Abstract
Background. Spirometry patterns suggesting restrictive and obstructive pulmonary dysfunction have been reported in Parkinson’s disease (PD). However, the patterns’ precise relation to PD pathophysiology remains unclear. Purpose/Aim. To assess ON- versus OFF-state pulmonary function, the quality of its spirometric evaluation, and the quality of longitudinal spirometric findings in a large sample of PD patients with motor fluctuations. Methods. During a placebo-controlled trial of an inhaled levodopa formulation, CVT-301, in PD patients with ≥2 h/d of OFF time, spirometry was performed by American Thoracic Society (ATS) guidelines at screening and throughout the 4-week treatment period. Results. Among 86 patients, mean motor impairment during an OFF state at screening was moderately severe. However, mean spirometry results at screening were within normal ranges, and in a mixed model for repeated measures (MMRM), the results at screening were not dependent on motor state (ON vs. OFF). In the placebo group (n = 43), 76% of ON-state and 81% of OFF-state examinations throughout the study met ATS quality metrics, and in an MMRM analysis, mean findings at these patients’ arrivals for treatment-period visits showed no significant 4-week change. Across all 86 patients, flow-volume curves prior to any study-drug administration showed only a 3% incidence of “sawtooth” morphology. Conclusions. In PD patients with motor fluctuations, longitudinal spirometry of acceptable quality was generally obtained. Although mean findings were normal, about a quarter of spirograms did not meet ATS quality criteria. Spirogram morphology may be less indicative of various forms of respiratory dysfunction than has previously been reported in PD.
Introduction
In patients with Parkinson’s disease (PD), characteristic clinical features such as rigidity and bradykinesia may impair the tone, contractility, and coordination of thoracic musculature, affecting respiratory mechanics and pulmonary function [Citation1]. Indeed, respiratory dysfunction can contribute to morbidity and mortality in advanced PD [Citation2–Citation4]. PD may also affect a patient’s ability to perform the respiratory maneuvers required for pulmonary function tests (PFTs) such as spirometry [Citation5]. In PD patients, PFT patterns suggesting restrictive pulmonary dysfunction and central- or upper-airway obstruction have been described [Citation1,Citation6–Citation9]. However, the pathophysiologic basis for differing patterns and the precise relation of each such pattern to PD motor dysfunction remain unclear [Citation1]. Parkinsonian fluctuations in motor function [Citation10] may further complicate the clinical interpretation of spirometry data.
The potential for OFF-episode reversal via intrapulmonary drug delivery has been the impetus for clinical studies of inhaled levodopa (LD) [Citation11,Citation12]. CVT-301 is an LD powder formulation for inhalation. During a phase 2b randomized, double-blind, placebo controlled, multinational, multicenter trial of CVT-301 in PD patients with motor fluctuations, spirometry was performed at screening and at each of four visits throughout a 4-week treatment period [Citation13,Citation14]. Here we report the pretreatment findings assessing ON- versus OFF-state pulmonary function and the quality of spirometric evaluation across all study participants before any exposure to study drug. We also report the longitudinal spirometric findings from the study’s placebo group, who were never exposed to the active study drug, and assess the quality and reproducibility of these results.
Methods
Study participants
All patients were required to be aged 30–80 years and have idiopathic PD at a modified Hoehn and Yahr (H&Y) stage [Citation15] of 1–3 in the ON state. Patients were also required to have recognizable, predictable OFF episodes totaling at least 2 h/d (excluding early-morning OFF time). Unified Parkinson’s Disease Rating Scale (UPDRS) Part III scores [Citation16] were required to show a ≥25% difference between each patient’s ON and OFF states. In the ON state, each patient’s forced expiratory volume during the first second (FEV1) was required to be >60% of the value predicted by the patient’s age, sex, height, and race [Citation17], and the ratio of FEV1 to forced vital capacity (FVC) was required to be ≥75%. The patient’s PD medications were required to include oral LD/carbidopa taken at least four times daily in a regimen that had been stable for at least the 2 weeks before screening. Patients were excluded for a history of chronic respiratory disease within the preceding 5 years and for a Mini-Mental State Examination score [Citation18] <25.
Study design
The study included a screening period lasting 2–4 weeks, a treatment period lasting 4 weeks, and a follow-up safety visit 1 week after the end of treatment. During the screening period, spirometry was performed in each patient’s ON state. At the same visit, spirometry was repeated when the patient had entered an OFF state, in the judgment of an investigator. Spirometry was performed at a subsequent screening visit only if needed for assessing eligibility. In a 1:1 randomization ratio, enrolled patients were assigned to self-administer inhaled CVT-301 or placebo as needed up to three times per day for the treatment of OFF episodes. In the active-treatment group, the dose was increased at the end of week 2. Placebo-treated patients used inhalation-grade lactose monohydrate at dose levels and a particle size selected to approximate the upper-airway powder-load deposition of the active drug and mimic the sensation of active-drug inhalation but not enter the lungs. For each study-drug dose level, the drug’s use began with an in-clinic self-administration.
Patients were assessed at the visit initiating study-drug use (treatment-period baseline visit), at the end of treatment weeks 1, 2, and 4, and at the follow-up visit. At each visit, spirometry was performed at the patient’s arrival, preferably in an ON state. At the baseline visit, spirometry was also performed immediately predose (regardless of the patient’s ON/OFF status) and at 15, 30, and 60 min postdose. At the end-of-week-2 visit, initiating the increased study-drug dose, spirometry was performed at arrival, predose (in an OFF state, as judged by an investigator), and 60-min postdose. In all patients, all oral PD medications were held constant throughout the study. The study was registered with ClinicalTrials.gov (identifier: NCT01777555).
Spirometry procedure
At each study site, spirometry was performed by trained and qualified personnel using standardized equipment (6800 Fleisch Pneumotach; Vitalograph, Inc., Lenexa, KS, USA) under guidelines specified by the Third National Health and Nutrition Examination Survey (NHANES III), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) [Citation5,Citation17]. Spirometry values (FEV1, FVC, and the FEV1/FVC ratio) were obtained from each patient’s best effort, predefined as the acceptable effort yielding the highest sum of FEV1 and FVC. All spirometry data were reviewed by a central spirometry laboratory (Biomedical Systems, Inc., St. Louis, MO, USA), which provided an over-read based on ATS/ERS quality standards. The ATS/ERS criteria [Citation5] require that at least three of the patient’s efforts be acceptable (including an exhalation lasting at least 6 s) and that two be repeatable (as shown by a difference of <0.15 L between the two FEV1 values and between the two FVC values).
Spirometry morphology analyses
The shape of the flow–volume curve produced by each patient at the first treatment visit, prior to active-drug or placebo administration, was interpreted post hoc by a pulmonologist (NBH) blinded to the patient’s treatment assignment. Each curve was given one of four classifications. “Normal” curves were those that exhibited a sharp peak in expiratory flow rate (PEFR) followed by a relatively linear decline to residual volume (Figure A). “Sawtooth” curves demonstrated a sawtooth shape during exhalation, typically after the PEFR (Figure B). This pattern has been attributed to upper-airway obstruction [Citation6–Citation9]. “Low PEFR, reproducible” curves had a rounded peak but were consistently reproducible (Figure C), as is typically seen in patients with neuromuscular weakness [Citation6–Citation9]. “Low PEFR, not reproducible” was applied to curves with a slow rise to peak flow, a rounded peak, and inconsistent reproducibility, suggesting poor effort, difficulty with the test maneuver, or detriments such as pain (Figure D).
Figure 1. Examples of flow–volume curves categorized as (A) normal; (B) sawtooth; (C) low PEFR, reproducible; or (D) low PEFR, not reproducible. PEFR, peak expiratory flow rate.
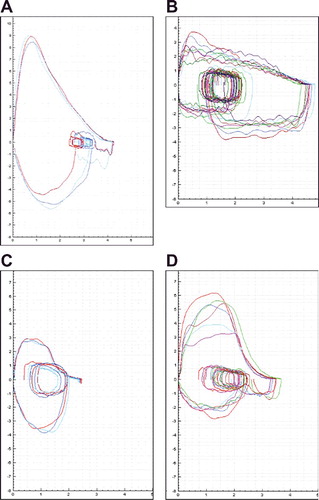
Table 1. Demographic and baseline PD characteristics.
Table 2. Spirometry results obtained at the screening of all treated patients. Data are presented as means with standard deviations and as estimated ON-vs.-OFF differences with 95% confidence intervals calculated using an MMRM.
Statistical methods
Descriptive analyses are presented for ON-state versus OFF-state spirometry findings from all study participants at their screening visits; for spirometry-quality metrics in placebo-group patients at all spirometry time points; for longitudinal spirometry findings in placebo-group patients, as obtained upon their arrivals at treatment-period visits; and for flow–volume-curve morphology in all patients, as obtained prior to active-drug or placebo administration at their first treatment visit. All analyses were of observed cases, with no imputation of data.
To complement the descriptive approach, 95% confidence intervals were calculated for the ON-vs.-OFF differences at screening and for the placebo group’s longitudinal changes. The ON-vs.-OFF calculations used a mixed model with repeated measures (MMRM) with H&Y stage, country, and motor state (ON vs. OFF) as fixed factors. The longitudinal-change calculations used a similar MMRM, with H&Y stage, country, and study visit as fixed factors and baseline value as a covariate. The changes themselves were estimated using contrasts.
As a post-hoc analysis, the variability of spirometric findings when a given parameter is measured multiple times in an individual patient was estimated by calculating the within-subject coefficient of variability (CV) for each parameter among all patients with at least two ON-state examinations before any study-drug exposure. For each patient, up to four such assessments were available: one or two at screening, plus arrival and predose assessments on the first day of treatment. The subset of examinations meeting ATS/ERS quality criteria was analyzed separately.
Results
Study participants
In all, 134 patients were screened at 20 sites, beginning in April 2013. Of the 45 screening failures, 11 (8.2% of 134) were unable to achieve an FEV1 and/or an FEV1/FVC ratio meeting eligibility criteria and another 4 (3.0% of 134) were unable to perform the spirometry maneuver. The remaining 89 patients were enrolled and randomized, and 86 used at least one dose of study drug. By UPDRS Part III score [Citation16], their mean motor impairment during an OFF state at screening was moderately severe [Citation19]. The randomized patients included 45 patients allocated to placebo, of whom 43 used at least one dose and 36 completed the 4-week treatment period (Supplemental Figure A). The study’s last patient completed treatment in January 2014. Table summarizes the demographic and PD characteristics of the 86 study-drug users and the placebo-user subset, which resembled those of the full group.
Spirometry at screening
Table compares the ON-state and OFF-state spirometry results obtained at the screening of all treated patients. By MMRM analysis, FEV1 and FVC exhibited no significant difference between the patients’ ON and OFF states. Although the 95% confidence interval for ON-versus-OFF difference in the FEV1/FVC ratio did not cross zero, the mean OFF-state value showed a decrease of only 1.1% points, to 78.5% from a mean ON-state value of 79.6%. In both ON and OFF states, the variability among FEV1 and FVC values was greater than that for the FEV1/FVC ratio, but for all parameters, the observed mean values were well within commonly utilized normal ranges (for FEV1 and FVC, ≥80% of the predicted value, and for the FEV1/FVC ratio, ≥70%) [Citation20].
Spirometry-quality metrics
Among all spirometry measurements performed during the study in the placebo group, motor state did not predict the ability to obtain adequate spirometry. Of all ON-state examinations, 76% (238 of 314) met ATS/ERS criteria (three acceptable and two repeatable efforts as defined above, under “Spirometry procedure”). Of all OFF-state examinations, the frequency was 81% (192 of 236). In both motor states, the most common reason for failure was lack of repeatable efforts. This problem occurred in 41% of the deficient ON-state examinations (31 of 76) and 48% of the deficient OFF-state examinations (21 of 44). Among all examinations rejected by ATS/ERS criteria, 43% (52 of 120) had three acceptable efforts but less than two repeatable efforts. Another 41% (49) had neither three acceptable nor two repeatable efforts. The remaining 16% (19) had two repeatable efforts but lacked three acceptable efforts.
Longitudinal spirometry
Table presents the spirometry results obtained from the study’s placebo users upon their arrival at study visits during the 4-week treatment period. At the end of week 4, there was no significant change from baseline (the beginning of week 1) in either FEV1, FVC, or the FEV1/FVC ratio.
Table 3. Longitudinal spirometry results in placebo-treated patients, as obtained upon their arrival for treatment-period visits. Data are presented as means with standard deviations and as estimated changes with 95% confidence intervals calculated using an MMRM (observed cases).
Spirogram morphology
Of the flow–volume curves obtained from patients at their first treatment visit, 45% (39 of 86) were classified as having normal morphology. Another 3% (3 curves) were classified as “sawtooth,” 19% (16 curves) as “low PEFR, reproducible,” and 34% (29 curves) as “low PEFR, not reproducible.”
Within-subject variability
Among the 86 patients studied, 74 had a total of at least two ON-state spirometry evaluations prior to any study-drug exposure. For all such patients, and for the subset of 49 patients whose spirometry met ATS/ERS quality standards, Table presents within-subject CVs for FEV1, FVC, and the FEV1/FVC ratio. Across the patients whose spirometry met the quality standards, all of the CVs were <6%. Across all of the patients, all of the CVs were <8%.
Table 4. Within-subject coefficients of variability for ON-state spirometry results prior to study-drug exposurea.
Discussion
This report represents the largest cohort of PD patients studied to date in which pulmonary function was assessed prospectively, and provides the first longitudinal spirometric data from PD patients with motor fluctuations. Furthermore, the study included same-day evaluations of PD patients in both their ON and OFF states and reviewed spirometry metrics using ATS/ERS quality standards.
A large majority of spirometric evaluations (78% overall) met the ATS/ERS criteria, confirming the ability of PD patients to perform the spirometry maneuver, regardless of their motor state. The most frequent reason for failure of a tracing to meet ATS/ERS criteria was a patient’s inability to provide repeatable efforts. Overall, mean spirometry values were within normal ranges at presentation, and remained normal in patients’ OFF states. Moreover, spirometry findings remained unchanged over 4 weeks of longitudinal evaluation.
In prior spirometric studies of PD patients, several patterns of abnormalities have been described. When present, the most common patterns have been characterized as restrictive pulmonary dysfunction, obstructive airflow disease, and upper airway obstruction [Citation1,Citation7–Citation9,Citation21–Citation24]. Abnormal ventilatory control has also been described, even though respiratory flows and volumes were normal in most of the patients [Citation25]. Most of the available studies have been cross-sectional in design. None have provided data on the intra- or inter-subject variability of repeated observations, or any ascertainment of ATS/ERS quality metrics. Three of the studies [Citation21,Citation23,Citation24] presented results for both ON and OFF states. Among these studies, two described restrictive patterns, more severe in the OFF state. In one such report [Citation23], the OFF-state findings among 12 patients showed declines of approximately 10% in both FEV1 and FVC, to mean values of 77.9% and 76.8% of predicted, with no significant change in the FEV1/FVC ratio. In the other report [Citation24], LD dosing of 53 patients led to a 13% improvement in FVC, from a mean 62.8% of predicted to a mean 71.4% of predicted, with no change in the FEV1/FVC ratio. In both studies, however, total lung capacity was not assessed. Other possible explanations for the absence of analogous findings in the current study include its stringent eligibility criteria and substantial differences from the other studies in patient characteristics such as age (at a mean of 67.7 years [Citation23] and 53.3 years [Citation24] in the other studies, compared with 62.4 years in the current study) and PD duration (at a mean of 14.5 years [Citation23] and 3.1 years [Citation24] in the other studies, compared with 9.4 years in the current study). In the third study reporting ON-state and OFF-state results [Citation21], mean spirometry findings among 10 patients, presented only as absolute values, were higher during the OFF state, by amounts described as too small to meet ATS/ERS criteria for clinical relevance [Citation26].
The present study excluded patients with an FEV1 ≤60% or an FEV1/FVC ratio <75%, patients unable to perform the spirometry maneuver, patients with chronic respiratory disease, and patients with more than moderate PD-related disability, as defined by an H&Y stage >3 during an ON state. Hence, the study’s findings do not permit exploration of the pathophysiology of respiratory dysfunction in PD patients or the extent to which such dysfunction represents PD, associated physical decline, or normative aging. PD has been found to exacerbate the normative age-related loss of respiratory muscle strength [Citation27], and PD-associated deficits in maximum inspiratory and expiratory pressures have been attributed to rigidity and bradykinesia of thoracic musculature [Citation1]. Nevertheless, the precise causal relation of parkinsonism to respiratory dysfunction remains unknown [Citation1]. The present findings are also limited by the absence of an active-treatment comparator. The aim here, however, was to investigate spirogram quality and reproducibility in the ON and OFF states of PD patients free of exposure to an investigational drug.
In a post-hoc analysis, we categorized the morphologies of the flow–volume curves produced by the patients at a point in the trial when they would have become familiar with the spirometry procedure but would not yet have received any study drug. Prior studies in smaller groups of PD patients had reported a 29% to 67% incidence of “sawtooth” morphology [Citation6–Citation9]. We found only a 3% incidence, undoubtedly related at least in part to differing methods of patient selection. In the current study, all patients were asymptomatic for respiratory dysfunction, regardless of flow–volume-curve morphology. Accordingly, an emphasis on the morphology seems unwarranted. Interpretation of the clinical significance of sawtoothing or other abnormalities was also limited by unavailability of comments from the spirometry technician, which might have provided insight. Of perhaps greatest significance for longitudinal spirometry in a PD population was the relatively frequent lack of test repeatability, a factor requiring consideration when planning evaluations of pulmonary function in PD studies. A lack of spirometry repeatability raises the possibility that PD patients could potentially experience difficulty with other complex PFTs, such as diffusing capacity.
Conclusions
The present study provides strong evidence that longitudinal spirometry measurements of acceptable quality can be obtained in studies of PD patients with motor fluctuations, using trained staff and strict quality controls. In contrast to prior studies, spirometry results were within normal ranges and did not differ significantly between the patients’ ON and OFF states. The results also suggest that flow–volume-curve morphology, which has received much attention in the PD literature, may be less well associated with respiratory dysfunction than has been described. Some patients had difficulty performing repeatable spirometry maneuvers, implying that other complex forms of PFT might be problematic. In planning future clinical studies, these issues should be considered.
Declaration of Interest
The authors alone are responsible for the content and writing of this paper. Neil B. Hampson worked as a noncompensated pulmonary consultant to Civitas Therapeutics during the time of the study and has no conflicts to disclose; Karl D. Kieburtz is a consultant for the National Institutes of Health (NINDS), Acorda, Astellas Pharma, AstraZeneca, Auspex, Biotie, Britannia, Cangene, CHDI, Civitas, Clearpoint Strategy Group, Clintrex, Cynapsus, Intec, Isis, Lilly, Lundbeck, Medavante, Medivation, Melior Discovery, NeuroDerm, Nueromedix, Omeros, Otsuka, Pfizer, Pharm2B, Prothena/Neotope/Elan Pharmaceutical, Raptor Pharmaceuticals, Roche/Genentech, Sage Bionetworks, Serina, Stalth Peptides, Synagile, Teikoku Pharma, Titan, Turing Pharmaceuticals, Upsher-Smith, US WorldMeds, Vaccinex, Voyager, and Weston Brain Institute. He received grants/research support from the Michael J. Fox Foundation, the National Institutes of Health (NEI, NINDS, NIA, NICHD), and Teva; Peter A. LeWitt served as a consultant or advisor for Acorda, Biogen Idec, Britannia, Cynapsus, Depomed, Impax, Insightec, Intec, Kyowa Hakko, Lundbeck, Luye, NeuroDerm, Osmotica, Pfizer, ProStrakan, Prothena, SynAgile, Teva, UCB Pharma, and USWorldMeds, and has received speaker honoraria from The International Parkinson’s and Movement Disorders Society, Lundbeck, and USWorldMeds. He is compensated for services as Editor-in-Chief of Clinical Neuropharmacology and also serves without compensation on the editorial boards of Journal of Neural Transmission, Translational Neurodegeneration, and Journal of Parkinson’s Disease. The Parkinson’s Disease and Movement Disorders Program that Dr LeWitt directs has received clinical research grant support (for conducting clinical trials and other research) from AbbVie, Acorda, Adamas, Biotie, Kyowa Hakko, The Michael J. Fox Foundation for Parkinson’s Research, the Parkinson Study Group, Pharma 2B, UCB, and USWorldMeds; Mika Leinonen is a biostatistician at 4Pharma AB, a contract research organization that provided compensated statistical consultation services to Civitas Therapeutics and also provides similar services to Acorda Therapeutics; Martin I. Freed was an employee of Civitas Therapeutics, the study sponsor that was responsible for the conduct of the study. This study was funded in part from a grant from the Michael J Fox Foundation for Parkinson's Research. Editorial assistance was provided by The Curry Rockefeller Group, LLC, which was funded by Acorda Therapeutics.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/00207454.2016.1194274.
Acknowledgements
The authors wish to acknowledge Tia DeFeo-Fraulini and Amanda Gentile for expert operational assistance in the conduct of the study and Jessica Roland of Acorda Therapeutics for editorial assistance. The authors would also like to acknowledge the efforts of the CVT-301-003 Study Group for their invaluable assistance in conducting this trial.
Additional information
Funding
References
- Sabate M, Gonzalez I, Ruperez F, et al. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J Neurol Sci 1996;138(1–2):114–9.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(5):427–42.
- Brown LK. Respiratory dysfunction in Parkinson’s disease. Clin Chest Med 1994;15(4):715–27.
- Shill H, Stacy M. Respiratory function in Parkinson’s disease. Clin Neurosci 1998;5(2):131–5.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Resp J 2005;26(2):319–38.
- Vincken WG, Gauthier SG, Dolfuss RE, et al. Involvement of upper-airway muscles in extrapyramidal disorders; a cause of airflow limitation. N Engl J Med 1984;311(7):438–42.
- Hovestadt A, Bogaard JM, Meerwaldt JD, et al. Pulmonary function in Parkinson’s disease. J Neurol Neurosurg Psychiatr 1989;52(3):329–33.
- Izquierdo-Alonso JL, Jiménez-Jiménez FJ, Cabrera-Valdivia F, et al. Airway dysfunction in patients with Parkinson’s disease. Lung 1994;172(1):47–55.
- Herer B, Arnulf I, Housset B. Effects of levodopa on pulmonary function in Parkinson’s disease. Chest 2001;119(2):387–93.
- Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci 2000;23(Suppl):S2–S7.
- Freed MI, Batycky R, Merica E. Safety, tolerability, and pharmacokinetics following inhaled administration of CVT-301, a levodopa powder, aerosol, in healthy adult subjects. Mov Disord 2013;28(Suppl 1):S154.
- Freed MI, Grosset DG, Worth PF, et al. Rapid levodopa augmentation following inhaled CVT-301 results in rapid improvement in motor response when administered to PD patients in the OFF state. Neurology 2014;82(10 Suppl):S7.007.
- LeWitt P, Saint-Hillaire M-H, Grosset DG, et al. Inhaled levodopa (CVT-301) provides rapid improvement of OFF states in Parkinson’s disease. Poster Presented at the 19th International Congress of Parkinson’s Disease and Movement Disorders, 2015 June 14–18, San Diego, CA [Poster 260].
- LeWitt PA, Hauser RA, Grosset DG, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. 2016 Apr 19. doi: 10.1002/mds.26611.
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19(9):1020–8.
- Fahn S, Elton RL. UPDRS program members. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent developments in Parkinson’s disease. Volume 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–63.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a general sample of the U.S. population. Am J Respir Crit Care Med 1999;169(1):179–87.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–98.
- Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the Unified Parkinson’s Disease Rating Scale. Arch Neurol 2010;67(1):64–70.
- Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J 2010;36(1):12–9.
- Lim A, Leow L, Huckabee M, et al. A pilot study of swallowing and respiration in Parkinson’s disease: “on” and “off” levodopa. Dysphagia 2008;23(1):76–81.
- Polatli M, Akyol A, Cildag O, et al. Pulmonary function tests in Parkinson’s disease. Eur J Neurol 2001;8(4):341–5.
- De Pandis MF, Starace A, Stefanelli F, et al. Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurol Sci 2002;23(Suppl 2):S69–70.
- Pal PK, Sathyaprabha TN, Tuhina P, et al. Pattern of subclinical pulmonary dysfunctions in Parkinson’s disease and the effect of levodopa. Mov Disord 2007;22(3):420–4.
- Seccombe LM, Giddings HL, Rogers PG, et al. Abnormal ventilatory control in Parkinson’s disease—further evidence for non-motor dysfunction. Respir Physiol Neurobiol 2011;179(2–3):300–4.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–68.
- Sanches VS, Santos FM, Fernandes JM, et al. Neurodegenerative disorders increase decline in respiratory muscle strength in older adults. Respir Care 2014;59(12):1838–45.
