Abstract
Purpose
Microglia-mediated inflammation is associated with perioperative neurocognitive disorders (PNDs) caused by sevoflurane. Dexmedetomidine has been reported to protect against sevoflurane-induced cognitive impairment. In this study, we investigated the effects and underlying mechanisms of dexmedetomidine on sevoflurane-induced microglial neuroinflammation and PNDs.
Methods
Wild-type and purinergic ionotropic 4 receptor (P2X4R) overexpressing C57/BL6 mice were intraperitoneally injected with 20 μg/kg dexmedetomidine or an equal volume of normal saline 2 h prior to sevoflurane exposure. The Morris water maze (MWM) test was performed to assess cognitive function. Immunofluorescence staining was employed to detect microglial activation. The expression levels of proinflammatory cytokines were measured by real-time quantitative PCR (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA). The protein levels of P2X4R and NOD-like receptor protein 3 (NLRP3) were detected by Western Blotting.
Results
Sevoflurane increased the number of microglia, upregulated the levels of proinflammatory cytokines, elevated the protein levels of P2X4R and NLRP3 in the hippocampus and induced cognitive decline, while pretreatment with dexmedetomidine downregulated the protein levels of P2X4R and NLRP3, alleviated sevoflurane-induced microglial neuroinflammation and improved cognitive dysfunction. Moreover, overexpression of P2X4R weakened the neuroprotective effect of dexmedetomidine.
Conclusions
Dexmedetomidine protected against sevoflurane-induced neuroinflammation and neurocognitive disorders by suppressing the P2X4R/NLRP3 pathway.
Introduction
Perioperative neurocognitive disorders (PNDs), which manifest as declines in perioperative attention, memory, executive ability and other cognitive functions, are a severe complication after surgery and anesthesia in elderly patients [Citation1]. PNDs delay the postoperative recovery process, deteriorate life quality and even increase the mortality of patients [Citation2, Citation3]. Accumulating evidence has revealed that the inhaled anesthetic sevoflurane causes neuroinflammation by stimulating microglial activation, which leads to hippocampal neuronal apoptosis, synaptic loss and cognitive dysfunction [Citation4–6]. Of note, microglial activation is closely related to sevoflurane-induced neuroinflammation and PNDs.
Dexmedetomidine, an alpha2 adrenoceptor agonist, is widely used in the sedation of intensive care patients because of its long-lasting sedative effect. However, it has not been commonly used for intraoperative sedation because some anesthesiologists are concerned about causing a delay in postoperative awakening. Recently, dexmedetomidine has been confirmed to inhibit neuroinflammation and improve postoperative cognitive dysfunction in clinical practice [Citation7]. Several studies have shown that dexmedetomidine alleviates sevoflurane-induced hippocampal neuronal apoptosis and neurocognitive impairment through anti-inflammatory and antioxidant properties [Citation8, Citation9]. This indicates that intraoperative dexmedetomidine may alleviate sevoflurane-induced neurotoxicity. However, the underlying mechanism of the neuroprotective effects of dexmedetomidine is unclear.
Purinergic ionotropic 4 receptor (P2X4R), a ligand-gated nonselective cation channel receptor for endogenous ATP, is abundant and widely distributed in activated microglia in the brain [Citation10]. P2X4R plays an important role in microglia-mediated neuroinflammation, which is closely related to the progression of neurological diseases such as stroke, neuralgia, Parkinson’s disease and Alzheimer’s disease [Citation11]. As one of the downstream genes of P2X4R, NOD-like receptor protein 3 (NLRP3) is upregulated with increased P2X4R expression when microglia are activated [Citation12]. Moreover, NLRP3 is a multimolecular scaffold that aggravates neuroinflammation by promoting inflammatory cytokine secretion [Citation13]. Importantly, the specific P2X4R inhibitor 5-BDBD downregulated the protein levels of P2X4R and NLRP3, and alleviated the microglia-mediated inflammation and cognitive decline induced by surgery and sevoflurane, suggesting that the P2X4R/NLRP3 pathway may play a crucial role in PNDs [Citation14].
In our earlier study, we found that dexmedetomidine reduced the increased P2X4R and NLRP3 expression caused by sevoflurane. Thus, we aimed to determine whether dexmedetomidine alleviates sevoflurane-induced neuroinflammation and neurocognitive disorders by downregulating P2X4R and NLRP3 expressions.
Materials and methods
Animals
Sixteen-months-old male C57BL/6 mice were purchased from the Experimental Animal Center of Shandong University. Before the study, the mice were housed for 1 week under 12:12 h light and dark cycles at a temperature of 22–25 °C.
Experimental procedures
The experiments were approved by the Ethics Committee for Animal Experimentation of the Second Hospital of Shandong University (KYLL-2020(KJ)A0073), and the procedures followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
In the first experiment, animals were randomly allocated into four groups: a Control group (Control), a dexmedetomidine group (Dex), a sevoflurane group (Sevo) and a sevoflurane + dexmedetomidine group (Sevo + Dex). On Day 1, mice were intraperitoneally injected with 20 μg/kg dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd.) or an equal volume of normal saline 2 h prior to sevoflurane exposure [Citation15]. Then, they were subjected to inhalation of air or 3% sevoflurane (2 L/min) plus 30% oxygen for 6 h in a temperature-controlled box. The concentrations of both O2 and sevoflurane were monitored using a gas analyzer (Mindray, Shenzhen, China). When all treatments were completed, the mice were placed back in their cages. On Day 4, some mice in each group were anesthetized by intraperitoneal injection of 100 mg/kg sodium pentobarbital and euthanized by perfusion with 4% paraformaldehyde (PFA), then the brain or hippocampus was dissected out on ice for further biochemical experiments. The others were subjected to the Morris water maze (MWM) test to assess cognitive function.
In a separate experiment, animals were divided into the following five groups: Control + NC group; Control + P2X4ROE group; Sevo + NC group; Sevo + Dex + NC group and Sevo + Dex + P2X4ROE group. On Day 1, animals were injected intracerebroventricularly with 2 μL adeno-associated virus nonspecific control (AAV-NC) or adeno-associated virus carrying the P2X4R overexpression target gene (AAV-P2X4R) (GeneChem, Shanghai, China) using a Hamilton syringe as previously described [Citation16]. On Day 4, they were intraperitoneally injected with 20 μg/kg dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd.) or an equal volume of normal saline. Two hours later, the mice were subjected to inhalation of air or sevoflurane as described in the first experiment. On Day 7, some mice in each group were euthanized for further biochemical experiments as previously described, while the others were subjected to the MWM test to assess cognitive function.
Morris water maze
The MWM test was used to investigate cognitive decline 3 days after sevoflurane exposure as previously described [Citation17]. In brief, a circular pool filled with 22 ± 1 °C water to a depth of 40 cm was divided into four quadrants, and a hidden platform was placed 0.5–1 cm below the water surface in the center of one of the quadrants. On Days 1–5, the mice were allowed to swim freely for 60 s to find the platform. If a mouse failed to find the platform within 60 s, it was guided to the target and allowed to stay for 15 s. On Day 6, the platform was removed from the pool to assess reference memory. The escape latency, swimming speed, number of times the mice crossed the platform and time in the target quadrant within 60 s were recorded by an automatic video tracking system (SuperMaze Video Tracking System, Shanghai, China).
Tissue preparation and immunofluorescence staining
Mice were anesthetized, and then cardiac perfusion was sequentially performed with phosphate-buffered saline (PBS) and a 4% PFA solution. After fixation with 4% PFA, dehydration and embedding, coronal slices were prepared at a 20 μm thickness and frozen for preservation (−80 °C).
For immunofluorescence staining, frozen brain sections were washed three times with PBS, blocked with 10% goat serum at 37 °C for 1 h and incubated with an anti-Iba-1 antibody (1:200, Cell Signaling Technology, USA) overnight at 4 °C. Next, sections were incubated with Alexa Fluor 488-conjugated AffiniPure goat anti-rabbit IgG (1:200, Yeasen, China) at 37 °C for 1 h. After staining with DAPI in an anti-fluorescence quenching solution, photographs were taken under a laser scanning confocal microscope (ZEISS, Oberkochen, Germany).
Protein extraction and western blot
The hippocampal tissue was triturated and homogenized in radio immunoprecipitation assay (RIPA) buffer, and the lysate was centrifuged at 12,000 ×g at 4 °C for 25 min. The protein concentration was determined using a BCA protein assay. Next, the samples were mixed with 5× loading buffer and heated at 99 °C for 10 min. After that, equal amounts of proteins (30 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skim milk for 1 h at room temperature, the membranes were incubated with the primary antibodies anti-P2X4 and anti-NLRP3 (1:1000, Cell Signaling Technology, USA) at 4 °C overnight. Subsequently, the membranes were incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit IgG secondary antibodies (1:5000, Proteintech, China) for 1 h. Finally, a chemiluminescent detection kit (Beyotime Biotechnology, Jiangsu, China) was utilized to observe the bands, and ImageJ software was employed for protein quantification.
RNA extraction and real-time quantitative PCR (qRT-PCR) assays
Total RNA was isolated from hippocampal tissues with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The mRNA expression levels were determined by qRT-PCR assays. Briefly, total RNA was reverse transcribed to generate complementary DNA (cDNA) by the PrimeScript RT Reagent Kit (Takara Co, Ostu, Japan). Then, PCR was performed using a SYBR Green PCR kit (Takara Co, Otsu, Japan). The mRNA expression levels were detected by a LightCycler 480 system (Roche, Mannheim, Germany) and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The following primers were used: IL-6, forward: 5′-CAATGAGGAGACTTGCCTGG-3′, reverse: 5′-TGGGTCAGGGGTGGTTATTG-3′; IL-1β, forward: 5′-CCACCTCCAGGGACAGGATA-3′, reverse: 5′-TGGGATCTACACTCTCCAGC-3′; TNF-α, forward: 5′-TGGGACAGTGACCTGGACTGT-3′, reverse: 5′-TTCGGAAAGCCCATTTGAGT-3′; GAPDH, forward: 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Enzyme-linked immunosorbent assay (ELISA)
Hippocampal tissues were collected and stored at −80 °C. According to the manufacturer’s instructions, ELISA kits (R&D Systems, Minnesota, USA) were used to measure the concentrations of IL-6, IL-1β and TNF-α. Subsequently, the optical density (OD) values were read at 450 nm, and the concentration was expressed in pg/mg.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 software (La Jolla, CA, USA). Data are presented as the means ± standard deviations (SD). The significance of differences between two groups was assessed using a two-tailed, nonpaired Student’s t test. Escape latency times were analyzed using two-way repeated-measures ANOVA followed by Tukey’s post hoc analysis. The other multiple comparisons were measured using one-way ANOVA followed by Tukey’s post hoc analysis. A p value less than 0.05 was considered statistically significant.
Results
Dexmedetomidine attenuated sevoflurane-induced cognitive impairment in aged mice.
The MWM test was performed to investigate the effect of dexmedetomidine on sevoflurane-induced cognitive dysfunction in mice. Sevoflurane prolonged the escape latency in the place navigation compared to the Control group, but these parameters were mitigated in mice treated with dexmedetomidine before sevoflurane exposure (). No significant difference was detected in swimming speed among the groups (). In the spatial probe test, the number of times crossing the platform and dwelling time in the target quadrant were reduced in the Sevo group compared to the Control group, whereas these parameters were recovered in the Sevo + Dex group (). Thus, these findings support a protective role for dexmedetomidine in sevoflurane-induced neurocognitive disorders.
Figure 1. Dexmedetomidine improved sevoflurane-induced cognitive impairment in aged mice. (A) The escape latency and (B) swimming speed in the place navigation trial (n = 8). (C) The number of platform crossings and (D) dwelling time in the target quadrant in the spatial probe test (n = 8). (E) The representative swimming paths in the spatial probe test. All values are expressed as the mean ± SD. $p < 0.05, $$$p < 0.001 Sevo group vs Control group; ##p < 0.01, ###p < 0.001 Sevo + Dex group vs Sevo group; *p < 0.05, **p < 0.01, ***p < 0.001.
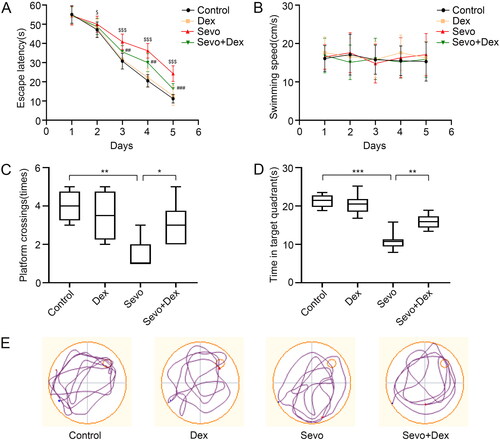
Dexmedetomidine alleviated microglial neuroinflammation in the hippocampus after sevoflurane exposure.
Considering the key effect of microglia on neuroinflammation during PND, Iba1+ cells in the hippocampus were assessed to detect microglial activation. Meanwhile, the mRNA levels and concentrations of the proinflammatory factors IL-6, IL-1β and TNF-α were examined by RT-PCR and ELISA. In contrast with the Control group, more Iba1+ cells were observed in the hippocampal DG area in the Sevo group. Moreover, the number of Iba1+ cells was reduced when the mice were treated with dexmedetomidine before sevoflurane exposure (). The mRNA levels of IL-6, IL-1β and TNF-α in the Sevo group were significantly increased compared to those in the Control group, whereas the levels were lower in the Sevo + Dex group than in the Sevo group (). Consistently, sevoflurane exposure elevated these proinflammatory cytokine levels in the hippocampus compared with the Control group, but the levels were decreased in mice injected with dexmedetomidine before sevoflurane exposure (). Altogether, these results indicated that dexmedetomidine attenuated microglial neuroinflammation caused by sevoflurane.
Figure 2. Dexmedetomidine alleviated sevoflurane-induced microglial activation and neuroinflammation in the hippocampus. (A) The number of Iba1+cells (green) in hippocampal DG region were detected by immunofluorescence staining (n = 6). (B) The mRNA levels of IL-6, IL-1β and TNF-α in the hippocampus among the four groups (n = 3). (C) The protein concentrations of IL-6, IL-1β and TNF-α in hippocampus among the four groups (n = 8). All values are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. scale bar = 50 μm.
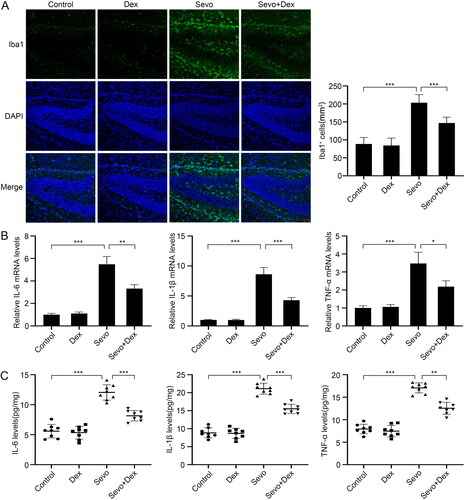
Dexmedetomidine reduced the increased protein levels of P2X4R and NLRP3 in the hippocampus caused by sevoflurane.
To explore the functions of sevoflurane and dexmedetomidine on P2X4R and the NLRP3 inflammasome in the hippocampus, we detected the P2X4R and NLRP3 protein levels using western blotting. Both the P2X4R and NLRP3 protein levels were higher in the Sevo group than in the Control group. In contrast, the protein levels of P2X4R and NLRP3 were reduced in the Sevo + Dex group compared with the Sevo group (). These results suggested that dexmedetomidine inhibited sevoflurane-induced activation of the P2X4R/NLRP3 pathway.
Figure 3. Dexmedetomidine attenuated sevoflurane-induced P2X4R and NLRP3 overexpression in the hippocampus. (A) The protein expression levels of P2X4R and NLRP3 were detected by western blot (n = 3). The protein levels of (B) P2X4R and (C) NLRP3 were quantified using ImageJ software and normalized to β-actin. The data are shown as fold changes relative to proteins from Control group (n = 3). All values are expressed as the mean ± SD. **p < 0.01, ***p < 0.001.
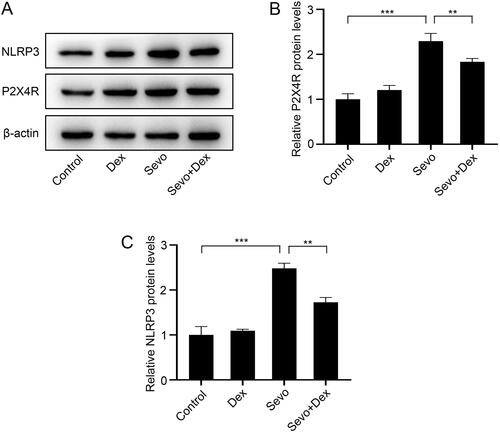
Overexpression of P2X4R abolished the protective effect of dexmedetomidine on neurocognitive disorders caused by sevoflurane.
To evaluate the role of P2X4R in the neuroprotective effect of dexmedetomidine, mice were injected intracerebroventricularly with AAV-P2X4R to overexpress P2X4R before treatment with dexmedetomidine and sevoflurane. The MWM test showed that both overexpression of P2X4R and sevoflurane exposure prolonged the escape latency in the place navigation compared to the Control + NC group, whereas dexmedetomidine reduced the escape latency but overexpression of P2X4R reversed the protective effect of dexmedetomidine compared to the Sevo + NC group. In addition, no significant difference was detected in swimming speed among the five groups (). Consistently, in the spatial probe test, both the number of times crossing the platform and the dwelling time in the target quadrant in the Control + P2X4ROE and Sevo + NC groups were less than those in the Control group. Moreover, these parameters were improved in the Sevo + Dex + NC group compared to the Sevo + NC group. However, the conditions were exacerbated in the Sevo + Dex + P2X4ROE group even though the mice were treated with dexmedetomidine before sevoflurane exposure (). Here, we concluded that dexmedetomidine may protect against sevoflurane-induced cognitive decline by suppressing P2X4R expression.
Figure 4. Overexpression of P2X4R weakened the protective effect of dexmedetomidine on cognitive decline caused by sevoflurane. (A) The escape latency and (B) swimming speed in the place navigation trial (n = 8). (C) The number of platform crossings and (D) dwelling time in the target quadrant in the spatial probe test (n = 8). (E) The representative swimming paths in the spatial probe test. All values are expressed as the mean ± SD. #p < 0.05, ###p < 0.001, Control + P2X4ROE group vs Control + NC group; $$p < 0.01, $$$p < 0.001, Sevo + NC group vs Control + NC group; &p < 0.05, &&&p < 0.001, Sevo + Dex + NC group vs Sevo + NC group; ^^p < 0.01, ^^^p < 0.001, Sevo + Dex + P2X4ROE group vs Sevo + Dex + NC group. *p < 0.05, ***p < 0.001.
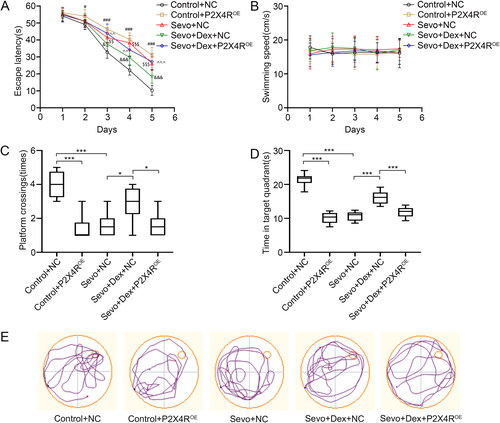
Overexpression of P2X4R aggravated sevoflurane-induced microglial neuroinflammation, which was attenuated by dexmedetomidine.
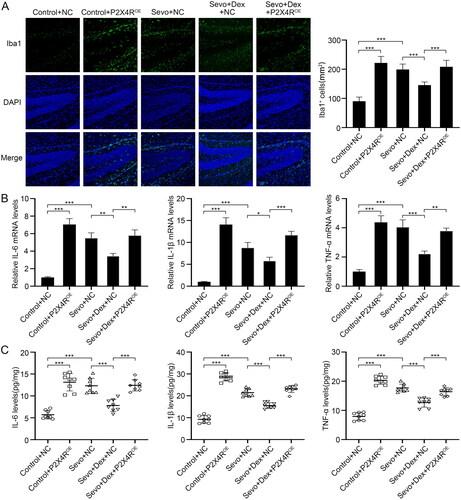
To further explore the involvement of P2X4R in the inhibitory effect of dexmedetomidine on microglial activation and neuroinflammation caused by sevoflurane, we overexpressed P2X4R by intracerebroventricular injection with AAV-P2X4R, detected Iba1+ cells in the hippocampus using immunofluorescence staining, and measured the mRNA levels and concentrations of IL-6, IL-1β and TNF-α by RT-PCR and ELISA. Compared to the Control + NC group, the number of Iba1+ cells in the hippocampal DG region were increased in both the Control-P2X4ROE and Sevo + NC groups. Compared with the Sevo + NC group, fewer Iba1+ cells were observed in the Sevo + Dex + NC group. However, overexpression of P2X4R significantly increased the number of Iba1+ cells in the hippocampal DG region compared to the Sevo + Dex + NC group (). Similarly, both overexpression of P2X4R and sevoflurane exposure increased the mRNA levels and concentrations of IL-6, IL-1β and TNF-α compared to those in the Control group. Moreover, pretreatment with dexmedetomidine decreased the mRNA levels and concentrations of IL-6, IL-1β and TNF-α compared to the Sevo + NC group, whereas overexpression of P2X4R enhanced the neuroinflammatory response compared with the Sevo + Dex + NC group (). Altogether, these results showed that P2X4R overexpression weakened the inhibitory effect of dexmedetomidine on microglial neuroinflammation caused by sevoflurane.
Figure 5. Overexpression of P2X4R deteriorated sevoflurane-induced microglial neuroinflammation, which was attenuated by dexmedetomidine. (A) The number of Iba1+ cells (green) in hippocampal DG region were detected by immunofluorescence staining (n = 6). (B) The mRNA levels of IL-6, IL-1β and TNF-α in hippocampus among the four groups (n = 3). (C) The protein concentrations of IL-6, IL-1β and TNF-α in hippocampus among the four groups (n = 8). All values are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. scale bar = 50 μm.
Overexpression of P2X4R weakened the inhibitory effect of dexmedetomidine on the P2X4R/NLRP3 pathway which was activated by sevoflurane.
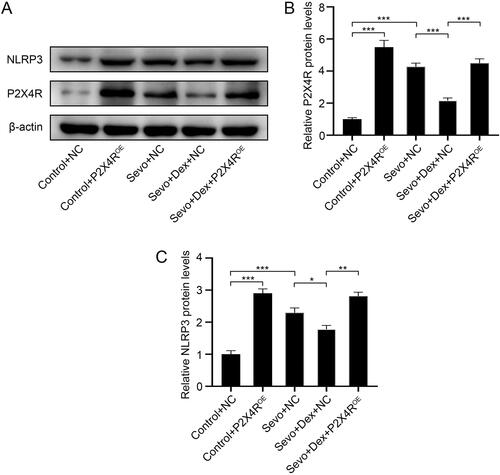
To further investigate the overexpression effect of AAV-P2X4R and to determine whether overexpression of P2X4R activated the NLRP3 inflammasome, we detected the protein levels of P2X4R and NLRP3 by western blotting. Notably, the protein levels of P2X4R and NLRP3 in the Control-P2X4ROE group were higher than those in the Control + NC group, indicating that P2X4R was successfully overexpressed in the hippocampus and that the NLRP3 inflammasome was activated when P2X4Rs were overexpressed. In contrast with the Control + NC group, the levels of P2X4R and NLRP3 in the Sevo + NC group were elevated, while dexmedetomidine reduced the increased expression levels of P2X4R and NLRP3 induced by sevoflurane. Conversely, overexpression of P2X4R increased the levels of P2X4R and NLRP3 compared to the Sevo + Dex + NC group (). Taken together, these results demonstrated that dexmedetomidine inhibited sevoflurane-induced activation of the NLRP3 inflammasome by downregulating the expression of P2X4R.
Figure 6. Overexpression of P2X4R abolished the inhibitory effect of dexmedetomidine on P2X4R/NLRP3 pathway which was activated by sevoflurane. (A) The protein expression levels of P2X4R and NLRP3 were detected by western blotting (n = 3). The protein levels of (B) P2X4R and (C) NLRP3 were quantified using ImageJ software and normalized to β-actin. The data are shown as fold changes relative to proteins from Control + NC group (n = 3). All values are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
PNDs exacerbate surgical outcomes, delay the recovery process and even increase mortality in elderly patients, but their pathogenesis remains unclear. In this study, we demonstrated that sevoflurane upregulated the levels of P2X4R and NLRP3, exaggerated microglial inflammation in the hippocampus and induced neurocognitive disorders in aged mice. Moreover, pretreatment with dexmedetomidine attenuated neuroinflammation and improved cognitive dysfunction caused by sevoflurane. In addition, overexpression of P2X4R weakened the protective effect of dexmedetomidine, indicating that dexmedetomidine exerts neuroprotective effects in sevoflurane-induced PNDs by suppressing the P2X4R/NLRP3 signaling pathway.
Accumulating evidence has indicated that neuroinflammation plays a core role in neurocognitive disorders caused by surgery and anesthesia. Microglia, which are the intrinsic immune cells of the brain, are essential for maintaining tissue homeostasis [Citation18]. Abnormal microglial activation induced by inhaled anesthetics such as isoflurane and sevoflurane lead to a severe neuroinflammatory response, neuronal apoptosis and cognitive impairment [Citation19]. A previous study reported that sevoflurane induced microglial neuroinflammation and learning and memory deficits by upregulating Sirtuin 2 levels in developing rats [Citation20]. Similarly, sevoflurane was found to activate caspase-3 and increase inflammatory cytokine levels by upregulating ATPase inhibitory factor 1 (ATPIF1) expression in microglia, which led to memory deficits and anxiety behavior in mice [Citation21]. In both clinical trials and animal studies, dexmedetomidine was found to attenuate neuroinflammation and emergence delirium induced by sevoflurane [Citation7, Citation15]. In vitro, dexmedetomidine alleviated neuroinflammation by reversing microglial M1 polarization via the mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) pathway [Citation22]. However, the downstream regulatory mechanisms of dexmedetomidine in sevoflurane-induced microglial neuroinflammation remain unclear.
As a member of the ionotropic receptor family, P2X4R has been implicated in microglia-mediated neuroinflammation and various neurodegenerative diseases in recent years [Citation23–25]. It has been proven that P2X4R-mediated calcium entry changes the membrane potential and promotes microglial activation into a proinflammatory subtype [Citation26]. In a mouse model of intracerebral hemorrhage, activation of P2X4R increased the proinflammatory activity of microglia and exacerbated brain edema, neural death and acute neurodeficits, whereas the specific P2X4R inhibitor 5-BDBD attenuated acute brain injury after intracerebral hemorrhage [Citation27]. Thus, P2X4R plays an important role in microglial activation and neuroinflammation. In this study, we found that sevoflurane upregulated P2X4R expression, increased the number of microglia and elevated proinflammatory factor levels in the hippocampus. Moreover, dexmedetomidine reduced P2X4R expression and attenuated neuroinflammation and neurocognitive impairment induced by sevoflurane. However, overexpression of P2X4R abolished the protective effect of dexmedetomidine, indicating that dexmedetomidine played a neuroprotective role in sevoflurane-induced cognitive decline by inhibiting P2X4R.
The NLRP3 inflammasome amplifies neuroinflammation by promoting the maturation of proinflammatory cytokines such as IL-1β and driving inflammatory cascade responses [Citation28]. According to a previous study, sevoflurane increased the expression of the proinflammatory cytokines IL-6, IL-18 and IL-1β, promoted hippocampal neuron apoptosis and led to cognitive impairment through the NLRP3/caspase-1 pathway in aged rats [Citation29]. In addition, sevoflurane induces neuronal pyroptosis and exacerbates the progression of Alzheimer’s disease by upregulating NLRP3 inflammasome and caspase-1 expression [Citation30]. Moreover, inhibition of the NLRP3 inflammasome ameliorates neuroinflammation and cognitive decline induced by both sevoflurane and isoflurane [Citation31–33]. These results suggest that the NLRP3 inflammasome is involved in neurotoxicity caused by inhalation anesthetics. Recently, dexmedetomidine was found to attenuate surgery-induced microglial inflammation in the hippocampus and restore cognitive decline by promoting NLRP3 inflammasome degradation [Citation34]. In vitro, dexmedetomidine alleviated lipopolysaccharide (LPS)-induced inflammation in microglia by suppressing the c-Fos/NLRP3/caspase-1 pathway [Citation35]. However, the underlying molecular mechanisms by which dexmedetomidine inhibits the NLRP3 inflammasome have not been fully clarified. It is well known that ascending P2X4R activates the NLRP3 inflammasome. A previous study reported that extracellular ATP mediated high glucose-induced inflammatory responses and activation of the NLRP3 inflammasome by upregulating P2X4R expression [Citation36]. In a rat model of Parkinson’s disease, the ATP-P2X4R signaling pathway regulated NLRP3 inflammasome activation, resulting in neuroinflammation and dopaminergic neurodegeneration [Citation37]. Moreover, inhibition of P2X4R attenuated microglial inflammation and improved neurological deficits by repressing NLRP3 expression in rats after traumatic brain injury [Citation38]. In our study, sevoflurane increased both P2X4R and NLRP3 expression, stimulated microglial activation, and increased the levels of IL-1β, IL-6 and TNF-α in the hippocampus. Moreover, dexmedetomidine reduced P2X4R and NLRP3 expression and alleviated neuroinflammation induced by sevoflurane. However, even when the mice were treated with dexmedetomidine before sevoflurane exposure, overexpression of P2X4R increased NLRP3 expression and enhanced neuroinflammation. These results suggested that the P2X4R/NLRP3 pathway played an important role in the anti-inflammatory effects of dexmedetomidine.
In conclusion, our study implied that dexmedetomidine protected against sevoflurane-induced neuroinflammation and neurocognitive disorders by suppressing the P2X4/NLRP3 pathway. Moreover, dexmedetomidine should be more widely used in surgery, which may help prevent and treat sevoflurane-induced PNDs.
Acknowledgment
We would like to thank the technical platforms of Key Laboratory of Urinary Precision Diagnosis and Treatment of Qilu Hospital.
Data availability statement
The data that support the findings of this study are available from either the corresponding author or the first author upon request.
Disclosure statement
The authors confirm that there are no conflicts of interest to declare.
Additional information
Funding
References
- Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;121(5):1005–1012.
- Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775.
- Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–491.
- Lv G, Li C, Wang W, et al. Silencing SP1 alleviated sevoflurane-induced POCD development via cholinergic anti-inflammatory pathway. Neurochem Res. 2020;45(9):2082–2090.
- Yu X, Zhang F, Shi J. Effect of sevoflurane treatment on microglia activation, NF-kB and MAPK activities. Immunobiology. 2019;224(5):638–644.
- Zhu Y, Wang Y, Yao R, et al. Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period. J Neuroinflammation. 2017;14(1):6–16.
- Shi M, Miao S, Gu T, et al. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Dev Ther. 2019;13:897–905.
- Zhang Y, Li M, Cui E, et al. Dexmedetomidine attenuates sevoflurane‑induced neurocognitive impairment through α2‑adrenoceptors. Mol Med Rep. 2021;23(1):1–8.
- Xu S, Gao R, Chen L. Dexmedetomidine regulates sevoflurane-induced neurotoxicity through the miR-330-3p/ULK1 axis. J Biochem Mol Toxicol. 2021;35(12):e22919.
- Zabala A, Vazquez-Villoldo N, Rissiek B, et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10(8):1–20.
- Illes P, Rubini P, Ulrich H, et al. Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 2020;9(5):1108–1123.
- Molnár K, Nógrádi B, Kristóf R, et al. Motoneuronal inflammasome activation triggers excessive neuroinflammation and impedes regeneration after sciatic nerve injury. J Neuroinflammation. 2022;19(1):1–22.
- Milner MT, Maddugoda M, Götz J, et al. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr Opin Immunol. 2021;68:116–124.
- Yuan H, Lu B, Ji Y, et al. Role of P2X4/NLRP3 pathway-mediated neuroinflammation in perioperative neurocognitive disorders. Mediators Inflamm. 2022;2022:6355805.
- Xie X, Shen Z, Hu C, et al. Dexmedetomidine ameliorates postoperative cognitive dysfunction in aged mice. Neurochem Res. 2021;46(9):2415–2426.
- Ling KK, Jackson M, Alkam D, et al. Antisense-mediated reduction of EphA4 in the adult CNS does not improve the function of mice with amyotrophic lateral sclerosis. Neurobiol Dis. 2018;114:174–183.
- Meng B, Li X, Lu B, et al. The investigation of hippocampus-dependent cognitive decline induced by anesthesia/surgery in mice through integrated behavioral Z-Scoring. Front Behav Neurosci. 2020;13:1–13.
- Tay TL, Savage JC, Hui CW, et al. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595(6):1929–1945.
- Wang Z, Meng S, Cao L, et al. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. J Neuroinflammation. 2018;15(1):1–12.
- Wu Z, Zhang Y, Zhang Y, et al. Sirtuin 2 inhibition attenuates sevoflurane-induced learning and memory deficits in developing rats via modulating microglial activation. Cell Mol Neurobiol. 2020;40(3):437–446.
- Xu Y, Gao G, Sun X, et al. ATPase inhibitory factor 1 is critical for regulating sevoflurane-induced microglial inflammatory responses and caspase-3 activation. Front Cell Neurosci. 2021;15:1–13.
- Qiu Z, Lu P, Wang K, et al. Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res. 2020;45(2):345–353.
- Montilla A, Mata GP, Matute C, et al. Contribution of p2x4 receptors to CNS function and pathophysiology. Int J Mol Sci. 2020;21(15):1–16.
- Bertin E, Deluc T, Pilch KS, et al. Increased surface P2X4 receptor regulates anxiety and memory in P2X4 internalization-defective knock-in mice. Mol Psychiatry. 2021;26(2):629–644.
- Duveau A, Bertin E, Boué-Grabot E. Implication of neuronal versus microglial P2X4 receptors in Central nervous system disorders. Neurosci Bull. 2020;36(11):1327–1343.
- Nguyen HM, di Lucente J, Chen YJ, et al. Biophysical basis for Kv1.3 regulation of membrane potential changes induced by P2X4-mediated calcium entry in microglia. Glia. 2020;68(11):2377–2394.
- Jin TW, Han R, Yao N, et al. Activation of P2X4 receptor exacerbates acute brain injury after intracerebral hemorrhage. 2022;28(7):1008–1018.
- Haneklaus M, O’Neill LAJ. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62.
- Shao A, Fei J, Feng S, et al. Chikusetsu saponin IVa alleviated sevoflurane-induced neuroinflammation and cognitive impairment by blocking NLRP3/caspase-1 pathway. Pharmacol Rep. 2020;72(4):833–845.
- Tian D, Xing Y, Gao W. Sevoflurane aggravates the progress of Alzheimer’s disease through NLRP3/caspase-1/gasdermin D pathway. Front Cell Dev Biol. 2022;9:1–12.
- Fang P, Chen C, Zheng F, et al. NLRP3 inflammasome inhibition by histone acetylation ameliorates sevoflurane-induced cognitive impairment in aged mice by activating the autophagy pathway. Brain Res Bull. 2021;172:79–88.
- Fan Y, Du L, Fu Q, et al. Inhibiting the NLRP3 inflammasome with MCC950 ameliorates isoflurane-induced pyroptosis and cognitive impairment in aged mice. Front Cell Neurosci. 2018;12:1–9.
- Ye JS, Chen L, Lu YY, et al. Honokiol-mediated mitophagy ameliorates postoperative cognitive impairment induced by surgery/sevoflurane via inhibiting the activation of NLRP3 inflammasome in the hippocampus. Oxid Med Cell Longev. 2019;2019:8639618.
- Zhang L, Xiao F, Zhang J, et al. Dexmedetomidine mitigated NLRP3-mediated neuroinflammation via the ubiquitin-autophagy pathway to improve perioperative neurocognitive disorder in mice. Front Pharmacol. 2021;12:1–17.
- Li H, Zhang X, Chen M, et al. Dexmedetomidine inhibits inflammation in microglia cells under stimulation of LPS and ATP by C-FOS/NLRP3/CASPASE-1 Cascades. EXCLI J. 2018;17:302–311.
- Chen K, Zhang J, Zhang W, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45(5):932–943.
- Wang J, Zhang XN, Fang JN, et al. The mechanism behind activation of the nod-like receptor family protein 3 inflammasome in Parkinson’s disease. Neural Regen Res. 2022;17(4):898–904.
- He W, Wang Q, Sha W, et al. P2X4 inhibition reduces microglia inflammation and apoptosis by NLRP3 and improves nervous system defects in rat brain trauma model. J Clin Neurosci. 2022;99:224–232.
