Abstract
Acute basilar artery occlusion (ABAO) accounts for 1% of all ischemic stroke cases, but has a high rate of severe complications and mortality (75-91%). Intracranial atherosclerosis is an significant cause of ischemic stroke. Revascularization using stents has shown good efficacy. However, intra-stent thrombosis and in-stent restenosis (ISR) are significant complications following stent placement. Drug-coated balloons (DCB), coated with the anti-proliferative drug paclitaxel (an inhibitor of endothelial proliferation), can prevent in-stent restenosis. Successful use of DCB dilation in the coronary and lower extremity vasculature has been reported. In our case, a 68-year-old Chinese male with ABAO was successfully revascularized by DCB dilation and showed dramatic improvement in stroke symptoms. This report may inform future treatment of patients with ABAO.
1. Introduction
The pathogenesis of stroke with acute basilar artery occlusion (ABAO) is complex. Intracranial atherosclerosis is a significant cause of ABAO and is associated with high rates of disability and death. Inadequate perfusion of cerebral tissue, increased thrombus load, and poor collateral circulation are the main causes of functional deterioration. Immediate treatment is required to open the occluded vessels and restore blood perfusion in a timely manner [Citation1, Citation2]. Current treatments can lead to in-stent restenosis (ISR), delayed stent endothelialization, and in-stent thrombosis. In addition, blind stent implantation can cause late acquired malapposition and increase the risk of ISR [Citation3, Citation4]. This case report evaluates the effectiveness and safety of drug-coated balloons (DCB) for treatment of ABAO. Drug-coated balloons represent ‘intervention without implantation’ as an improvement over other treatment strategies.
2. Case description
A 68-year-old Chinese man presented to the Affiliated Hospital of Weifang Medical University with acute onset right-sided weakness and speech impairment, and right facial paralysis five hours prior to presentation. He was in good health and did not have a history of hypertension, type II diabetes mellitus, or coronary heart disease. He smoked 10 cigarettes per day for 50 years and was a current smoker. His father and one brother had histories of cerebral infarction. Physical examination resulted in the following measurements: temperature of 37 °C, pulse 98 beats/min, respiratory rate 20 breaths/min, blood pressure 128/88 mmHg. On examination he was noted to be in a drowsy state, exhibited dysarthria, eye gaze palsy to the right side, right-sided facial paralysis, right upper and lower limb weakness, muscle strength grade 0/5, and positive Babinski’s sign on the right. The NIHSS score was 23. The patient had an initial LDL-C level of 3.31 mmol/l. Complete blood count, electrolytes, glucose, liver function, renal function, and coagulation cascade were within normal limits. An electrocardiogram was normal. Head CT (HCT) showed multiple prior lacunar cerebral infarcts in the bilateral basal ganglia and radiocoronal areas. The posterior circulation acute stroke prognosis early CT score (PC-ASPECTS) was 10. The diagnosis at admission was cerebral infarction. The TOAST classification was large artery atherosclerotic type.
3. Treatment
3.1. Mechanical thrombectomy
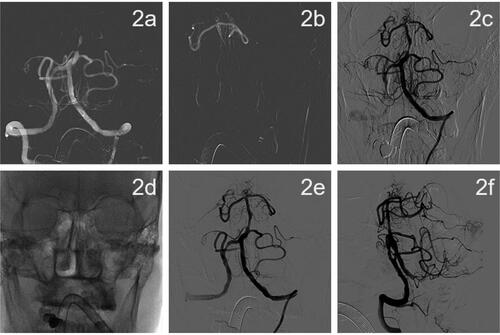
The time from arrival to the hospital to perform the femoral artery puncture was 55 min. General anesthesia under tracheal intubation with intraoperative DSA showed that the left vertebral artery was dominant, the left vertebral artery opening was normal, and the basilar artery was occluded. A small amount of posterior inferior cerebellar artery (PICA) flow compensated for the occluded segment of the basilar artery to the far side, and the lumen of the basilar artery was able to be intermittently visualized (1a, 1b). The right vertebral artery opening was normal, the basilar artery was occluded, and there was a small amount of antegrade compensation of the PICA branch (1c, 1d). No significant abnormalities were observed in the right internal carotid artery system, and an embryonic posterior cerebral artery was observed (1e, 1f). No significant abnormalities were seen in the left internal carotid artery system, and an embryonic posterior cerebral artery was observed (1 g, 1h). Intraoperative balloon dilation angioplasty was performed on the basilar artery. The left vertebral artery approach was selected, 8F Guiding was placed, and a 5 F*115 cm Navien intermediate catheter was selected to the V2 end of the left vertebral artery for fixation (2a). A 3 m Synchro microguide with a Rebar18 microcatheter was carefully passed through the occluded segment and an intracatheter angiogram indicated that the catheter was in the true lumen of the vessel (2b). the microguide wire reached the left superior cerebellar artery and was secured. Gateway balloons (1.5 × 9 mm and 2.5 × 15 mm) were selected and accurately positioned for dilation, and angiography showed a significant improvement in stenosis in the middle of the basilar artery. Ten minutes after balloon dilation, an angiogram showed significant lumen retraction at the stenosis (2c). A 2.5 × 20 mm paclitaxel drug-coated balloon was selected for dilation, and the pressure pump was slowly pressurized to 8 atm for 1 min (2d), after which the balloon was depressurized and removed. Angiography suggested good lumen formation, no obvious local residual stenosis or entrapment, and distal flow patency with modified thrombolysis in cerebral infarction (mTICI) grade 3. Eight milliliters of tirofiban was slowly pushed through the intermediate catheter and angiographic observations were reviewed every 10–15 min for a total of 50 min. The lumen of the basilar artery was well formed at the end of the procedure, with no retraction and unobstructed distal flow (2e, 2f) ().
Figure 1. (a–h). Angiography showing acute basilar artery occlusion.
(a, b) The left vertebral arteriogram in the frontal and lateral view shows occlusion of the basilar artery, with a small amount of PICA flow compensating for the occluded segment of the basilar artery and intermittent visualization of the lumen of the basilar artery. (c, d) A frontal and lateral view of the right vertebral artery angiogram shows occlusion of the basilar artery with a small amount of antegrade compensation of the PICA branch. (e, f) A right internal carotid artery system frontal and lateral angiogram shows no significant abnormalities, and an embryonic posterior cerebral artery was observed. (g, h) A front and side view of the left internal carotid artery system showed no significant abnormalities, and the embryonic posterior cerebral artery was observed ().
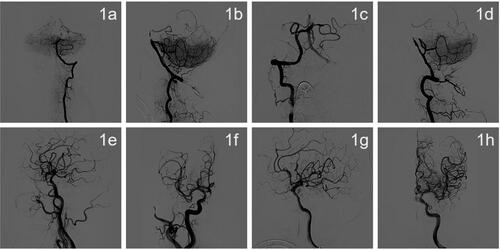
Figure 2. DSA imaging during drug-coated balloon dilatation angioplasty.
(a) A 5F*115 cm Navien intermediate catheter selected to the V2 end of the left vertebral artery for fixation. (b) A 3-m Synchro microguide with Rebar18 microcatheter fed through the occluded segment followed by intracatheter angiogram showing that the catheter was located in the true lumen of the vessel. (c) Ten minutes after 2.5 × 15 mm Gateway balloon dilation, the lumen of the stenosis was retracted significantly. (d) The microguide wire was fixed in the left superior cerebellar artery, and a 2.5 × 20 mm paclitaxel drug-coated balloon was used for dilation. (e, f) After drug-coated balloon dilation, the patient was observed for 50 min and re-imaged. The patient had good lumen formation of the basilar artery, no retraction, no artery dissection, distal flow patency, and mTICI grade 3.
4. Outcome and follow-up
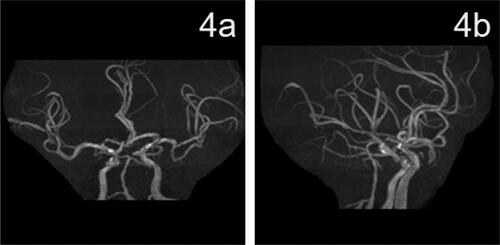
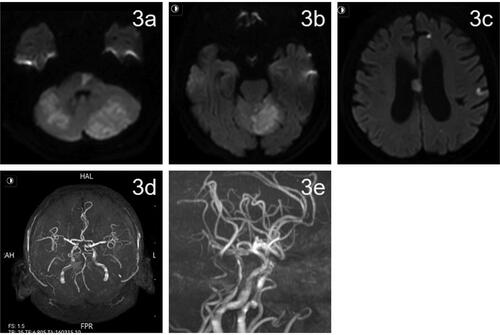
DynaCT was used to rule out intracranial hemorrhage. Tirofiban was continuously administered intravenously for 24 h, and aspirin (100 mg qd) and hydroclopidogrel (75 mg qd) were administered 4 h prior to discontinuation of tirofiban. The patient was given atorvastatin (80 mg qd) during hospitalization. Postoperatively, blood pressure was monitored and systolic blood pressure was controlled below 120 mmHg using urapidil. On postoperative day 1 a repeat head CT showed new bilateral cerebellar acute ischemic stroke. On postoperative day 2, physical examination indicated clear consciousness, incomplete gaze of both eyes to the right, and right limb muscle strength grade 2. The NIHSS score was 12. On postoperative day 3, magnetic resonance imaging of the head showed multiple fresh cerebral infarcts in the pons, cerebellar vermis, bilateral cerebellar hemispheres, frontoparietal temporal lobe, and corpus callosum (3a,3b,3c). Local luminal stenosis of the MRA basilar artery was observed (3d,3e). On postoperative day 13, physical examination showed clear consciousness and right limb muscle strength grade 4. NIHSS score was 5. MRS score was 3. Thromboelastogram indicated that aspirin and clopidogrel effectively inhibited platelet aggregation. At follow-up LDL-C level was 1.61 mmol/l. After discharge aspirin (100 mg qd), hydroclopidogrel (75 mg qd), and atorvastatin (20 mg qn) were prescribed. On postoperative day 43, physical examination showed clear consciousness, mild dysarthria, and normal muscle strength in the extremities. NIHSS score was 2. MRS score was 1. At follow-up 2 months after surgery the patient had recovered completely and MRS score was 0. At follow-up 90 days after surgery review of cranial MRA showed good lumen of the basilar artery (4a, 4b). After the 90-day follow-up the patient was treated with aspirin (100 mg qd) to prevent platelet aggregation ( and ).
Figure 3. Cranial magnetic resonance imaging on postoperative day 3.
(a–c) Postoperative day 3 MRI DWI showed multiple fresh cerebral infarcts in the pons, cerebellar vermis, bilateral cerebellar hemispheres, frontoparietal temporal lobe, and corpus callosum. (d, e) MRA on postoperative day 3 showed local luminal stenosis of the middle basilar artery (shown by red arrows).
5. Discussion
Recent landmark studies on thrombectomy for acute basilar artery occlusion patients with basilar-artery occlusion, who presented 6 to 24 h after symptom onset, thrombectomy led to a higher percentage with good functional status at 90 days than medical therapy but was associated with procedural complications and more cerebral hemorrhages [Citation5, Citation6]. In our case,use of a DCB was chosen because this case of ABAO was a result of atherosclerotic disease, and thromboembolism and artery dissection were excluded. The patient had a progressive course of neurological deficits, which was inconsistent with embolism. In addition, the patient had no history of atrial fibrillation or other related medical conditions. During hospitalization, a 24 h dynamic electrocardiogram did not show arrhythmia and no unstable plaques were observed on cervical vascular ultrasound. After the micro-guide wire passed through the occluded segment of the vessel, contrast agent showed obvious arterial stenosis, and local balloon dilation resulted in sustained clear vessels. Embolization can cause severe proximal vascular stenosis, and high density shadows can be observed in the occluded segment during angiography, and the lumen can only be maintained temporarily following balloon dilation. Artery dissection is common at the beginning of the artery and shows a ‘double cavity sign’ [Citation7, Citation8].
Acute basilar artery occlusion due to atherosclerotic disease is not uncommon, but it can significantly damage the nervous system, and the rate of death and disability is very high if timely revascularization is not performed [Citation9]. Conventional intravenous drug thrombolytic therapy and antiplatelet drug therapy are typically ineffective interventions for ABAO because of the narrow temporal therapeutic window and high thrombus load [Citation10]. Therefore, stent implantation is often required. However, the incidence of in-stent thrombosis and ISR is high. Development of paclitaxel DCBs is an alternative non-stent method for treatment of atherosclerosis-induced vascular occlusion/stenosis [Citation11].
Drug coated balloons are an alternative treatment modality for symptomatic intracranial high-grade atherosclerotic stenosis. A retrospective, single center cohort study of patients with sICAS treated with DCB showed a relatively low intracranial complication rate of 6% with a symptomatic recurrence rate of 12% [Citation12]. Between September 2016 and September 2017, details of 30 patients with 31 arteries treated with DCBs for symptomatic severe intracranial atherosclerotic disease (≥70% stenosis or chronic total occlusion) were retrospectively collected. All arteries were successfully dilated with DCB and 93.5% of arteries showed good antegrade perfusion. This study suggested that DCB dilation may be a safe and effective alternative treatment for intracranial atherosclerotic disease [Citation13]. In addition, a study was performed to evaluate DCB for treatment of nonacute symptomatic internal carotid artery terminus occlusion (sICATO) that was refractory to medical therapy. This study included 30 patients with nonacute sICATO treated with DCBs and/or remedial stenting. Drug-coated balloon (DCB) dilation of nonacute sICATO resulted in a 100% rate of successful recanalization, with a low restenosis/reocclusion rate (3.45%) [Citation14]. A retrospective analysis of the feasibility and safety of DCB treatment for non-acute intracranial arterial occlusions in 7 patients aged 21-32 years with severe symptomatic atherosclerotic MCA stenosis showed good perfusion in 5 patients. Two additional patients underwent remedial stenting due to residual stenosis >50% and unstable antegrade perfusion after DCB dilation, but none of these patients had perioperative complications [Citation15]. These studies informed our use of DCB to treat our case of ABAO, and our case demonstrated the viability of this approach.
An advantage of DCB is reduced duration of dual anti-platelet therapy (DAPT) after surgery. Dual anti-platelet therapy is an important treatment to prevent in-stent restenosis after stent placement [Citation16]. Treatment of coronary lesions without implantation of foreign polymer may reduce duration of DAPT treatment. The German Consensus Group recommends DAPT for 4 weeks if DCB is used as a standalone procedure. In contrast, DAPT is necessary for 3 months to 1 year after stent placement, particularly in elderly patients. However, some scholars believe that DAPT treatment after DCB should be used for a similar period of time as DAPT following drug-eluting stent placement [Citation17]. We took a conservative approach and prescribed DAPT for 90 days after DCB treatment. Paclitaxel is an antiproliferative drug with anti-inflammatory activity that can inhibit smooth muscle cell proliferation and migration by irreversibly stabilizing intracellular microtubules [Citation18, Citation19]. Paclitaxel has been shown to improve parameters related to atherosclerotic lesions in rabbits, rats, and mice and reduced neointima formation in experiments that evaluated prevention of post-stenting restenosis [Citation20]. Finally, we considered that luminal improvement on MRA 4a,4b than MRA 3d,3e was due to the joint effect of DCB + DAPT. Additional studies are needed to confirm our findings.
Drug coated balloon treatment of ABAO due to atherosclerotic disease is superior to stenting in patients who may not tolerate metallic foreign bodies, particularly tortuous vascular anatomy, or who cannot take DAPT for long periods of time. Furthermore, DCB treatment is superior for lesions located at the bifurcation of vessels that cannot be stented and patients with long tandem stenoses with incomplete stent coverage. Finally, DCB it also reduces the impact on branch arterial [Citation21, Citation22]. However, balloon angioplasty is subject to several limitations such as hyperperfusion syndrome, unsatisfactory angioplasty vascular, wall retraction-induced re-occlusion, vessel dissection/rupture, distal embolization, and occlusion of the pontine perforators [Citation23]. To minimize the risk of these potential adverse effects smaller balloon diameters can be used. The balloon should be about 50%–80% of the vessel diameter and should be inflated slowly. The most common complication of balloon angioplasty is vascular dissection. If forward blood flow cannot be maintained, stents can be used [Citation22].
6. Conclusion
In the present report, we successfully treated a patient with ABAO due to atherosclerotic disease using DCB dilation. Postoperative results and follow-up confirmed the feasibility of DCB dilation angioplasty for treatment of ABAO. Successful use of this approach is clinically significant in that it has potential to overcome the limitations of stenting or balloon dilation alone. However, use of DCB for treatment of intracranial arterial occlusion is in the exploratory stage, and its safety and efficacy requires further study.
Authors’ contributions
Yingzhe Zhao and Xiaomei Cui conceptualized the research. Xiaomei Cui and Shuai Jia communicated with the patient’s family and obtained informed consent. Yingzhe Zhao and Xiaomei Cui drafted the manuscript. Jianhong Geng and Jian Li revised the manuscript. Yingzhe Zhao and Yanqiang Wang performed literature searches and final proofreading. All authors contributed to the article and approved the submitted version.
Ethics statement
Written informed consent was obtained from the individual(s) and/or next of kin for the publication of any potentially identifiable images or data included in this article.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
All datasets presented in this study are included in the article.
Additional information
Funding
References
- Lindsberg PJ, Pekkola J, Strbian D, et al. Time window for recanalization in basilar artery occlusion: speculative synthesis. Neurology. 2015;85(20):1806–1815. Nov 17eng Cited in: pubmed; PMID 26574535.
- Mak CH, Ho JW, Chan KY, et al. Intra-arterial revascularization therapy for basilar artery occlusion-a systematic review and analysis. Neurosurg Rev. 2016;39(4):575–580. OctengEpub 20160125 Cited in: pubmed; PMID 26810313.
- Gory B, Eldesouky I, Sivan-Hoffmann R, et al. Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry. 2016; May87(5):520–525. engEpub 20150518 Cited in: pubmed; PMID 25986363.
- Le TM, Gaughen JR, Jensen ME, et al. Symptomatic wingspan stent stenosis and occlusion: stent-in-stent rescue. J Neurointerv Surg. 2010;2(4):348–350. DecengEpub 20100604 Cited in: pubmed; PMID 21990644.
- Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to Basilar-Artery occlusion. N Engl J Med. 2022;387(15):1373–1384. Oct 13eng. Cited in: pubmed; PMID 36239645.
- Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute Basilar-Artery occlusion. N Engl J Med. 2022;387(15):1361–1372. Oct 13eng. Cited in: pubmed; PMID 36239644.
- Chen WH, Yi TY, Zhan AL, et al. Stent-unsheathed effect predicts acute distal Middle cerebral artery atherosclerotic disease-related occlusion. J Neurol Sci. 2020;416:116957. 2020 Sep 15 eng. Epub 20200607. Cited in: pubmed; PMID 32535360.
- Yi TY, Chen WH, Wu YM, et al. Microcatheter "First-Pass effect" predicts acute intracranial artery atherosclerotic Disease-Related occlusion. Neurosurgery. 2019;84(6):1296–1305. Jun 1eng Cited in: pubmed; PMID 29790969.
- Liu Z, Liebeskind DS. Basilar artery occlusion and emerging treatments. Semin Neurol. 2021; Feb41(1):39–45. engNoneEpub 20210114 Cited in: pubmed; PMID 33469899.
- Chandwar K, Shah C. Trial of endovascular treatment of Basilar-Artery occlusion. N Engl J Med. 2021; Sep 2385(10):959. eng. Cited in: pubmed; PMID 34469659.
- Ang H, Koppara TR, Cassese S, et al. Drug-coated balloons: technical and clinical progress. Vasc Med. 2020;25(6):577–587. Deceng. Epub 20200707. Cited in: pubmed; PMID 32634046.
- Remonda L, Diepers M, Berberat J, et al. Drug-Coated balloon treatment in symptomatic intracranial high grade stenosis: a retrospective study of 33 patients. Clin Neuroradiol. 2021;31(1):45–49. MarengEpub 20200720 Cited in: pubmed; PMID 32691077.
- Han J, Zhang J, Zhang X, et al. Drug-coated balloons for the treatment of symptomatic intracranial atherosclerosis: initial experience and follow-up outcome. J Neurointerv Surg. 2019; Jun11(6):569–573. engCompeting interestsNone declared. Epub 20181018. Cited in: pubmed; PMID 30337378.
- Yin H, Zhang J, Zhao W, et al. Drug-Coated balloon for the treatment of nonacute symptomatic intracranial carotid artery terminus occlusion: initial experience and Follow-Up outcome. Front Neurol. 2022;13:840865. engThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Epub 20220211. Cited in: pubmed; PMID 35222260.
- Zhang Y, Chu X, Meng Y, et al. Drug-Coated Balloon-Oriented angioplasty for severe symptomatic atherosclerotic MCA stenosis in young adults. Front Neurol. 2021;12:743851. engThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Epub 20220201. Cited in: pubmed; PMID 35178020.
- Sella G, Gandelman G, Tuvali O, et al. Utilization of Drug-Coated balloons for the treatment of coronary lesions in the elderly population. JCM. 2022;11(9):2616. May 6engThe authors declare no conflict of interestEpub 20220506. Cited in: pubmed; PMID 35566739.
- Zhang Y, Zhang X, Dong Q, et al. Duration of dual antiplatelet therapy after implantation of Drug-Coated balloon. Front Cardiovasc Med. 2021;8:762391. eng. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Epub 20211201. Cited in: pubmed; PMID 34926613.
- Cremers B, Speck U, Kaufels N, et al. Drug-eluting balloon: very short-term exposure and overlapping. Thromb Haemost. 2009;101(1):201–206. Janeng. Cited in: pubmed; PMID 19132209.
- Pósa A, Nyolczas N, Hemetsberger R, et al. Optimization of drug-eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries. Catheter Cardiovasc Interv. 2010;76(3):395–403. Sep 1eng Cited in: pubmed; PMID 20839356.
- Maranhão RC, Tavares ER. Advances in non-invasive drug delivery for atherosclerotic heart disease. Expert Opin Drug Deliv. 2015; Jul12(7):1135–1147. engEpub 20150114 Cited in: pubmed; PMID 25585820.
- Gruber P, Braun C, Kahles T, et al. Percutaneous transluminal angioplasty using the novel drug-coated balloon catheter SeQuent please NEO for the treatment of symptomatic intracranial severe stenosis: feasibility and safety study. J Neurointerv Surg. 2019;11(7):719–722. JulengCompeting interestsNone declared. Epub 20181110. Cited in: pubmed; PMID 30415229.
- Stapleton CJ, Chen YF, Shallwani H, et al. Submaximal angioplasty for symptomatic intracranial atherosclerotic disease: a Meta-Analysis of Peri-Procedural and Long-Term risk. Neurosurgery. 2020;86(6):755–762. Jun 1eng Cited in: pubmed; PMID 31435656.
- Zhao W, Chu X, Song Y, et al. Drug-Coated balloon treatment for delayed recanalization of symptomatic intracranial artery occlusion. Transl Stroke Res. 2023;14(2):193–199. Apr 23. engEpub 20220423 Cited in: pubmed; PMID 35460456.