Abstract
Objective
The aim of this study was to evaluate the safety and effectiveness of rotigotine under daily clinical practice in Parkinson’s disease patients.
Methods
The study was a prospective, non-interventional, observational study targeting patients who were treated with rotigotine for the first time, with a 1-year follow-up period from September 2013 to August 2016.
Results
There were 603 patients in the safety population and 599 patients in the effectiveness population. The mean age was 71.6 years, and the age group of ≥65 and ≥80 years accounted for 80% and 18.6% of all patients, respectively. The frequency of adverse drug reaction (ADR) was 34.3%, and common ADRs were application site reaction (20.2%), typical for transdermal patches. However, the majority of patients recovered or was recovering from these ADRs and were non-serious. Although ADRs related to non-motor symptoms of Parkinson’s disease were observed, most of them were non-serious. Total scores of the Unified Parkinson's Disease Rating Scale Part III (UPDRS-III) (ON-time) significantly decreased from baseline in the effectiveness population. In the analysis of overall improvement in 12 months of post-treatment, ≥70% of patients achieved mild or greater improvement. The safety profiles and improvements in the UPDRS-III score were similar in both the ≥80 years of age group and younger age group.
Conclusion
There were no new or notable safety concerns observed, and the effectiveness of rotigotine was suggested in daily clinical practice.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by motor symptoms such as tremor, rigidity, akinesia, and postural instability [Citation1]. Such symptoms of PD are associated with the depletion of dopamine in the striatum following the degeneration of neurons of substantia nigra [Citation2]. The loss of neurons of the substantia nigra is also caused by increasing age, and studies have elucidated that overall numbers and prevalence of PD increases with age [Citation3,Citation4]. Consequently, the number of patients with PD is increasing with the aging population in Japan.
l-Dopa, a dopamine precursor that compensates for depleted dopamine levels, has been in use for many years as replacement therapy for the treatment of PD. However, the half-life blood elimination of l-dopa is short, and thus a long-term use of this drug could cause motor complications such as dyskinesia and wearing-off, resulting in inadequate efficacy [Citation5,Citation6]. Consequently, treatments such as ergot dopamine receptor agonists (DA), including pergolide and cabergoline and non-ergot DAs including pramipexole and ropinirole were developed to resolve such issues [Citation7]. A significantly lower frequency of dyskinesia was reported in patients with PD with the use of DA at an early stage compared with the use of l-dopa [Citation8,Citation9], and improvements in motor complications were observed with concomitant use of DA with l-dopa in advanced patients with PD [Citation10].
Taking account of the above findings, the Parkinson’s Disease Guideline 2018 [Citation11] states that treatment with DA or monoamine oxidase-B inhibitors should be initiated for patients under 65 years of age, who are expected to have a higher risk of motor complications, and if the response is inadequate, treatment with other drugs or combination therapy should be considered. On the other hand, since elderly patients tend to have more rapid progress in motor symptoms and have a lower rate of dyskinesia, treatment with l-dopa is beneficial for these patients.
Rotigotine (Neupro® Patch) is a non-ergot DA that has been approved and marketed in more than 70 countries as a treatment for PD. In Japan, rotigotine was launched from 2013, indicated for PD, and moderate to severe idiopathic restless leg syndrome [Citation12].
Rotigotine is characterized by its affinity to all dopamine receptor subtypes (D1–D5). It is the first transdermal formulation of anti-parkinsonian drugs that are expected to display stable and continuous effects due to its capability of maintaining constant blood plasma concentration throughout the day [Citation13]. Moreover, since rotigotine has been characterized by its transdermal formulation, rotigotine could possibly relieve the burden of patients with PD who take multiple drugs or have dysphagia or both [Citation14].
Efficacy and safety profiles of rotigotine have been evaluated in clinical studies in many countries, including in Japan. Studies have shown that rotigotine significantly improves activities of daily living (ADL) and motor function in patients with PD in the early stage, and in the advanced stage [Citation15–18].
In addition, a 52-week long-term follow-up study on rotigotine showed that the effect is maintained longitudinally [Citation12]. Reported common adverse drug reactions (ADRs) were application site reaction, nausea, hallucination, dyskinesia, somnolence, and vomiting. Other than application site reaction, which is a characteristic of transdermal formulation, these events were similar to those commonly observed in patients treated with DA [Citation11,Citation12].
However, the severity of the disease and dose were strictly regulated in the above studies (e.g. no data on patients ≥80 years of age). Thus, the results could not have thoroughly reflected the use of rotigotine under actual clinical practice. This study was conducted to evaluate the long-term effectiveness and safety of rotigotine in patients with PD under clinical practice. The results of post-marketing surveillance with a 1-year follow-up period were evaluated.
Methods
Study design and participants
This was a prospective, non-interventional, observational study targeting patients who were newly prescribed to rotigotine.
The planned population size was 600 patients. Patients were enrolled in the study within 14 days from the start of rotigotine treatment. The study period was from September 2013 to August 2016, and the follow-up was to be conducted for 1 year. However, follow-up was set to be until discontinuation for patients whose treatment period is less than a year by reasons such as lack of visits and treatment discontinuation.
This study was conducted in compliance with the Japanese Ministry of Health, Labour and Welfare (MHLW)’s Ordinance on Good Post-marketing Study Practice (GPSP): MHLW Ordinance Related to Standards for Conducting Post-Marketing Surveys and Studies on Drugs; MHLW Ordinance No. 171 issued by the MHLW on December 20, 2004. Because informed consent and ethics committee approval are not required under the GPSP, those were not mandatory in this study.
Dosage and administration
The initial adult dose for rotigotine is 2 mg/24 h once daily. The maintenance dose (standard dose: up to 16 mg/24 h) was increased as needed by 2 mg/24 h at weekly intervals by observation. The dose may be adjusted according to the patient’s condition and age; however, an increase beyond the dose of 16 mg/24 h was not permitted. The application sites of rotigotine are intact healthy skin on the shoulder, upper arm, front of your abdomen, flank, hip, or thigh. The new patch is to be used each day, and the application site should be moved (rotation) on a daily basis.
Survey items
Demographic characteristics
Sex, presence/absence of pregnancy, age, visit status (inpatient/outpatient), purpose of use, duration of disease, history of treatment with l-dopa, history of treatment with other dopamine agonists, complication (liver dysfunction, kidney dysfunction, others), medical history, hypersensitivity, Modified Hoehn and Yahr Scale (H&Y scale) for severity, Unified Parkinson's Disease Rating Scale Part III (UPDRS-III) total score, prior treatment for PD (PD treatment used 1 month prior to the start of rotigotine), and concomitant therapy were included as baseline demographic information.
Status of rotigotine administration
Status of treatment with rotigotine included daily dose in the form of daily levodopa equivalent dose (LED) [Citation19], duration of treatment, status (continue/discontinue), the reason for discontinuation (if discontinued), and adherence to application site rotation.
Safety assessments
Adverse events (AEs) were evaluated as safety assessments, and AEs were defined as medically undesirable symptoms and diseases (including abnormal clinical laboratory values) occurring or aggravated after starting rotigotine. Data were collected, and AEs were assessed according to the seriousness and causality of the event, as noted by the physician, and any causal relationship to rotigotine that could not be ruled out.
Effectiveness assessment
Clinical symptoms were evaluated by the UPDRS-III at the start of treatment (baseline), 3 months, 6 months, 9 months, and 12 months post-treatment or at discontinuation. Each UPDRS-III items were assessed in 5 levels, ranging from 0-4. Similarly, UPDRS-III total score of the 5 items possibly related to ADL [Citation20], ‘facial expression’, ‘arising from a chair’, ‘gait’, ‘postural stability’ and ‘body bradykinesia and hypokinesia’, were assessed.
Overall improvement was assessed by the physician comprehensively at 6 and 12 months post-treatment, or at discontinuation using the following 8 scales: (1) Marked improvement, (2) Moderate improvement, (3) Mild improvement, (4) Unchanged, (5) Mild worsening, (6) Moderate worsening, (7) Marked worsening, (8) Unevaluable.
Statistical analysis
AEs were coded by the Japanese Medical Dictionary for Regulatory Activities (MedDRA/J) version 19.1 in System Organ Class (SOC) and Preferred Term (PT). The occurrence of ADRs and association with demographic factors were assessed using the Cochran-Armitage trend test and Fisher's exact test. Changes in the UPDRS-III score before and after rotigotine treatment was analyzed using paired t test, and association to demographic factors were analyzed using Kruskal–Wallis test, Jonckheere–Terpstra trend test, or Fisher’s exact test. A two-sided 5% significance level was used for all analyses.
All data were analyzed using the SAS version 9.2 (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Patient disposition
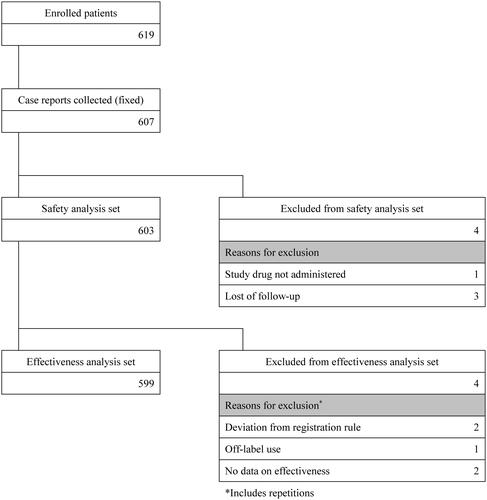
Of the 619 patients enrolled during the study, 607 case reports were collected. As a result, the safety analysis set consisted of 603 patients, and the effectiveness analysis set consisted of 599 patients ().
Demographic characteristics
Demographic characteristics are summarized in . Age distribution showed a skew in the ≥65 age group accounting for 80% of all patients. There were 112 patients (18.6%) who were ≥80 years of age. Baseline modified H&Y data showed that 70% of the patients had ≥3 severity. The mean baseline UPDRS-III total score was 33.4 ± 17.4.
Table 1. Demographic characteristics.
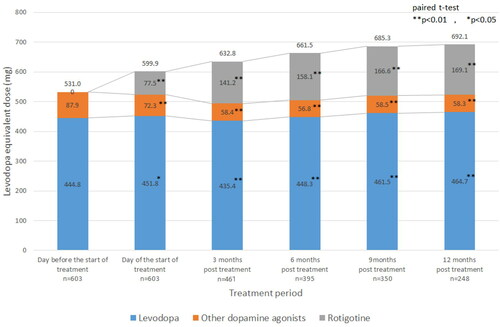
The mean initial daily dose was 2.6 ± 2.0 mg/24 h, and the mean daily dose was 4.5 ± 2.6 mg/24 h, mean maximum daily dose was 5.4 ± 3.2 mg/24 h, mean most frequent daily dose was 4.8 ± 2.9 mg/24 h, and the mean actual days of administration was 264.3 ± 162.5 days. Application site rotation was adhered to by 556 patients (92.2%). The LED at baseline, starting day of rotigotine, and 3, 6, 9, 12 months post-treatment are shown in . The overall LED increased by approximately 160 mg/day (531.0 mg/day to 692.1 mg/day). The dosage of l-dopa remained almost unchanged (approximately 450 mg/day) throughout the study. On the other hand, the mean dosage of other dopamine agonists significantly decreased compared to the baseline (87.9 mg/day) over the course of the study (p < 0.01) (). The mean dose of rotigotine was 135.7 mg/day (77.5 mg/day to 169.1 mg/day).
There were 273 patients who discontinued the treatment, and major reasons for discontinuation (multiple choices allowed) were AEs (139 patients), requested by the patient or his/her family (73 patients), hospital transfer (34 patients), and inadequate effect (25 patients). There was no notable trend observed in the timing of discontinuation.
Safety
Adverse drug reactions
ADRs of ≥1% frequency are shown in . The frequency of patients who experienced ADRs in the current study was 34.3%. The majority of events was non-serious, and there were no serious ADRs reported in ≥2 patients. Other ADRs characteristic of DA included were constipation (2 patients, 0.3%), vomiting (1 patient, 0.2%), sudden onset of sleep (1 patient, 0.2%). And binge eating and gambling disorder (1 patient, 0.2%, each) were reported as obsessive–compulsive disorder/impulse control disorder related events. Subgroup analysis stratified by age was performed, and no statistical significance in the incidence of ADRs was observed ().
Table 2. Adverse drug reactions with an incidence of ≥1%.
Table 3. Adverse drug reactions by age subgroups.
Application site reaction as adverse drug reactions
The incidence of application site reaction specifically reported in transdermal patch formulation was 170 events in 122 patients (20.2%), and all were non-serious. The most frequent application site reaction was application site pruritus (62 patients, 10.3%), followed by application site erythema (47 patients, 7.8%), application site dermatitis (34 patients 5.6%), and application site rash (11 patients, 1.8%). The most frequent daily dose of the 122 patients experiencing application site reaction at occurrence was ≥2 mg/24h to <4 mg/24h (37 patients, 30.3%), followed by ≥4 mg/24h to <6 mg/24h (30 patients, 24.6%), ≥6 mg/24h to <8 mg/24h (25 patients, 20.5%), and ≥8 mg/24h to <10 mg/24h (11 patients, 9.0%).
In the subgroup analysis by demographic characteristics, analysis with Fisher’s exact test showed significance in the category of hypersensitivity “yes” (14/34 patients, 41.2%) and “no” (105/544 patients, 19.3%) (p = 0.0042), and application site rotation adhered (113/556 patients, 20.3%) and not adhered (5/10 patients, 50.0%) (p = 0.0373).
Within 122 patients experiencing application site reactions, which were determined to be ADRs, 85 patients (69.7%) discontinued, 32 patients (26.2%) continued, 3 patients (2.5%) reduced their doses, and 2 patients (1.6%) were given a washout period. All patients recovered or were recovering regardless of the presence or absence of measures taken. For the patients who had continued the study, majority of the events (31 cases of all events, 77.5%) were treated with a topical treatment.
Effectiveness
Unified Parkinson's disease rating scale part III total score (on time)
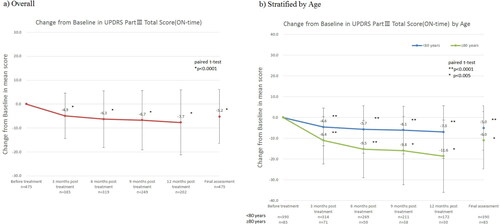
Regarding the analysis using UPDRS-III, a statistically significant reduction in the score was observed for all time points (p < 0.0001) (). The baseline mean score of 33.4 ± 17.2 decreased to 28.2 ± 16.2 at the final assessment (p < 0.0001), demonstrating improvement in the score throughout the follow-up.
In a subgroup analysis stratified by age (<80 and ≥80 years of age), a statistically significant reduction in the score was observed for all time points (). A trend in the level of effectiveness relative to the age groups was not observed.
In a subgroup analysis comparing the changes in the mean UPDRS-III scores from baseline to the final assessment stratified by demographic factors, significant differences were observed in history of treatment with other DAs “yes”/“no” (p = 0.0030), days administered (p < 0.0001), baseline UPDRS-III score (p < 0.0001), and history of treatment of PD (p = 0.0127). The mean change in score for the history of treatment with other DAs with an answer of “yes” was −4.2 ± 10.5, and “no” was −7.0 ± 12.7, and history of treatment of PD with an answer of “yes” was −5.0 ± 11.5 and “no” was −7.3 ± 9.2.
Score related to activities of daily living
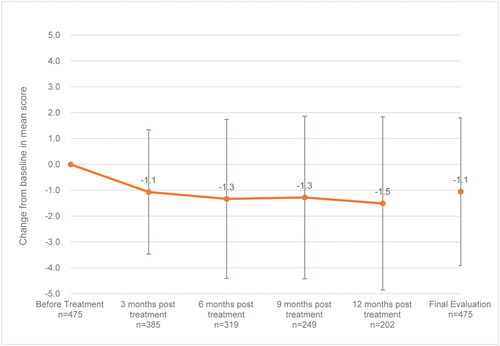
Each of the UPDRS-III items possibly related to ADL, “facial expression” (baseline: 1.5 ± 1.0, final assessment: 1.3 ± 0.9 [mean change: −0.2]), “arising from chair” (baseline: 1.6 ± 1.3, final assessment: 1.4 ± 1.2 [mean change: −0.2]), “gait” (baseline: 1.8 ± 1.1, final assessment: 1.6 ± 1.0 [mean change: −0.2]), “postural stability” (baseline: 1.7 ± 1.2, final assessment: 1.5 ± 1.1 [mean change: −0.2]) and “body bradykinesia and hypokinesia” (baseline: 2.0 ± 1.0, final assessment: 1.7 ± 1.0 [mean change: −0.2]), showed reduction in the mean scores, and the total score of the above 5 items (baseline: 8.6 ± 4.7, final assessment: 7.5 ± 4.6 [mean change: −1.1]) showed improvement ().
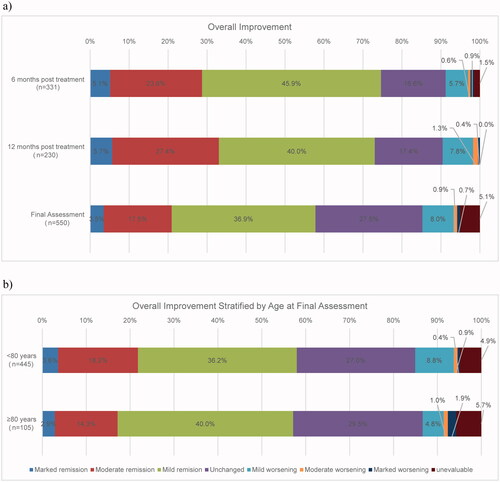
Overall improvement
In the analysis of overall improvement 12 months post-treatment, ≥70% of the patients achieved mild improvement or higher. The improvement rates were 5.7% (13/230 patients) for marked improvement, 27.4% (63/230 patients) for moderate improvement, 40.0% (92/230 patients) for mild improvement, 17.4% (40/230 patients) for unchanged, 7.8% (18/230 patients) for mild worsening, 1.3% (3/230 patients) for moderate worsening, and 0.4% (1/230 patients) for marked worsening. The rate of marked improvement and moderate improvement combined was 28.7% (95/331 patients) at 6 months post-dose, 33.0% (76/230 patients) at 12 months post-dose, and 20.9% (115/550 patients) at final assessment including discontinued cases (). An association with age was not observed in the analysis comparing the overall improvement rate of different age subgroups (<80 and ≥80) ().
Discussion
The current post-marketing surveillance study evaluated the safety and effectiveness of rotigotine in clinical practice, prescribed to patients with PD who were naive to the drug for the first time.
In the phase II/III study of Japanese patients with early-stage PD [Citation15] (early-stage PD study) and the phase III study in patients with advanced-stage PD [Citation16] (advanced-stage study), the percentage of enrolled patients ≥65 years of age were 59.1% and 56.7%, respectively. In this study, the percentage of patients ≥65 years of age in the safety analysis set was 80%. The mean ages of the above studies are 65.1 ± 8.1 years, 64.8 ± 8.8 and 71.6 ± 9.1 years, respectively. In addition, target patients for the 2 preceding studies were under 80 years of age, but our study included 18.6% of patients who were ≥80 years of age. Moreover, there were 29 patients (4.8%) who were ≥85 years of age, showing the high number of elderly patients in this study compared with that of populations generally enrolled in clinical trials. The inclusion of a wide age range of patients was possible due to the nature of this study being a surveillance study without an age restriction, and the formulation of rotigotine being a transdermal patch. Since rotigotine is a transdermal patch, it could relieve the burden of drug-taking and dysphagia in patients with PD as mentioned above [Citation14]. In addition, unlike an oral drug or injection, patch formulations have an advantage over those drugs because removal of the patch allows for the rapid lowering of drug levels. Therefore, rotigotine could be used safely by elderly patients [Citation12].
In this study, 64.0% of enrolled patients had a history of treatment with DAs other than rotigotine, in addition to having more severe PD at baseline compared with those in the early-stage study [Citation15] and the advanced stage study [Citation16]. Novel drugs are commonly used preferentially for patients facing difficulties in their treatment. This could have been one of the reasons for the high enrollment rate of patients with high severity of the disease in the study of rotigotine.
The frequency of patients experiencing an ADR was 34.3% (207/603 patients). Although the results of a clinical study and our surveillance study should not be taken as directly comparable data, the patients who experienced ADRs in the current study were lower than those reported at the time of approval (83.5%) [Citation12]. ADRs which are commonly observed in DAs are gastrointestinal symptoms, including nausea, vomiting, constipation, and autonomic disorders, including dizziness and orthostatic hypotension, and psychiatric symptoms including hallucination, delusion, and dyskinesia, sudden onset of sleep, and somnolence. Such ADRs are known to occur more frequently in patients taking a DA than in those taking l-dopa [Citation21]. The frequency of patients experiencing hallucination and delusions from taking other DAs include ropinirole [Citation22], talipexole [Citation23], cabergoline [Citation24], and pramipexole [Citation25], ranging from 1.98–8.24% to 0.40–2.38% respectively. However, the frequencies were 1.7% (10 patients) and 0.5% (3 patients) in our study. This shows that the frequency of psychiatric symptoms tends to be lower in patients taking rotigotine than in those taking other DAs. Factors associated with the above phenomena are suggested to be the pharmacodynamics quality [Citation26] of rotigotine and concentration of rotigotine in plasma that increasing gradually compared with oral drugs [Citation13], owing to its nature of being a transdermal formulation. Moreover, the high affinity of rotigotine to all dopamine receptor subtypes (D1–D5) is also considered to be an associated factor. The latest study reports that cholinesterase inhibitors are effective against psychiatric symptoms of PD [Citation27]. Although the following results are sub-group analyses of primary studies, rotigotine and apomorphine, both D1/D2 dopamine receptor stimulators, were reported to increase the concentration of intracerebral acetylcholine [Citation28,Citation29]. The affinity profile of rotigotine could have had a causative effect on the relatively low frequency of ADRs associated with psychiatric symptoms.
Application site reactions observed in the study were non-serious ADRs, and most of the patients had recovered. As reported in the results section, of patients who had continued treatment after the event of an application site reaction, 26.2% of patients were able to continue the treatment with rotigotine by using topical treatments. This shows that, although it may depend on the severity of the cutaneous AE, proper care could lead to the successful management of it. Although the numbers were low, patients who had not adhered to patch site rotation had significantly higher occurrences of application site reactions. Thus, the importance of continuous communications with the patients on proper use of the patch, especially regarding adherence to patch site rotation, was reconfirmed.
The mean change in the UPDRS-III total score from baseline to 12 months post-treatment was 7.7 ± 13.5, which was lower compared with the improvement of symptoms observed in the advanced PD study (10.9 ± 8.1) [Citation16]. This could have been due to a higher baseline UPDRS-III total score in this study (33.4 ± 17.2) than in the advanced PD study (25.8 ± 10.6) [Citation16], suggesting that our analysis set had a higher proportion of severe patients and patients with a history of DA treatment. Some of these patients could have possibly switched from or added on another DA to rotigotine, expecting an increased effect, and this could have possibly had a negative effect on overall effectiveness. In the subgroup analysis, the presence or absence of a history of treatment with other DAs was detected as one of the factors affecting the outcome, resulting in a smaller reduction in the change in score from baseline in the group with a history of other DA treatments compared with the group without the said treatment history. However, the changes in score for each assessment point showed adequate reduction from baseline for experiencing effectiveness in clinical practice, and the 5 items of UPDRS-III related to ADL showed reductions. The analysis in groups stratified by age showed that patients ≥80 years of age had no significant differences in the frequency of ADRs, compared with the younger patients (<80 years of age). Both age groups showed significant improvements in the effectiveness assessments. A recent statistical study in 2017 showed that the majority of patients with PD are in their 80 s, and 44.4% of the patients are ≥80 years of age [Citation4]. The results of this study confirmed that rotigotine improves PD symptoms in both non-elderly and elderly patients, and effects of the drug are maintained for 1 year.
Throughout the follow-up period, the dosage of l-dopa remained almost unchanged (approximately 450 mg/day). The overall LED increased by approximately 160 mg/day; however, rotigotine was of low dose. This finding could have been caused by (1) low, additional doses of rotigotine causing an improvement in symptoms by, (2) the slow progression of PD [Citation30], and there was no necessity for immediate dose increase from a risk–benefit perspective based on the physicians from their observation of the symptoms and progression status of the patients, and (3) many elderly patients in the study population, and (4) possibility of the follow-up ending during the switching of drugs. If a certain degree of improvement in symptoms was achieved by a low dose of rotigotine and improvement in the Quality of Life were achieved, a careful determination of the DA dose increases according to the patient’s condition while maintaining a uniform l-dopa dose is considered. This would reflect the actual situation in clinical practice, which would leave the door open for further dose increases in cases of disease progression.
Limitations
The limitation of the current study included the study design being a non-interventional study without a control group. In this study, factors such as the type of concomitant therapies taken during the study or changes in the dose which may have had an impact on the data were not considered. Thus, there are limitations to this report, and the interpretation of the results must be interpreted carefully.
Conclusion
This was a prospective, non-interventional, observational study targeting patients with PD with a 1-year follow-up period, evaluating the safety and effectiveness of rotigotine use in daily clinical practice. Our study included a high percentage of elderly patients compared to other clinical studies. There were no new or notable safety concerns observed in the frequency and type of ADRs, and the effectiveness of rotigotine was suggested under daily clinical practice.
Author contributions
Hidefumi Ito and Hiroyuki Kondo contributed to analysis and interpretation of the data. Tomoyo Takayama contributed to study design and data collection. All authors contributed to drafting and revising the manuscript and provided final approval of the publication version.
Acknowledgments
The authors would like to thank all patients who participated in this study, as well as supporters and physicians of the study sites. The authors received medical writing assistance from Shiori Mikami (SunFlare Co., Ltd., Tokyo, Japan) for the preparation of the initial and final drafts of the manuscript, and the fee was funded by Otsuka Pharmaceutical Co., Ltd.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zigmond MJ, Burke RE, et al. Pathophysiology of Parkinson's disease. In: Davis KL, Charney D, Coyle JT, editors. Neuropsychopharmacology: The fifth generation of progress. Philadelphia (PA): Lippincott Williams & Wilkins; 2002. p. 1781–1793. Available from https://acnp.org/wp-content/uploads/2017/11/C123_1781-1794.pdf.
- Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26 Suppl 1:S1–S58.
- Yamawaki M, Kusumi M, Kowa H, et al. Changes in prevalence and incidence of Parkinson's disease in Japan during a quarter of a century. Neuroepidemiology. 2009;32(4):263–269.
- Ministry of Health, Labour and Welfare. [Summary of statistical study on patients 2017]. https://www.e-stat.go.jp/stat-search/files?page=2&layout=dataset&toukei=00450022&tstat=000001031167&stat_infid=000031790685 [Japanese].
- Schapira AH. Present and future drug treatment for Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76(11):1472–1478.
- Huot P, Johnston TH, Koprich JB, et al. The pharmacology of l-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev. 2013;65(1):171–222.
- Brooks DJ. Dopamine agonists: their role in the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2000;68(6):685–690.
- Rascol O, Brooks DJ, Korczyn AD, et al. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342(20):1484–1491.
- Group PS. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. JAMA. 2000;284(15):1931–1938.
- Lieberman A, Olanow CW, Sethi K, the Ropinirole Study Group, et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Neurology. 1998;51(4):1057–1062.
- Japanese Society of Neurology. [Practical guideline for Parkinson's Disease 2018]. https://www.neurology-jp.org/guidelinem/parkinson_2018.html (Japanese).
- Otsuka Pharmaceutical Co., Ltd. [Neupro® Patch Interview form, revised March 2016 (version 7)].
- Elshoff JP, Braun M, Andreas JO, et al. Steady-state plasma concentration profile of transdermal rotigotine: an integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Ther. 2012;34(4):966–978.
- Hatano T, Hattori N. [Questionnaire survey on adherence in Parkinson’s disease patients]. Pharma Medica. 2013;31(5):101–107 (Japanese).
- Mizuno Y, Nomoto M, Kondo T, Rotigotine Trial Group, et al. Transdermal rotigotine in early stage Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Mov Disord. 2013;28(10):1447–1450.
- Mizuno Y, Nomoto M, Hasegawa K, Rotigotine Trial Group, et al. Rotigotine vs ropinirole in advanced stage Parkinson's disease: a double-blind study. Parkinsonism Relat Disord. 2014;20(12):1388–1393.
- Giladi N, Boroojerdi B, Korczyn AD, et al. Rotigotine transdermal patch in early Parkinson's disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord. 2007;22(16):2398–2404.
- LeWitt PA, Lyons KE, Pahwa R, SP 650 Study Group. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology. 2007;68(16):1262–1267.
- Tomlinson LC, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653.
- Nomoto M, Iwaki H, Kondo H, et al. Efficacy and safety of rotigotine in elderly patients with Parkinson's disease in comparison with the non-elderly: a post hoc analysis of randomized, double-blind, placebo-controlled trials. J Neurol. 2018; 265(2):253–265.
- Zhang J, Tan LC. Revisiting the medical management of Parkinson's disease: Levodopa versus dopamine agonist. Curr Neuropharmacol. 2016;14(4):356–363.
- GlaxoSmithKline plc. [ReQuip CR Tablets 0.25 mg, 1 mg, 2 mg Interview form_Oct.2019] [Japanese].
- Nippon Boehringer Ingelheim Co., Ltd. [Domin® Tablets 0.4 Interview form_Aug.2019] [Japanese].
- Pfizer Inc. [Cabasar® Tablets 0.25 mg, 1.0 mg Interview form_Sep.2019] [Japanese].
- Nippon Boehringer Ingelheim Co., Ltd. [BI Sifrol® Tablets 0.125 mg, 0.5 mg Interview form_Aug.2019] [Japanese].
- Walker DK. The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. Br J Clin Pharmacol. 2004;58(6):601–608.
- Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015;86(2):135–143.
- Day JC, Fibiger HC. Dopaminergic regulation of septohippocampal cholinergic neurons. J Neurochem. 1994;63(6):2086–2092.
- Sohya K, O'Hashi K, Toma H, et al. Different effects of rotigotine and ropinirole on cholinergic transmission in the mouse medial prefrontal cortex. Psychogeriatrics. 2017;17(6):502–503.
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14.