Abstract
Background: Despite the increase in ventriculoperitoneal shunt surgeries performed for children with hydrocephalus, the potential complications and survival of patients after the procedure remains the major challenge for both clinical and public health aspects. This study intends to assess the survival status and scrutinize the predictive factors of mortality among children after a ventriculoperitoneal shunt.
Methods: A retrospective cohort study was employed by reviewing charts of 337 systematically selected children who have undergone a ventriculoperitoneal shunt from 2016 to 2018 in Addis Ababa. The extraction tool was used for data collection, Epi-data version 4.4.2 for data entry, and Stata version-14 for cleaning and analysis. Kaplan-Meier curve, log-rank test, and life table were used to describe the data. Cox proportional hazard regression model was used for analysis. Any variable at p < 0.25 in the bi-variable analysis was fitted to multivariate analysis, and significance was declared at p ≤ 0.05. Then, AHR with 95% CI was used to report the association and test the statistical significance. Finally, texts and tables were used to present the results.
Results and Conclusion: The incidence rate of mortality was 58.4 per 1000 child-months of observation with a median survival time of 12 months (95%CI: 9.04–14.96). Communicative hydrocephalus (AHR: 1.99, 95% CI: 1.18–3.36), post-traumatic brain injury (AHR: 7.43, 95% CI: 3.21–16.88), emergency surgery (AHR: 1.86, 95% CI: 1.17–3.13) as well as revised shunt procedure (AHR: 8.01, 95% CI: 6.12–13.43) were independent predictors of death. Besides, sunset eye (ARH: 2.01; 95% CI: 1.17–3.47), rapidly increased head size (ARH: 2.05, 95% CI: 1.14–3.37), prolonged antibiotics treatment (AHR: 2.46, 95% CI: 1.82–7.37), and gram-negative infections (AHR: 1.95, 95% CI: 1.60–12.64) were also significantly associated. Hence, health professionals ought to give special attention to patients with identified predictors.
Introduction
Ventriculoperitoneal shunting is a sterile procedure that involves the insertion of a tube inside the brain ventricles and tunnels underneath the skin via the abdominal cavity to enable CSF reabsorption [Citation1–5]. Ventriculoperitoneal (VP) shunt surgery is the primary treatment option for patients with hydrocephalus throughout the world. According to a survey of pediatric acute care facilities, over 19,000 CSF shunts are placed annually in Africa, where 17,789 were ventriculoperitoneal [Citation5,Citation6].
In the past five decades, VP shunt became the most commonly performed type of shunt procedure (accounts for 98.4% of all shunt types) for the management of hydrocephalus globally [Citation3,Citation7,Citation8]. Currently, the VP shunt system has three parts, including an inflow (proximal) catheter, a valve mechanism, and an outflow durable plastic tube [Citation9,Citation10]. The shunting system can be classified as ventriculoperitoneal, ventriculoatrial, ventriculopleural, and limboperitoneal [Citation11].
Globally, the survival rate of children at 2–5 years after undergoing VP shunt procedure ranges from 67.6–79.14% [Citation2,Citation12,Citation13]. Despite the undeniable improvement in the coverage of shunting treatment for pediatric patients with hydrocephalus, the survival of patients remains the major challenge for both clinical and public health aspects [Citation14,Citation15]. It is associated with potential complications that may require multiple clinical interventions throughout the patient’s lifetime, with reported frequency ranging from 45–59% in the pediatrics population [Citation16–18]. The most commonly identified complications include shunt obstruction, shunt infection, wound infection, disconnection, dislocation, migration breakage, and excessive drainage [Citation19].
In Ethiopia, neurosurgical procedures were not carried out until the late 1960s [Citation20]. But, the current well-structured VP shunt surgical management was started in March 2006 [Citation20,Citation21]. VP shunting is the advanced and preferred treatment of hydrocephalus regardless of etiologies. It accounts for 71.8% of the primary neurological procedures for pediatric patients [Citation6,Citation20,Citation22]. Nevertheless, the factors affecting the survival status of children with hydrocephalus after VP shunt surgery remain equivocal. Available studies worldwide reported mainly on complications and infection rates after VP shunt operation rather than survival status. Furthermore, we could not find a single study conducted to assess the survival status of children after ventriculoperitoneal shunt surgery and explore factors that predispose death throughout the country except for prevalence studies. Thus, the current study aims to fill the abovementioned gap.
Methods
Study area, period, and design
A hospital-based retrospective follow-up study was conducted in Addis Ababa, the capital city of Ethiopia, by reviewing three years of follow-up data on charts of children aged below 15 years with hydrocephalus who started to have ventriculoperitoneal shunt surgery from 2016 to 2018. Addis Ababa was located at the heart of the country, with an estimated population of 7.8236 Million divided into ten sub-cities [Citation23]. The city has twelve governmental and nine private hospitals, of which seven hospitals (Zewuditu Memorial, St. Peter, Korean missionary hospital, Teklehaymanote general hospital, Charismas neurosurgical center, Betel, and St. Yared general hospital) provide VP shunt service.
Study population
All children who underwent VP shunt surgery from January 2016 to December 30, 2018, at selected hospitals in Addis Ababa and whose chart is complete and available during data collection time were incorporated. Charts of children with incomplete forms and not available during data collection were excluded.
Sample size determination and sampling procedure
A single proportion population formula was used to estimate the sample size by considering the following statistical assumptions: Zα/2 = 1.96 (at 95% confidence level), 32.4% proportion of death (p), and 67.6% proportion of survivors (1-p) among children managed with VP shunt surgery from a study conducted in Uganda [Citation12], and 5% degree of freedom which brought 337. Thus, 337 charts were required and included for review.
Among those hospitals providing VP shunt service, three hospitals were selected randomly using the lottery method. The total VP shunt insertion procedures performed at each hospital were 548 at Zewuditu memorial hospital, 236 at St. Peter hospital, and 221 at Korea Missionary hospital. Next, 337 subjects were proportionally allocated to each selected hospital (Zewuditu memorial hospital = 184, St. Peter hospital = 79, and Korea Missionary hospital = 74) with their respective number of cases. A systematic sampling technique was used to select charts to be reviewed at each hospital, with every three charts incorporated after determining the first case by lottery method. Whenever there appear missed charts or charts with incomplete data, the next charts were considered.
Operational definitions and measurements
The event was considered when the death occurred on follow-up after the initial VP shunt placement to the end of the study. Those patients who recovered, remain on following during study period, lost, transferred outs, and those exceed 15 years birthday during follow up were considered as censored. A ventriculoperitoneal Shunt is small tubing placed inside the brain’s ventricle and tunneled underneath the skin to the abdominal cavity. Shunt Failure was defined as any blockage, disconnection, displacement, shunt extrusion, migration, over drainage, and shunt site infection requiring revision of the shunt system. Survival time was the time from initial VP shunt placement to death or censored and is measured in months.
Data collection tools and procedure
The information available in the patient charts was checked first, and an appropriate data extraction checklist was prepared in English. The checklist was adapted from the neurologic clinic follow-up registration book and by reviewing different related articles. The extraction tool comprised of socio-demographic characteristics, obstetric factors, diseases and diagnostic related factors, as well as management-related variables. The lists of charts were retrieved from the database in the electronic recording system and follow-up registration logbooks in each selected hospital using the patient’s medical registration number. Three data collectors (BSc nurses working out of the selected hospitals) and one supervisor (MSc student) were hired, and the data collection was accomplished within a month from March 1–30, 2019.
Data quality control
To maintain data quality, the checklist was pretested on 17 (5% of participants) randomly selected charts at Teklehaymanote general hospital and Charismas neurosurgical center before starting actual data collection. Two days of training was given for three data collectors (BSC nurses) and one supervisor (BSC nurse, MSc fellow) working outside the selected hospitals concerning the data collection tool and data collection process before the actual data collection period. The principal investigator and supervisor crosschecked the collected data to the source document on randomly selected charts for completeness and consistency, and charts with incomplete data during data collection were excluded.
Data processing and analysis
After the completion of data collection, the collected data were checked thoroughly by observation for any unfilled or inappropriate responses. Next, the data were entered into Epi-data manager version 4.4.2.1 and exported to SPSS version 25 and STATA version 14 for cleaning, edition, coding, and analysis. The nature of data such as normality and presence of outliers were determined before data analysis. Then, the data were described using relative frequency, percent, mean with standard deviation, and median based on its applicability. Life-table was used to estimate the cumulative probabilities of death at different time intervals. Kaplan Meier’s survival curve was considered to estimate median survival time during the follow-up period and log-rank tests to compare survival curves for possible differences in mortality among the groups.
The bi-variable analysis was carried out to identify possible associations between mortality and each covariate using Cox proportional hazard model. Those variables having p ≤ 0.25 were fitted to the final model to identify independent predictors of mortality.
Multi co-linearity between independent variables was checked using the variance inflation factor that all outputs were within the acceptable range. The proportional hazard assumptions were also tested using the global test with a value of p = 0.9224, which was highly insignificant. Finally, the Cox regression model for its fitness to the data was checked using the log-likelihood ratio. Generally, we could conclude that the final model fits successfully. In the multivariate analysis, any statistical test was considered significant at p ≤ 0.05. Then, the association between death and independent variables was declared using an adjusted hazard ratio with 95% CI. Finally, tables and texts were used to present the results.
Ethical consideration
Ethical clearance was obtained from the Institutional Review Board of Addis Ababa University, College of Health sciences. A cooperation letter, written by the Nursing and Midwifery departments, was submitted to each selected hospital. Permission for data collection was obtained from hospital administrators on behalf of patients since the study was conducted through a review of medical records. For certainty of anonymity and confidentiality, data were coded and reported as combined.
Results
A total of 337 charts of children with hydrocephalus having VP shunt inserted were included for analysis. Thirteen [Citation13] charts were excluded during data extraction; five for incomplete data and eight due to missed charts at the time of data collection. In this case, charts with medical record numbers next to the missed chart or those with incomplete data were incorporated for review.
Socio-demographic characteristics of the study participants
In this study, 197 (58.5%) of the study participants were males, of which 51 (25.9%) children died whereas 48 (34.3%) female participants passed away. Regarding the age of study subjects, the majority of deaths, 42 (33.1%), were recorded among children before six months of age, while the minimum proportion of mortality, 12 (23.5%), occurred among those aged above five years. The mean weight of children at admission was found to be 10.9536 kgs (SD ±6.34546) with a minimum of 3.1kgs and a maximum of 48 kgs ().
Table 1. Distribution of socio-demographic characteristics among children with hydrocephalus managed with VP shunt insertion at selected hospitals, Addis Ababa, Ethiopia, 2020 (N = 337).
Obstetric factors
Of the total 337 participants, 189 (56.08%) were delivered spontaneously per vagina, 108 (32.05%) by cesarean section, and 40 (11.9%) were born after instrument-assisted delivery. The study also revealed that the proportion of death among children delivered spontaneously per vagina, by cesarean section, and using instrumental delivery was 31.2%, 28.7%, and 22.5% consecutively. On the other hand, 82 (24.3%) children born before 37 weeks of gestation, and seven (2.1%) participants were with an unknown maturity level.
Diseases and diagnostic related factors
The study result showed that 117 (34.7%) study subjects were diagnosed as having hydrocephalus using CT scan, 95 (28.2%) with the help of MRI, 70 (20.8%) by U/S, and 55 (16.3%) were diagnosed by using both MRI and CT. Among 269 (79.9%) children investigated for CSF culture, 130 (48.3%) were found to be culture-positive, of those 31 (23.8%, n = 130) caused by gram-negative microbes. Moreover, 210 (62.3%) children developed hydrocephalus congenitally, of which 51 (22.9%) died. Again, 112 (33.2%) acquired from secondary causes, with 44 (39.3%) deaths recorded. The majority of the participants, 214 (63.5%), were diagnosed with obstructive hydrocephalus. Nevertheless, the proportion of death was higher among children with communicative hydrocephalus (31.1%) ().
Table 2. Diseases related characteristics of children with hydrocephalus who were undergoing VP shunt at selected hospitals, Addis Ababa, Ethiopia, 2020 (N = 337).
Management-related factors
The mean hospital stay was 9.42 ± 5.501SD days with a minimum of two and a maximum of 30 days. Similarly, the duration of antibiotic therapy ranged from 2 to 30 days with a mean of 8.33 ± 5.144 SD days. The mean duration of patients since the initial shunt was 4.74 ± 3.82 SD months with a range of 15 days to 26 months. Based on the finding, the common causes of hydrocephalus that require emergency VP shunt surgery were post-traumatic brain injury, hemorrhage, and aqueduct stenosis. The proportion of death was higher (53%) among children who had undergone emergency VP shunt surgery than those operated electively (25.3%). Of the total 337 procedures performed, only 29 (8.6%) were performed by a pediatrics neurosurgeon with eight deaths, while the rest 91 (92%) deaths were recorded after surgery performed by a non-pediatrics neurosurgeon. Even though only 27 (8%) inserted shunts were revised, the proportion of death was higher (48.1%) than those who did not require revised shunt insertion (27.7%). Likewise, the proportion of children who died after occipital (57.1%) and left-side insertion (30.8%) was higher than among those shunted on the right side (28.8%) ().
Table 3. Distribution of management-related factors among children diagnosed with hydrocephalus who had undergone VP shunt surgery at selected hospitals, Addis Ababa, Ethiopia, 2020 (N = 337).
When we see patients complaints during follow up; 45 (13.4%) had sunset eyes, 85 (25.2%) rapid head size increment, 23 (6.9%) had visual deficit, 15 (4.4%) tense anterior fontanel, 15 (4.4%) distention of scalp vein, 15 (4.4%) loss of consciousness, 77 (22.8%) fever, 29 (8.7%) abdominal distension, and 34 (10%) abdominal tenderness. After shunt insertion, children who took gentamycin, ampicillin, furosemide, and tramadol had a better chance of survival compared to those who took other antibiotics and paracetamol. Besides, 99 (29.3%) developed sign of shunt failure such as obstruction, shunt infection, excessive drainage, and others (dislocation, migration, disconnection) among 40 (11.7%), 32 (9.5%), 5 (1.4%), and 8 (2.3%) children respectively.
Comparison of survival status
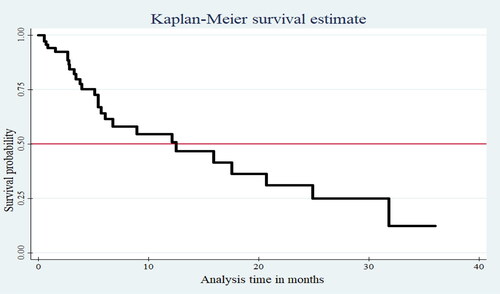
The Kaplan Meier survival curve decreased stepwise as the follow-up time increased, and it crosses the survival function at a survival probability of 0.5 (). The log-rank test also showed that most variables had statistically significant survival differences.
Survival function and incidence density rate of mortality
Children were followed for a minimum of half a month and a maximum of 36 months, with the majority of them followed for three months after VP shunt insertion. The finding also revealed that 99 (29.4%) children died during the follow-up period, and 238 (70.6%) were censored. Among those considered as censored, 204 (85.7%) have been alive up to the end of the follow-up period, 27 (11.6%) lost, and seven (2.7%) were transferred to other institutions. The total person-time observation was 1695.39 child-months with cumulative incidence mortality of 58.4 per 1000 child-month observations or 70 per 100 child-year observations. The incidence of mortality among children diagnosed with congenital hydrocephalus was 30.1 per 1000 child-month observations whereas it was 26 per 1000 child-month observations among those with acquired hydrocephalus. The median survival time of patients after VPS surgery was 12 months (95%CI: 9.04–14.96). In addition, the cumulative probabilities of survival at the end of 6, 12, 18, and 24 months were found to be 0.75, 0.55, 0.30, and 0.13 consecutively. The overall cumulative survival at 36 months of follow-up after VPS surgery was 13% (95% CI, 6.09 to 18.67%).
Predictors of mortality
After data description, the bi-variable analysis was conducted using Cox proportional hazard regression model to determine the variables that should be included in the final model for multivariate analysis. Some variables (site of the shunt, past procedure, and causes of hydrocephalus) were left out of the final model since they have less than 20% expected events per cell. However, the type of hydrocephalus was fitted in the final model despite it had a cell containing four counts. This is because the type of hydrocephalus was expected to be the most significant variable, and its effect should have been determined. This was done by loaning two cases for the normal-pressure group by subtracting one case from each of the communicative and obstructive categories after computing descriptive statistics.
Finally, communicative type of hydrocephalus, post-traumatic brain injury, emergency shunt insertion, revised shunt procedure, rapid increases in head size during follow-up, taking antibiotics ≥7 days, having sunset eye appearance during follow-up, and infected with gram-negative pathogen were found to be independent predictors of mortality in children with hydrocephalus having VP shunt inserted at 95% confidence level ().
Table 4. Bi-variable and multivariable analysis output for children with hydrocephalus who had undergone VP shunt insertion operation at selected hospitals, Addis Ababa, Ethiopia, 2020 (N = 337).
Children with a diagnosis of communicative hydrocephalus were 1.99 times at higher hazard of death than their counterparts did (AHR: 1.99, 95% CI: 1.18–3.36). Again, those children who develop hydrocephalus from post-traumatic brain injury were 7.43 (AHR: 7.43, 95% CI: 3.21–16.88) times at an increased hazard of death compared to those who did not have a traumatic brain injury. Likewise, the risk of death among patients who underwent emergency VP shunt operation was 1.86 (AHR: 1.86, 95% CI: 1.17–3.13) times greater than those managed with elective surgery. Furthermore, children with a revised shunt inserted had 8.01 times higher hazard to death than their counterparts (AHR: 8.01 95% CI: 6.12–13.43).
The other factor that independently associated with death was head size increment during follow-up. Patients who had rapid head size increment during follow-up were 2.05 more hazard of death than those who did not have (ARH: 2.05; 95% CI: 1.14–3.37). Similarly, participants who had sunset eye appearance during follow-up were 2.01 times at higher hazard of death than those without the symptom (ARH: 2.01; 95% CI: 1.17–3.47). On the other hand, the risk of death among patients who received antibiotics medications for ≥ seven days was 2.462 times greater than children who took for < 7 days (AHR: 2.46, 95% CI: 1.82–7.37). Additionally, children who were infected by gram-negative pathogens had 1.95 (AHR: 1.95, 95% CI: 1.60–12.64) times the higher hazard of mortality ().
Discussion
This retrospective study aimed to assess the survival status and the factors that predict mortality among children who had undergone VP shunt operation for hydrocephalus management. At the end of the follow-up, about 99 (29.4%) patients died. The incidence rate of mortality was 58.4 per 1000 child-months of observation. The median survival time was found to be 12 months (95%CI: 9.04–14.96). Having communicative hydrocephalus, post-traumatic brain injury, emergency shunt insertion, revised shunt procedure, rapid head size increment during follow-up, prolonged antibiotic therapy, and having sunset eye appearance during follow-up were found to be independent predictors of mortality.
The overall incidence of mortality in this study was 70 per 100 child-years observation, which is higher than findings in Korea 35.7 per 100 person-years observation [Citation24]. Apart from economic variety, this discrepancy might be due to the variation in the length of follow-up in which the previous study involved two years of follow-up. Therefore, that will reduce the total person-time observations that would inturn decrease the incidence of mortality. Again, the number of deaths was expected to increase proportionally with the length of time after VP shunt inserted, which could escalate the mortality rate. To support this, the cumulative probability of survival was decreased from 75% at six months to 13% after 36 months of follow-up from VP shunt insertion in our study.
Children having spinal Bifida were found to be at higher hazard of death than their counterparts did. Other studies conducted in Uganda, India, and Pakistan [Citation2,Citation25,Citation26] supported this finding. The possible justification could be because of the impaired circulation of CSF due to the mass that could alter the patency of the spinal cord. This blockade will lead to excess CSF leakage and accumulation in the brain ventricles, disease exacerbation, and an increased probability of death.
The study result also revealed that post-traumatic brain injury was a significant predictor of mortality among children who underwent VPS operation. This finding is consistent with reports of studies conducted in Pakistan, Uganda, and South Korea [Citation10,Citation26,Citation27]. Several reasons could be stated for this finding. For instance, the possible immediate complications such as acute infection (meningitis) and CSF leak could block brain nerve endings. Furthermore, limited accessibility of neurologic services for timely intervention might complicate the management and decrease the chance of children’s survival even though VP shunt insertion.
Those children diagnosed with a communicative type of hydrocephalus were at an increased hazard of death, which was congruent with reports from Bengihamin, Pakistan, and Sweden [Citation26,Citation28,Citation29]. The possible rationale could be the severity and the complicated nature of the communicative type of hydrocephalus. Unlike the other types of hydrocephalus, where symptoms resolved immediately after VP shunt insertion, the communicative type needs prolonged treatment and effective follow-up. Consequently, an extended hospital stay and an increased chance of complications further add a challenge for the patient as well as the healthcare system.
Moreover, emergency shunt insertion was reported as an independent predictor of mortality among children with hydrocephalus. The study conducted in India contradicts this finding [Citation25]. This difference might be due to setting variations in technology advancement with equipment and diagnostic aids to perform the procedure timely and as quickly as possible. On the other hand, children with a revised shunt inserted were more likely to die than those with an initial shunt inserted, which agreed with previous study findings [Citation24,Citation25]. Possibly, the reason for this could be repeated procedure that increases the chance of procedure-related infection acquisition. Besides, repeated insertion involves many body parts that might lead to the destruction of multiple ventricular and neural pathways serving for the CSF circulation system during shunt insertion. On the other hand, the time needed for reinsertion indicates the failure of the initial shunt that might be done with the appointment. Hence, the patient will remain without treatment for a while on transition in which the disease will progress rapidly.
Prolonged antibiotic therapy (≥7 days) increased the hazard of mortality by 2.46, which was incompatible with other study findings [Citation26,Citation30]. This effect of this predictor might be influenced by the presence or absence of complications during the procedure as well as after then. Logically, patients who took antibiotics for a shorter duration indicated a better response to the therapy and did not develop complications. On the contrary, the need for prolonged antibiotic intake might infer insufficient response to treatment and/or the presence of complications that could limit the chance of survival.
Furthermore, having a sunset eye appearance and a rapid increase in head sizes during follow-up were reported as significant predictors of death. This finding was conformable with previous study findings [Citation7,Citation22,Citation25]. The explanation could be, as those signs are cardinal signs of hydrocephalus though on VP shunt management, which could indicate ineffective treatment and recurrence of the diseases. This study also revealed that patients infected with gram-negative pathogens were found to be at an increased hazard of death. The result was also proved true in studies conducted in Hong Kong and Korea [Citation18,Citation24]. The possible justification might be the universal fatal nature of gram-negative pathogens compared to gram-positives despite the high prevalence of infection caused by gram positives.
Conclusion
The incidence of mortality among children undergoing VP shunt insertion procedure remains unacceptably high that children dying at a rate of 58.5%. Consequently, children who had communicative hydrocephalus, post-traumatic brain injury, emergency surgery, revised shunt procedure, the rapid increase in head size, sunset eye appearance, antibiotic therapy ≥ seven days, and gram-negative infections were at increased hazard of death after VPS surgery.
Ethical approval and consent to participate
Ethical clearance and ethical approval were obtained from the Intuitional Review Board (IRB) of Addis Ababa University. The IRB waived such that the research could be done by record review without contacting patients since the study was retrospective. A cooperation letter was obtained from the School of Nursing and Midwifery. Permission letters were obtained from each hospital administration and coordinators of the neurologic clinics. All information was kept confidential, and no individual identifiers were collected.
Authors’ contributions
ABW, WEA & YBA participated in the study conception and design as well as collection and interpretation of data. GS & FA advised during proposal development, edited the proposal, and advised during data analysis. MSM, ABW & TMN participated in the design, performed the statistical analysis, as well as drafted and critically revised the manuscript. MSM edited the manuscript and formatted it for publication. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Consent for publication
Not applicable.
| Abbreviations | ||
| BSc | = | bachelor of science |
| MSc | = | master of science |
| CSF | = | cerebrospinal fluid |
| CT | = | computed tomography |
| ETV | = | endoscopic third ventricles |
| EVD | = | extra ventricular drains |
| MMC | = | meningomyelocele |
| MRI | = | magnetic resonance imaging |
| NPH | = | normal pressure hydrocephalus |
| VP | = | ventriculoperitoneal |
Acknowledgments
The authors would like to thank data collectors, supervisors, and respective hospitals involved in the study for their wholehearted contributions. We also want to appreciate Addis Ababa University that offered the chance and sponsored this study.
Disclosure statement
The authors declared no conflict of interest
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. However, St Peter Specialized Hospital provided the financial backing of this research. The funder had no role in study design, data collection, analysis, preparation of the manuscript, and decision on publication.
Data availability statement
Extra data that support the findings of this study are available from the corresponding author upon reasonable request and can be shared upon legal request via [email protected]
References
- Drake J, Kestle J. Ventriculoperitoneal shunt 30-day failure rate. Neurosurgery. 2014;74(1):11–15.
- Khan F, Shamim MS, Rehman A, et al. Analysis of factors affecting ventriculoperitoneal shunt survival in pediatric patients. Childs Nerv Syst. 2013;29(5):791–802.
- Rinaldo L, Lanzino G, Elder BD. Predictors of distal malfunction after ventriculoperitoneal shunting for idiopathic normal pressure hydrocephalus and effect of general surgery involvement. Clin Neurol Neurosurg. 2018;174(9):75–79.
- Heyman J, Ved R, Amato-Watkins A, et al. Outcomes of ventriculoperitoneal shunt insertion in the management of idiopathic intracranial hypertension in children. Childs Nerv Syst. 2017;33(8):1309–1315.
- Nigim F, Critchlow JF, Kasper EM. Role of ventriculoperitoneal shunting in patients with neoplasms of the central nervous system: an analysis of 59 cases. Mol Clin Oncol. 2015;3(6):1381–1386.
- Laeke T, Tirsit A, Biluts H, et al. Pediatric hydrocephalus in Ethiopia: treatment failures and infections: a hospital-based, retrospective study. World Neurosurg. 2017;100:30–37.
- Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014;81(2):404–410.
- Adalberto PMS, Va FJ, Agustín C, et al. Ventriculo-peritoneal shunting devices for hydrocephalus. Cochrane Database Syst Rev. 2017;2017(7):CD012726.
- Segal-Gidan F. Neurology and family medicine at keck school of medicine, USC, and in gerontology at L. CE/CME. Allen Institute for AI proceedings. 2015:44–49.
- Khan F, Rehman A, Shamim MS, et al. Ventriculoperitoneal (VP) shunt survival in patients developing hydrocephalus after cranial surgery. J Neurol Neurophysiol. 2016;26(3):369–377.
- Christian EA, Melamed EF, Peck E, et al. Surgical management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant. PED. 2016;17(3):278–284.
- Warf BC, Dagi AR, Kaaya BN, et al. Five-year survival and outcome of treatment for postinfectious hydrocephalus in ugandan infants. J Neurosurg Pediatr. 2011;8(5):502–508.
- Paff M, Alexandru-Abrams D, Muhonen M, Loudon W. World journal of assessment of complications of VP shunt. Interdiscip. Neurosurg. 2018;4(8):60–2.
- Low D, Drake JM, Seow WT, et al. Management of ventriculo-peritoneal shunts in the paediatric population. Asian J Neurosurg. 2010;5(1):7–14.
- Hanak BW, Bonow RH, Harris CA, et al. Cerebrospinal fluid shunting complications in children. Pediatr Neurosurg. 2017;52(6):381–400.
- Kulkarni AV, Drake JM, Kestle JRW, et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children. Neurosurgery. 2010;67(3):588–593.
- Pal SS, Dubey S. A study of VP shunt in the management of hydrocephalus. Int Surg J. 2017;4(5):1697–1701.
- Woo PYM, Wong HT, Pu JKS, et al. Primary ventriculoperitoneal shunting outcomes: a multicentre clinical audit for shunt infection and its risk factors. Hong Kong Med J. 2016;22(5):410–419.
- Wasserman S, Faust K. Structural balance, and transitivity. Soc Netw Anal Methods Appl. 2014;41:220–248.
- Lund-Johansen M, Laeke T, Tirsit A, et al. An Ethiopian training program in neurosurgery with Norwegian support. World Neurosurg. 2017;99:2–5.
- Baird LC, Mazzola CA, Auguste KI, et al. Effect of valve type on cerebrospinal fluid shunt efficacy. PED. 2014;14(Supplement_1):35–43.
- Kim HM, Kim KH. Clinical experience of infantile posthemorrhagic hydrocephalus treated with ventriculo-peritoneal shunt. Korean J Neurotrauma. 2015;11(2):106–111.
- The population of Addis Ababa in the year 2019 as per estimates; 2019. Available from: http://worldpopulationreview.com/world-cities/Addis-Ababa
- Lee JK, Lee JH, Choi JH, et al. Incidence and risk factor study of ventriculoperitoneal shunt infections in children. Int J Infect Dis. 2012;16:e201.
- Pan P. Outcome analysis of ventriculoperitoneal shunt surgery in pediatric hydrocephalus. J Pediatr Neurosci. 2018;13(2):176–181.
- Ibrahimou B, Kodali S, Salihu H. Survival of preterm singleton deliveries: a population-based retrospective study. Adv Epidemiol. 2015;2015:1–6.
- Park MK, Kim M, Park KS, et al. A retrospective analysis of ventriculoperitoneal shunt revision cases of a single institute. J Korean Neurosurg Soc. 2015;57(5):359–363.
- Gora NK, Gupta A, Sinha VD. Cerebellar mutism syndrome following midline posterior fossa tumor resection in children. J Pediatr Neurosci. 2017;12(4):313.
- Hussain M, Raja RA, Shaikh AU, et al. Ventriculoperitoneal shunt blockage. J Ayub Med Coll Abbottabad. 2012;24:3–4.
- Lee L, Low S, Low D, et al. Late pediatric ventriculoperitoneal shunt failures: a Singapore tertiary institution’s experience. Neurosurg Focus. 2016;41(5):E7.