in isolates from agricultural soil samples had the lowest prevalence. Although Richter et al. [Citation28] detected other variants of pAmpC in members of Enterobacteriaceae from fresh vegetables retailed in Gauteng Province, South Africa, the EBC-type was dominant. The EBC-type pAmpC was also dominant in Enterobacteriaceae isolated at a tertiary hospital in South Western Uganda [Citation29]. But, EBC-, FOX-, and MOX-type pAmpC were only detected in low frequencies from Escherichia coli, Klebsiella spp., and Proteus mirabilis isolated from urinary tract infections in Egyptian Hospitals [Citation30]. This indicates that the epidemiological distribution of the various types of pAmpC varies with sample types (environmental or clinical), the bacteria that harbour the genes, and geographical location.
In this study, the prevalence of blaSHV ESBL in isolates from irrigation water and agricultural soil samples was low compared to other studies [Citation31,Citation32]. It was also found to be the most prevalent ESBL in E. coli O157:H7 isolated from three selected rivers in Osun State, Nigeria [Citation33]. Nonetheless, just like other ESBLs, their prevalence is contingent on their geographical location. This, therefore, requires broad and regular surveillance studies to investigate the prevalence of these genes in E. coli O157:H7 recovered from irrigation water and agricultural soil as well as other niches of the agro-ecosystem. This will help map the ecological distribution of these genes in E. coli O157:H7 and track their hotspots within the agro-ecosystem.
The multiple antibiotic resistance genotypic patterns in E. coli O157:H7
The multiple antibiotic resistance genotypic (MARGs) patterns in E. coli O157:H7 isolated from irrigation water and agricultural soil samples are shown in respectively. Isolates from the irrigation water samples portrayed 6 patterns of MARGs ranging from 2 to 4 ARGs made up of β-lactamases and non-beta lactamases. Three of MARGs patterns were uniquely observed; and 2 and 4 patterns occurred in duplicate and quadruplicate respectively. Isolates from the agricultural soil samples portrayed 19 patterns of MARGs ranging from 2 to 6 ARGs made up of both β-lactamases and non-beta-lactamases. The MARGs patterns occurred uniquely or in duplicate.
Table 5. Patterns of MAR genotypes of E. coli O157:H7 isolated from agricultural soil samples
Although the co-occurrence of beta-lactamases has been documented in some studies [Citation34,Citation35], this study observed the co-occurrence of other resistance genes together with beta-lactamases in E. coli O157:H7 from irrigation water and agricultural soil samples. Similar data are scarce in the literature. More patterns of MARGs were observed in isolates from agricultural soil samples than in irrigation water samples. This suggests that agricultural soil creates a better platform for the transfer and acquisition of resistance genetic materials. The most observed pattern of MARGs was tetA- catII in isolates from irrigation water samples. This is not entirely surprising because these two resistance genes were more prevalent than other resistance genes detected in the study. The co-occurrence of non-beta-lactamases with beta-lactamases in some of the isolates suggests why a high proportion of the isolates were multidrug-resistant. It also gives an insight into the level of genetic transfer of resistance genes within the agro-ecosystem.
Conclusion
This study revealed that MDR E. coli O157:H7 occurred in irrigation water and agricultural soil samples collected from Amathole and Chris Hani District Municipalities. These pathogens exhibited resistance against numerous test antibiotics and harboured multi-genetic repertoires including plasmid-mediated AmpC and ESBLs, which aided their resistance to the test antibiotics. Prudent use of antibiotics for medical, veterinary and metaphylactic purposes should be encouraged to diminish the emergence of resistance in E. coli O157:H7.
Abbreviations
| AMR | = | Antimicrobial-resistant |
| ARGs | = | Antibiotic resistance genes |
| CDC | = | Centers for Disease Control and Prevention |
| CLSI | = | Clinical Laboratory Standard Institute |
| DMs | = | District Municipalities |
| ESBLs | = | Extended spectrum beta lactamase |
| MARGs | = | Multiple antibiotic resistance genotypes |
| MARI | = | Multiple Antibiotic Resistance Index |
| MARPs | = | Multiple Antibiotic Resistance Phenotypes |
| MDR | = | Multidrug resistance |
| pAmpC | = | Plasmid mediated AmpC |
| PCR | = | Polymerase chain reaction |
| RTE | = | Ready-to-eat |
Author contributions
CDI, LK and AIO contributed to the conception and design of the study. CDI collected the data and prepared the draft manuscript. EDP, LK, AIO and CDI revised the manuscript, and all authors approved the submission of the manuscript.
Supplemental Material
Download MS Word (52 KB)Acknowledgments
We are grateful to the South African Medical Research Council, United States Agency for International Development and the Department of Science and Technology of South Africa for financial support; and to the Editor, Dr Michael Brett-Crowther for his many efforts.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- Aslam, B., Wang, W., Arshad, M.I., Khurshid, M., Muzammil, S., Rasool, M.H., Nisar, M.A., Alvi, R.F., Aslam, M.A., Qamar, M.U., Salamat, M.K.F., and Baloch, Z., 2018, Antibiotic resistance: A rundown of a global crisis. Infection and Drug Resistance 11, 1645–1658.
- Iwu, C.D. and Okoh, A.I., 2019, Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A review. International Journal of Environmental Research and Public Health 16, 1–34.
- Iwu, C.D. and Okoh, A.I., 2020, Characterization of antibiogram fingerprints in Listeria monocytogenes recovered from irrigation water and agricultural soil samples. PLoS ONE 15, 1–22.
- Adegoke, A.A., Faleye, A.C., Singh, G., and Stenström, T.A., 2017, Antibiotic resistant superbugs: Assessment of the interrelationship of occurrence in clinical settings and environmental niches. Molecules 22, 1–17.
- Bolukaoto, J.Y., Kock, M.M., Strydom, K.A., Mbelle, N.M., and Ehlers, M.M., 2019, Molecular characteristics and genotypic diversity of enterohaemorrhagic Escherichia coli O157:H7 isolates in Gauteng region, South Africa. Science of the Total Environment 692, 297–304.
- Bielaszewska, M., Idelevich, E.A., Zhang, W., Bauwens, A., Schaumburg, F., Mellmann, A., Peters, G., and Karch, H., 2012, Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrobial Agents and Chemotherapy 56, 3277–3282.
- Lupindu, A.M., 2018, Epidemiology of Shiga toxin-producing Escherichia coli O157:H7 in Africa in review. Southern African Journal of Infectious Diseases 33, 24–30.
- Chigor, V.N., Umoh, V.J., Smith, S.I., Igbinosa, E.O., and Okoh, A.I., 2010, Multidrug resistance and plasmid patterns of escherichia coli O157 and other E. coli isolated from diarrhoeal stools and surface waters from some selected sources in Zaria, Nigeria. International Journal of Environmental Research and Public Health 7, 3831–3841.
- EL-Alfy, S.M., Ahmed, S.F., Selim, S.A., Abdel Aziz, M.H., Zakaria, A.M., and Klena, J.D., 2013, Prevalence and characterization of Shiga toxin O157 and non-O157 enterohemorrhagic Escherichia coli isolated from different sources in Ismailia, Egypt. African Journal of Microbiology Research 7, 2637–2645.
- Iwu, C.D., Du Plessis, E.M., Korsten, L., Nontongana, N., and Okoh, A.I., 2020, Antibiogram signatures of some enterobacteria recovered from irrigation water and agricultural soil in two district municipalities of South Africa. Microorganisms 8, 1–19.
- Karama, M., Cenci-Goga, B.T., Malahlela, M., Smith, A.M., Keddy, K.H., El-Ashram, S., Kabiru, L.M., and Kalake, A., 2019, Virulence characteristics and antimicrobial resistance profiles of Shiga Toxin-producing escherichia coli isolates from humans in South Africa: 2006–2013. Toxins 11, 1–15.
- Maugeri, T.L., Carbone, M., Fera, M.T., Irrera, G.P., and Gugliandolo, C., 2004, Distribution of potentially pathogenic bacteria as free living and plankton associated in a marine coastal zone. Journal of Applied Microbiology 97, 354–361.
- Lopez-Saucedo, C., Cerna, J.F., Villegas-Sepulveda, N., Thompson, R., Velazquez, F.R., Torres, J., Tarr, P.I., and Estrada-Garcia, T., 2003, Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerging Infectious Diseases 9, 127–131.
- Wang, G., Clark, C.G., and Rodgers, F.G., 2002, Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. Journal of Clinical Microbiology 40, 3613–3619.
- CLSI. 2018, Clinical and laboratory standards institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th edition. CLSI supplement M100. Wayne: Clinical and Laboratory Standards Institute.
- Iweriebor, B.C., Iwu, C.J., Obi, L.C., Nwodo, U.U., and Okoh, A.I., 2015, Multiple antibiotic resistances among Shiga toxin producing Escherichia coli O157 in feces of dairy cattle farms in Eastern Cape of South Africa. BMC Microbiology 15(213), 1–9.
- Krumperman, P.H., 1983, Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Multiple antibiotic resistance indexing of escherichia coli to identify high-risk sources of fecal contamination of foods. Applied and Environmental Microbiology 46, 165–170.
- Titilawo, Y., Obi, L., and Okoh, A., 2015, Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: Implications for public health. Science of the Total Environment 523, 82–94.
- Dallenne, C., da Costa, A., Decré, D., Favier, C., and Arlet, G., 2010, Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. Journal of Antimicrobial Chemotherapy 65, 490–495.
- Galland, J.C., Hyatt, D.R., Crupper, S.S., and Acheson, D.W., 2001, Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Applied and Environmental Microbiology 67, 1619–1627.
- Iwu, C.D., Korsten, L., and Okoh, A.I., 2020, The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. MicrobiologyOpen 9, 1–28.
- Davies, J. and Davies, D., 2010, Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews 74, 417–433.
- Munir, M. and Xagoraraki, I., 2011, Levels of antibiotic resistance genes in manure, biosolids, and fertilized soil. Journal of Environmental Quality 40, 248–255.
- Wilkerson, C., Samadpour, M., Van Kirk, N., and Roberts, M.C., 2004, Antibiotic resistance and distribution of tetracycline resistance genes in Escherichia coli O157:H7 isolates from humans and bovines. Antimicrobial Agents and Chemotherapy 48, 1066–1067.
- Montso, K.P., Mlambo, V., and Ateba, C.N., 2019, The first isolation and molecular characterization of Shiga Toxin-producing virulent multi-drug resistant atypical enteropathogenic Escherichia coli O177 serogroup from South African Cattle. Frontiers in Cellular and Infection Microbiology 9, 1–11.
- Ng, K.H., Samuel, L., Kathleen, M.M., Leong, S.S., and Felecia, C., 2014, Distribution and prevalence of chloramphenicol-resistance gene in Escherichia coli isolated from aquaculture and other environment. International Food Research Journal 21, 1321–1325.
- Galopin, S., Cattoir, V., and Leclercq, R., 2009, A chromosomal chloramphenicol acetyl transferase determinant from a probiotic strain of Bacillus clausii. FEMS Microbiology Letters 296, 185–189.
- Richter, L., Du Plessis, E.M., Duvenage, S., and Korsten, L., 2019, Occurrence, identification, and antimicrobial resistance profiles of extended-spectrum and AmpC β-lactamase-producing enterobacteriaceae from fresh vegetables retailed in Gauteng Province, South Africa. Foodborne Pathogens and Disease 16, 421–427.
- Nakaye, M., Bwanga, F., Itabangi, H., Stanley, I., Bashir, M., and Bazira, J., 2014, AmpC-BETA lactamases among enterobacteriaceae isolated at a tertiary hospital, South Western Uganda. British Biotechnology Journal 4, 1026–1036.
- Helmy, M.M. and Wasfi, R., 2014, Phenotypic and molecular characterization of plasmid mediated AmpC β-lactamases among Escherichia coli, Klebsiella spp., and Proteus mirabilis isolated from urinary tract infections in Egyptian hospitals. BioMed Research International 2014, 1–8.
- Montso, K.P., Dlamini, S.B., Kumar, A., and Ateba, C.N., 2019, Antimicrobial resistance factors of extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae isolated from Cattle farms and Raw Beef in North-West Province, South Africa. BioMed Research International 2019, 1–13.
- Gundran, R.S., Cardenio, P.A., Villanueva, M.A., Sison, F.B., Benigno, C.C., Kreausukon, K., Pichpol, D., and Punyapornwithaya, V., 2019, Prevalence and distribution of bla CTX-M, bla SHV, bla TEM genes in extended- spectrum β- Lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Veterinary Research 15, 1–8.
- Bisi Johnson, M.A., Adedeji, A.A., and Sulaiman, A.A., 2018, Molecular characterization of extended spectrum beta-lactamase producing Escherichia coli O157:H7 from three selected rivers in Osun State, Nigeria. Journal of Environmental Research 2, 1–83.
- He, D., Partridge, S.R., Shen, J., Zeng, Z., Liu, L., Rao, L., Lv, L., and Liu, J.H., 2013, CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 group β-lactamases recovered from escherichia coli isolates in china. Antimicrobial Agents and Chemotherapy 57, 4068–4071.
- Li, S., Zhao, M., Liu, J., Zhou, Y., and Miao, Z., 2016, Prevalence and antibiotic resistance profiles of extended-spectrum β-lactamase-producing Escherichia coli isolated from healthy broilers in Shandong Province, China. Journal of Food Protection 79, 1169–1173.