Abstract
Targeting a series of advanced manufacturing technology (AMT) ‘interventions’ provides the potential for significant step changes across the pharmaceutical value chain, from early stage ‘system discovery’ and clinical trials, through to novel service supply models. This research explores future value network configurations which, when aligned with disruptive shifts in technology (process and digital), may enable alternative routes to medicines production and the delivery of additional value to ‘end-users’, i.e. patients and health care providers. We draw on a categorisation of AMTs that may enable a shift from the traditional ‘batch’ and centralised manufacturing paradigm of ‘make-to-stock’, towards more re-distributed ‘continuous’ manufacturing and ‘make-to-order’ models. Despite reported benefits in the academic literature (e.g. reduced footprints, improved quality, enhanced flexibility and inventory savings), current adoption rates of continuous technologies in this sector remain low (c. 5%). This paper presents new data sources, in our study of AMT adoption in a global pharmaceutical context – assessing the barriers to implementation, and the pathways to delivering future continuous manufacturing scenarios. Our findings capture the high level of disparity in viewpoints, highlighting the uncertainties and transformational challenges ahead – in terms of opportunity areas, technological readiness and a future vision for the sector, as a whole.
1. Introduction
The pharmaceutical industry is undergoing a period of great change, reacting to the ‘patent cliff’, with fewer blockbusters being launched, R&D productivity at record low levels and the requirement for more niche products to serve new markets (Dixon et al. Citation2010). Many organisations are now actively reviewing their global footprints and legacy supply chains, driven by, for example, growth in emerging markets, and questioning whether they have the optimal architecture for manufacturing in the future (Harrington and Srai Citation2016). This highlights a particular problem faced by the pharmaceutical sector – the very significant uncertainty around which new molecules and products are selected for development, and around clinical trial outcomes (Shah Citation2004). While the number of compounds in development has increased by approximately 60% and total R&D expenditures have doubled over the past 10 years, the average number of new drug entities approved each year has declined, with only one in 10 small molecules entering clinical development expected to advance to full FDA approval (Hay et al. Citation2014). With estimates that average drug development costs are now circa. $2600–2800M (Avorn, Citation2014) – an increase from averages of $800M in the early 2000s (DiMasi Citation2002; DiMasi, Hansen, and Grabowski Citation2003), it is growing more critical to balance risk and potential rewards, promote faster development timelines, and to make better informed and earlier decisions on the pipeline (DiMasi, Grabowski, and Hansen Citation2016).
The sector has a reputation for being conservative, compared to other related industries that have successfully implemented sophisticated advanced manufacturing technologies (AMTs) that increase both process and product understanding (Saberi and Yusuff Citation2012; Rantanen and Khinast Citation2015). While identification, selection, acquisition and implementation issues have been dominant topics to-date in AMT literature (Chan et al. Citation2001; Goyal and Grover Citation2012), currents trends across the pharmaceutical industry – in accelerating innovative technology delivery, and establishing new supply chains for medicines – can help address a key research gap of addressing the realities of modern manufacturing environments (Farooq and O’Brien Citation2012). Here, the sector is looking to reconfigure value networks, moving towards smaller and more agile facilities, and associated end-to-end (E2E) supply chains, to support more dispersed and globalised manufacturing models (Srai et al. Citation2015). However, limited attention has also been paid to the role of the ‘industrial system’ in ‘connecting’ technology developments to final products, and how the design of the value network needs to provide a link between the two (Harrington and Srai Citation2016). In this study, we draw on a categorisation of AMTs (Gunawardana Citation2006) that may enable the shift from the traditional centralised and batch manufacturing paradigm of ‘make-to-stock’, towards that of re-distributed ‘continuous’ manufacturing operations and ‘make-to-order’ models.
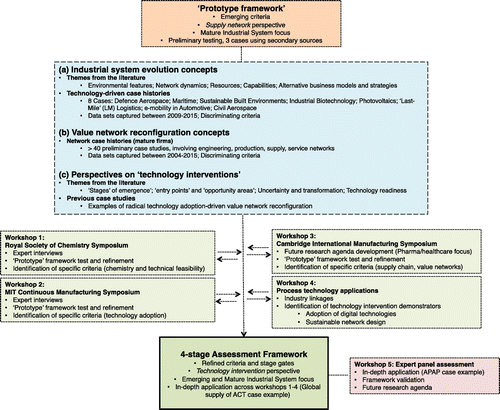
This research forms part of the on-going research agenda at the UK Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation. Research activities are targeting the adoption of continuous processing technologies across the pharmaceutical industry (see Figure ).
Figure 1. Continuous processing in the manufacture of pharmaceuticals – 10 focus areas targeting the adoption of AMTs (adapted from CMAC Citation2014).
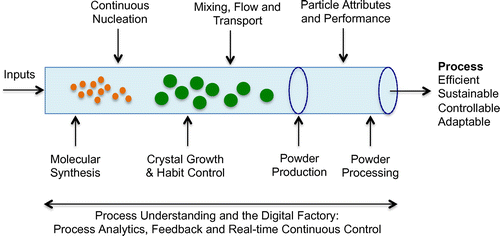
Specifically, this study focuses on AMTs, in the area of continuous crystallisation (CC), which is a critical unit operation in influencing the pharmacological properties and therapeutic efficacy of a final drug product (specifically respiratory and solid oral doses, which remain the prevalent dose forms today). Through design and control of this purification process, the aim is to promote improved crystal quality, in terms of targeted crystal size distribution, shape, polymorphic form and purity, which is critical for the efficiency of downstream processes from the crystallisation step (filtration and drying) (Laird Citation2013). Such characteristics have a strong effect on the properties of the final drug product, in terms of dissolution, bioavailability, quality and shelf life (Variankaval, Cote, and Doherty Citation2008). We extend an analytical framework – previously developed from a supply network perspective (Srai et al. Citation2015), using key literature and a series of case examples to develop assessments involving a series of technology interventions. In the context of this paper, we define ‘technology interventions’ as those which enable a significant breakthrough in one or more research areas involving continuous crystallisation-based AMTs (Brown et al. Citation2014; Callahan and Ni Citation2014; McGlone et al. Citation2015), and process analytical techniques (Powell et al. Citation2015, 2016). A series of workshop engagements involving industry and academic experts were used to then test, refine and validate the assessment framework and outputs.
The paper is structured as follows: first we review the key literature on industrial system evolution, value networks and reconfiguration concepts, and perspectives on technology interventions, which inform our framework extension. Section 3 summarises the investigative phase in developing a framework to assess technology interventions, involving a combination of literature synthesis, case histories and secondary data, and an outline of framework testing/refinement using expert panels. Next, the methodology section outlines the research strategy and process/methods of data collection and analysis. Section 5 demonstrates application of the four-step assessment framework, and criteria, in a pharmaceutical context. Section 6 presents new sources, of AMT adoption in a global context, assessing the barriers to implementation, and the pathways for delivering future continuous manufacturing scenarios. Finally, Section 7 presents conclusions, study limitations and future research activities.
2. Literature review
This section is organised around the following themes relevant to the extension of a prototype framework, which have emerged from literature on emerging technologies and industries, e.g.
| • | Industrial system evolution | ||||
| • | Value networks – reconfiguration concepts | ||||
| • | Perspectives on technology interventions | ||||
Key insights, from this review, are then integrated to inform the development of a assessment framework to better evaluate ‘technology interventions’ – from a value network perspective, in Section 3.
2.1 Industrial system evolution
Value networks and industrial systems continue to evolve, enabled by innovative manufacturing processes, and the emergence of new technologies (Royal Academy of Engineering (RAE) Citation2012). Traditional approaches, to the study of industrial systems in management literature, have often been developed on the assumption of ‘stable’ environments, which do not adequately provide theoretical or practical guidance on how to effectively capture new and emerging market opportunities (Zhang and Gregory Citation2011; Harrington and Srai Citation2012). Hence, a better understanding of the industrial system in which a network operates (capturing context, resources, activities, processes, actors and interdependencies) and an assessment of reconfiguration opportunities, which may result from an AMT intervention, provide valuable insights for both research and practice (Saberi and Yusuff Citation2012).
Within industrial systems, established firms are constantly reviewing and looking to reconfigure their legacy networks, leveraging existing capabilities where possible and creating advantages in terms of quality, flexibility and lead time, to support the emergence of new technologies and new operating or business models (Adner and Kapoor Citation2010). However, optimising the configuration of these fragmented networks is particularly challenging especially when there are ‘multiple tiers’ of partner firms within an industrial system, spanning component and intermediate goods supply, presenting a plethora of options on location and partnering models (Srai and Gregory Citation2008).
With the focus of this paper on reconfiguring value networks driven by AMTs, two common ‘enablers’ were identified that support the successful emergence of a new market, based on a radical technological intervention, and the ‘process’ of commercialising a new technology. Firstly, the successful emergence of any new market depends largely on the parallel development of a new supply or value chain to support commercialisation activities (Jacobides Citation2005; Sebastiao and Golicic Citation2008; Harrington and Srai Citation2016). Secondly, ‘new market creation’ has been considered as the overall process involving the coming together of a new network of stakeholders for the commercialisation of a new technology (Zahra and Nielsen Citation2002; Voelpel, Leibold, and Tekie Citation2004; Sarasvathy and Dew Citation2005). Hence, a key criterion in analysing technology interventions and the subsequent effect on emerging and evolving industrial systems is the role that the network of key institutional, industrial and supply network actors may play in the development of alternative business models, effective supply network strategies and viable products and services.
2.2 Value networks – reconfiguration concepts
For many decades, the drug product manufacturing model has been controlled by a regulatory framework – safeguarding the quality of the final product, by stringent process monitoring, testing of raw material, in-process material and end-product characteristics – using batch-based operations (Rantanen and Khinast Citation2015; Srai et al.Citation 2015). However, the sector needs to become more responsive to a changing environment, to devise new ways of providing patients with improved health ‘outcomes’, at a fraction of today’s cost (Erhard et al. Citation2013).
In studies of the pharmaceutical sector, ‘value network’ terminology has often been more applicable, given the complex interaction of clinical trials supply, primary (API) and secondary (drug product) manufacturing, and post-dosage activities being increasingly spread across networks of specialised firms, who may also be geographically dispersed and operating in mature and emerging economies (Edwards Citation2009). In terms of pharmaceutical value networks, new innovative manufacturing processes (such as ‘continuous’), the emergence of specialist CMOs in ‘continuous’, new supply chain partners (in distribution, logistics and the provision of digital technologies) are contributing to the need to reconfigure the pharmaceutical landscape (Srai et al.Citation 2015). One of the key drivers of ‘high value’ manufacturing is targeting AMT solutions that present both tangible and intangible benefits (Ariss, Raghunathan, and Kunnathar Citation2002). Previous studies have shown that many enterprises commonly focus on too narrow a segment of the value network, to an extent that the requirements of the ultimate ‘end-user’ (as opposed to the immediate customer for the product or technology) are often not adequately recognised (Harrington and Srai Citation2016). Hence, critical linkages, between continuous crystallisation-based processing and other continuous technologies both upstream and downstream, and any associated implications for patients and government health providers should be considered. There may be unseen benefits in a number of areas; product variety, consistency and functionality, energy and resource efficiency, inventory and customisation options, that may well contribute to a move towards more ‘continuous’ or ‘hybrid’ modes of processing becoming the norm and not the exception.
2.3 Perspectives on technology interventions
Recent advances in novel technologies (in areas such as ‘continuous’ processing, printing, sensing and diagnostics) are now creating the potential for significant step changes across the health care sector, with opportunities ranging from clinical trials, right through to the ‘end-user’ (Srai and Alinaghian Citation2013). In a recent review paper, examining the research and manufacturing potential of inkjet ‘2-D’ printing, for the development of pharmaceutical drug products (Daly et al. Citation2015), six ‘entry points’ and opportunity areas, in terms of research and specific technology ‘intervention’, were identified (see Appendix 1). These ‘interventions’ included the potential of the AMT in enabling breakthroughs in rapid prototyping for early stage ‘system’ discovery and clinical trials, instant and rapid changeovers within secondary processing, late customisation – leading to increased flexibility and service in terms of packaging/distribution, and the provision of personalised ‘dial-a-dose’ drug delivery to the patient. However, current adoption rates of continuous technologies within the pharmaceutical sector remain low, with industrial exemplars of ‘continuous’ manufacturing only operating at pilot and/or R&D lab scale capacities in many cases (Srai et al.Citation 2015). While evidence exists that continuous processing delivers financial benefits (mainly for single-purpose plants), studies and modelling have largely been focused at production and plant levels, with the business case, in each case, lacking any formal E2E network assessment (Srai et al. Citation2015).
Implementing such innovations require organisations, and their extended global networks, to proactively adapt their strategies to ensure an optimal fit between changing competitive priorities and the development of critical support structures (Nair and Boulton Citation2008). To illustrate these opportunities, examples of radical value network reconfiguration from other sectors, as a result of the emergence of a new technology are briefly outlined here:
| • | In vitro diagnostics (IVDs): here, new industrial actors have emerged such as IVD companies, specialist clinics and laboratory equipment manufacturers to support the emergence of a technology. Subsequent disaggregation of the value network has led to the reconfiguration of information, material, and revenue flows between industrial actors, resulting in radically different patient-centric supply chain models, and the potential for novel business models (Srai and Alinaghian Citation2013). | ||||
| • | Digital inkjet decoration and the industrial ceramics sector: a current drive to explore inkjet printing in many sectors is largely due its very successful implementation as part of late-stage customisation tactics, involving variant production at scale, in order to satisfy proliferation demands. Emergence has led to a revitalised industrial ceramics sector in Europe, characterised by vastly reduced inventory levels, yet with enhanced flexibility with respect to near-market supply (Daly et al. Citation2015). | ||||
| • | The Internet, digitisation and the recorded music sector: historically dominated by five major record labels, a series of innovations including the emergence of file compression techniques and the establishment of peer-to-peer (P2P) networks have radically transformed value networks within the recorded music sector. Furthermore, e-business models in this space, continue to open up opportunities for novel digital distribution channels involving music and film (Leyshon Citation2001). | ||||
In the context of the pharmaceutical sector, equivalent disruptions on a par with these three examples could enable the adoption of technologies compatible with (i) the rapid scale-up of new niche drug products in smaller volumes, (ii) novel delivery formats, with the option of late-stage personalisation and customisation and (iii) agile supply chains designed to manage the potential of significantly increased stock-keeping-units (SKUs).
The next section presents the development of an assessment framework to explore current and future configurations, which when aligned with disruptive shifts in technology, may enable alternative routes to drug product production.
3. Literature synthesis – framework extension
An analytical framework – developed from a supply network perspective (Srai et al.Citation 2015), was refined using key literature across a series of research domains (see Figure ). It is argued that it is critical to assess technology interventions in terms of the E2E supply chain benefits that breakthroughs may enable (e.g. time-to-patient; quality; inventory; enhanced volume flexibility and customisation; financial impact; return on investment). In summary, this synthesis phase examined:
| • | literature on emerging industry systems, in addition to eight technology-driven adoption/evolution case studies e.g. product-service systems in Defence Aerospace; niche high-specification production in Maritime; technologies for sustainable built environments; technology platform development in Industrial Biotechnology; next-generation technologies in Photovoltaics; e-Commerce driven ‘Last-Mile’ Logistics; e-mobility in Automotive, composite materials in Civil Aerospace (Harrington and Srai Citation2012, 2016; Harrington et al. Citation2016). These were selected in order to provide insights and inform the dimensions of analysis to consider in terms of technology adoption. | ||||
| • | literature on supply chain and value networks (configuration concepts, ‘states’, ‘archetypes’) supported by in excess of 40 network case studies and secondary data from the literature reflecting a number of diverse network forms (data-sets capturing engineering, production, supply and service networks) was used to explore dimensions of analysis as a basis to understanding the linkages between supply networks and technology interventions in a Pharmaceutical context (Srai et al.Citation 2015). | ||||
| • | Perspectives on ‘technology interventions’, examining previous studies on radical value network reconfigurations in other sectors, and themes from the literature which included ‘stages’ of emergence, maturity descriptors, entry points, opportunity areas and technology readiness (Daly et al. Citation2015; Harrington and Srai Citation2016). | ||||
The prototype framework was then tested and refined through a series of workshops – capturing rich insights and evidence from a variety of industrial practitioners on their specific AMT adoption and investment decisions, across a range of pharmaceutical drug products. Outputs from the case study interviews, in combination with case-specific secondary data, were then used to refine and finalise the assessment framework.
4. Methodology
This section presents an overview of the methodological approach used in his research paper. A mixed methodology was employed, involving expert group input, followed by a multiple case study method. This multiple case study strategy we adopt is in line with Yin’s definition (Citation2009) of it being an empirical inquiry that investigates a contemporary phenomenon both in-depth and within its real-life context. The approach is particularly appropriate here as this study seeks to explore both practice-based (where the insights of key industrial stakeholders are critical) and emerging phenomena when research and theory may be at an exploratory or formative stage (Eisenhardt and Graebner Citation2007; Yin Citation2009). The next section provides both details on the data collection process and methods, including information regarding the qualitative investigation and case studies development (interviewees and timescales) to inform the development of the technology intervention assessment framework.
4.1 Data collection and analysis – process
This process involved a series of workshop-type engagements, targeting specific outputs (see Figure ). Appendix 2 sets outs the 58 expert informants, by role, contribution, and workshop linkages where applicable. The expert engagements are summarised as follows:
| (1) | Royal Society of Chemistry workshop, on ‘redefining the twenty-first Century E2E pharmaceutical supply chain through enhanced manufacturing flexibility’ (Cambridge, March 2014). This initial stage involved 15 experts across industry and academia, active in the area of continuous AMTs in pharma, sharing insights from specific cases. Questionnaire outputs and a group discussion were then used to formulate a specific case review structure, to identify synergies across cases, with focus centred on assessment of the barriers to future continuous manufacturing scenarios. | ||||
| (2) | Workshop on ‘Reconfiguring pharmaceutical supply networks E2E through continuous manufacturing’, MIT Continuous Manufacturing Symposium (Boston, May 2014). This stage involved eight experts, active in the area of continuous AMTs, who shared insights on specific adoption and investment decisions, in assessing a series of future continuous manufacturing scenarios for the global supply of Artemisinin-based Combination Therapies (ACTs) – see Appendix 3. | ||||
| (3) | Workshop on ‘Pharmaceutical supply chains – setting the future research agenda’, Cambridge International Manufacturing Symposium (Cambridge, September 2015). This stage involved 12 academics – specialising in supply chain and global manufacturing domains – assessing a series of future continuous manufacturing scenarios, involving novel technologies (in areas such as ‘continuous’ processing, printing, sensing and diagnosing). | ||||
| (4) | Leveraging access to 24 industrial partners, aligned with the Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation (CMAC), and project ‘ReMediES’ (Re-configuring Medicines E2E Supply), this workshop explored (a) what firms are using to select continuous manufacturing technologies to meet specific E2E supply chain objectives and (b) linkages between in practice criteria and those derived from the literature (Glasgow, March 2016). | ||||
| (5) | The final validation workshop (Glasgow, June 2016) involved 12 experts, active in the area of continuous AMTs in pharma, who shared insights on the technology assessment process for a specific in-depth application e.g. CMAC continuous demonstrator, involving the manufacture of Paracetamol (APAP). | ||||
5. Assessment framework application
One of the main aims of this research is to highlight the management of AMTs in an increasingly globalised world. The case studies (outlined in Section 3) informing framework extension, suggest significantly enhanced product variety and/or flexibility to be key criteria in any future industry development. This together with the observed trend in the cases to locate production closer to the end-user suggest future manufacturing will support small-scale distributed operations where speed, and product/product-service customised solutions are more attractive value propositions, enabled by technology developments that provide these viable options.
In this section, the assessment framework is applied to demonstrate how one (or a combination of) continuous AMT ‘interventions’ may enable (a) reduced investment costs, through smaller production facilities with lower capital cost, reduced overall plant footprint; (b) operating costs through lower catalyst and solvent use, and by minimising total reaction times and (c) reducing inventory cost (less WIP inventory, with reduced material handling and transport).
A set of pre-screening criteria is first used to identify the barriers to adopting alternative product-process technologies and business models. Using basic operational and societal data inputs to explore future trends (where implementation of ‘continuous’ may have a cost advantage or increase speed to market for an entity) and market requirements (in terms of volume and product variety, affordability and unmet needs) the pre-screening stage facilitates the rapid analysis (‘go’ vs. ‘no-go’) of whether an existing drug product or new molecule may be a viable candidate to investigate further in terms of business case evaluation and AMT implementation. As outlined in Appendix 3, criteria should include:
| • | Therapy or disease area; patient population; treatment profile; volumes (current, projected); basic financials (price, cost, revenues, margin); SKU mix; inventory; CapEx and quality/waste. | ||||
| • | In addition, to inform workflow development for rapid product assessment and continuous process selection, 10 critical ‘attributes’ – in terms of technology feasibility and chemistry that may also be assessed at this pre-screening stage – were identified. Focus here looks at complexity and area(s) of opportunity (in the context of ‘continuous’ processing) with respect to molecule; polymorph; chirality; number of process steps; particle engineering; kinetics; stability; bioavailability; final dosage form and ease of scale-up/scale-out. | ||||
The next phase (stage 2) involves mapping the ‘current state’, in terms of technologies, unit operations and the supply network. This leads to the identification of critical ‘sub-systems’ (e.g. clinical trials, primary manufacturing, secondary processing, packaging and distribution, and E2E supply) that may be affected by a shift to a future, more continuous operating model. The first current state mapping exercise identifies those unit operations where an existing batch production process may be ‘pre-disposed’ to a series of continuous technologies (in terms of current state and future potential), namely, in synthesis; purification; isolation; formulation and packaging. Here, the specific implications of defined continuous technology developments and readiness levels may be assessed (are certain routes to manufacture pre-disposed to continuous processing? What alternative routes could be?).
An E2E network performance analysis is then used to define overall system metrics, and to evaluate the current state configuration and trade-offs being made. Current-state supply and value network mapping techniques are used to define the existing sub-systems and the drivers/design factors that predominate in each sub-system. Associated impact variables may then be scored, setting out the potential scale of the benefit to patients, government health service providers and industrial value network partners. Here, technology interventions are assessed in terms of 15 impact variables, namely: inventory; lead-time supply; lead-time to market; scale-up (going into); volume flexibility (mix and volume); process control (including reliability and safety); quality (purity); yield; IP protection and extension (including issue of counterfeits); cost (process, packaging and transport); investment cost (incorporating financial impacts and return on investment); fiscal/tax; environmental impact; viability/adaptability and asset utilisation.
Future state process and network design scenarios, based on the emerging process technology options emerging from stage 2 of the assessment framework and future industry trends are then assessed. Future state models, in the form of value stream maps, generally explore several potential future states prior to a final decision. Alternative states may be based on emerging process and production technologies or AMTs that are still yet to be fully developed. In addition to key industry reports, central to the decision-making process, is continued access to, and engagement with industry, in order to assess future scenarios and the subsequent effect on the wider pharmaceutical landscape.
The research strategy we adopt enables cross-case analysis, in order to identify emerging patterns and other pharmaceutical drug products that may benefit from similar value network design and reconfiguration opportunities. The process also facilitates the development of a series of ‘value network roadmaps’, which may be both generic sector summaries, and product (category) specific. In the context of this research, these roadmaps may be defined as a visual representation, through time, of (disruptive) changes in ‘activities’ and ‘actors’ across the value chain, typically as a result of multiple technological disruptions in process (i.e. T1 → T2 … Tn) and/or a network reconfiguration of actors (i.e. V1 → V2 … Vn), usually resulting in the emergence of new products (i.e. P1 → P2 … Pn) – see schematic representation in Figure .
Figure 3. Reconfiguring pharmaceutical value networks: targeted interventions (T1 → T2) changing the product and industrial landscape (P1 and V1 → P2 and V2).
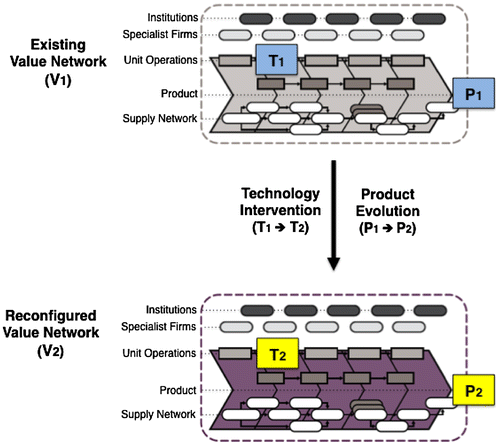
Within a pharmaceutical context, this stage can also support a performance assessment of several potential technological choices involving batch, continuous or hybrid routes, and involve a combination of:
| (a) | Providing a benchmark, in terms of potential yield and purity | ||||
| (b) | Evaluating various scenarios, which may involve alternative scale production footprints (dispersed, close-to-market, low-scale and integrated plants) and | ||||
| (c) | Developing alternative supply models that might now be possible, due to technological advances in ordering or replenishment. | ||||
In turn, architectural differences between batch, hybrid or continuous operations and associated implications for up-stream and down-stream value network configurations, structures, processes, and systems may be assessed across a series of product families. In practice, scenarios may depend on various disruptive influences that challenge the current value network model and introduce a series of possible product or product-service models.
Finally, stage 4 integrates business context/viability and technology readiness inputs (from supporting technology roadmaps) in order to evaluate value network reconfiguration opportunities, and develop a case for transformation. Inputs here include:
| • | Examining current and future states, in terms of key system metrics from stages 2 and 3 | ||||
| • | Value propositions in making the transformation from a business context for key value network players, versus the investments required (potential impact on revenue, margin and inventory reduction) | ||||
| • | Key economic evaluation criteria in terms of batch versus continuous, for example, direct fixed capital, plant throughput, manufacturing cost and unit production cost | ||||
| • | Finally, feasibility and timescales on specific AMT interventions | ||||
A series of emerging themes and insights from practitioners on the opportunities for technology-driven value network reconfigurations in the pharmaceutical sector are now outlined in the following section.
6. Results and implications
This section presents new sources in assessing the barriers to implementation, and the pathways for delivering future continuous manufacturing scenarios, using the framework and scenarios set out in Section 5.
While there is collective agreement from industry and academia that the funding model for drug discovery involving small molecules is becoming outdated and inefficient, there is level of disparity of viewpoints when it comes to adoption of ‘continuous’ manufacturing – in terms of opportunity areas, technological readiness levels and uncertainties and the transformation challenges (including behaviours) that lay ahead within the sector. Table summarises outputs from workshop 1 involving industrial and academic experts who are actively involved in the areas of continuous flow R&D and process-technology adoption. Overcoming these barriers requires a coordinated and systematic approach when redesigning the entire value network. Our research suggests that barriers may be real or perceived, and arise from combinations of socio-political, technical and regulatory factors. Content validity ratio (CVR) values were calculatedFootnote1 and range between −1 (perfect disagreement) and +1 (perfect agreement), with CVR values above zero indicating that over half of panel members were in agreement. For a panel size of 15, the critical level of agreement or disagreement (CVR critical) is +/−0.6 (Ayre and Scally Citation2014).
Table 1. Barriers to the continuous manufacture of pharmaceutical drug products.
Second, in assessing pathways to future continuous manufacturing scenarios, critical to this is how best to manage AMTs and best guide the selection of processes (continuous or batch). Identifying what adds value to the ultimate ‘end-customer’ is not just a technical consideration, but also requires a change in mind-set. The future influence of the patient was also cited as, potentially, the most disruptive factor across the entire industry. Hence, industry needs to design products and services based on patient needs, and not what an organisation ‘wants to make’. Key insights on how the sector should approach ‘manufacturing in a new way’ to deliver value are summarised as follows:
| • | Technology interventions linked to E2E supply chain analysis should be a key deliverable in assessing any business case (‘It’s more than just a ‘batch-to-continuous’ agenda’). | ||||
| • | While industry has committed to the principle of continuous processing, it may be better served to target ‘tactical’ AMT opportunities. Employing a phased approach, and scouting for opportunities where there are ‘low effort/high yield’ opportunities, confidence can be gained before applying more resources. | ||||
| • | It is argued that the industry, as a whole, doesn’t currently operate its batch processes at an optimum level. In the future, how can the sector guarantee that continuous processes, enabled by AMTs, will operate any better? A key consideration is to acknowledge that benefits may be achievable without moving to a fully continuous route for some products. For example, continuous, batch, or hybrid solutions may well drive agile supply chains of the future. | ||||
For many of the emerging niche and high-cost candidates discussed as part of this study, scenarios often involve exploring opportunities to reformulate in order to increase bioavailability. This opens up the potential to reduce ‘dosages’, hence, volumes are required for new formulations. There were cases where more combinations are likely to be required in the future, where single-use derivatives are actively discouraged due to risk of drug resistance (as in this case of ACTs in Appendix 3), complex hybrid solutions involving more combinations and reformulations and, finally, cases where significant increases in volumes will require additional capacity close to emerging markets. Combining such criteria may also be used to identify clusters of candidate drug products with attractive business cases that may exhibit similar areas of benefit and scale for patients and government health service providers, made possible in the context of adopting specific continuous processing technologies. This emerging ‘library’ of ‘product-process archetypes’ can inform a classification system that enables ease of comparability in identifying other drug candidates and new molecules that may benefit from specific technology interventions and opportunity areas.
Thirdly, in a future pharma context, it is argued that collaboration may be the key to success, but clear deliverables, timelines and specific targets must be established to make alliances work. The management of AMT is not so straightforward in this scenario. Decisions on partner selection should involve not only assessing the type of AMT that is appropriate for a particular manufacturing and business situation, but also its specification, integration and use. Key considerations from industry on ‘continuous capability and supplier development’ are as follows:
| • | Where are the ‘solution spaces’ (large volumes, elimination of working capital, speed of supply reducing inventories and customising for the patient) where continuous processing can deliver value? | ||||
| • | What are the specific continuous AMT interventions, and associated technical challenges, that need to be overcome to achieve delivery of these ‘solution spaces’? | ||||
It is suggested that the ideal supply chain may be composed of a mix of internal fixed-capacity, with some degree of external variable capacity. With a growing patient-centric focus and a move towards ‘outcomes-based’ business models, it is argued that future pharma may well be better served to focus on R&D and commercialisation, leaving emerging CMOs to provide the ‘solution space’ or capacity to become an attractive ‘test-bed’ for rolling out new drug products. There may be a requirement for more of a supplier development focus given that the industry needs to grow external manufacturer capability to enable continuous manufacturing to develop. A critical counter argument here is increased dependencies, especially external, may lead to suppliers of AMTs or input chemicals becoming future competitors as they develop more core competencies in the focal firm’s key processes.
A fourth theme that emerged was whether the real opportunities for AMT adoption lay in ‘development’ stages. With the transfer of many elements of clinical supply to commercial supply inherently built-in, should industry concentrate on alternative clinical supply chain designs at an early phase, which may drive technology solutions to deliver precision, speed, integration, inventory savings, increased flexibility, improved product quality and quicker response to new customer demands? This would involve a focus on speed-to-market for system or drug discovery, with commercial and ‘development’ phases using continuous technologies to enable rapid synthesis and speed/efficiency as an optimal pathway to E2E integration. The importance of continuous AMT implementation in ‘discovery’ could also enable rapidly shorter supply chains where there may be less of a stability requirement for a final drug product. This could open up potential opportunities for a new range of targeted molecules with a stability of a few weeks (i.e. radiopharmaceuticals).
Finally, while the adoption of specific AMTs in pharma may be used to serve existing markets more effectively, or deliver unmet end-user needs, individual drug products may also be assessed, in the context of new continuous capabilities and technology interventions that may create opportunities with respect to new and emerging markets. These could include (a) drug products previously considered uneconomical to deliver via traditional batch processing routes, (b) more established generic products which may need to ‘evolve’, in response to future trends and changing markets, in addition to (c) new chemical entities for an initial assessment. High levels of investment in continuous AMTs may well remove cost benefits (initially), but once a maturity level is achieved and a technology is no longer proprietary but widespread (like the example of laser printing), there will be opportunities for return on investment. However, continued uncertainty with respect to timescales on certain continuous technology developments and implementation, versus changing cost profiles of high cost and emerging countries remains a key concern for the sector.
7. Conclusions and future research agenda
Pharmaceutical organisations are now actively reviewing their global footprints and questioning whether they have the optimal architecture and technologies for manufacturing in the future. In this study, we draw on a categorisation of AMTs that may enable a shift from the traditional centralised and batch manufacturing paradigm of ‘make-to-stock’, towards more re-distributed ‘continuous’ manufacturing operations and ‘make-to-order’ models. The research strategy employed enabled an investigation of the scope, challenges and opportunities of specific AMT innovations, in the area of continuous manufacturing and crystallisation, where current adoption rates within the pharmaceutical sector remain low. This paper then presented new sources, in our study of AMT adoption in a global context – assessing the barriers to implementation, and the pathways for delivering future continuous manufacturing scenarios – using a four-stage assessment framework as a basis for data capture.
While our studies have highlighted a desire for improved integration, openness, collaboration and trust between stakeholders, our findings also capture the high level of disparity in viewpoints that exist across the pharmaceutical value network, emphasising the many uncertainties and transformational challenges that lay ahead. Secondly, the widespread take-up of AMTs, in the area of ‘continuous’ manufacturing, is not solely dependent upon the technical requirements identified. Overcoming the barriers to adoption requires a coordinated and systematic approach to understanding and quantifying the benefits across the entire value network, in local and global contexts, in order to develop the business case for the implementation of continuous AMT solutions.
In conducting this study, some limitations are evident which, however, present interesting opportunities for future research. First, and inherent to the nature of emerging AMTs in Pharma, was access to a significant number of case examples at this juncture. While the assessment framework was developed using an extensive literature review, testing and refinement was restricted to four workshops, with validation restricted to application using two in-depth case studies to-date that were assessed by an expert panel. The second limitation of this study is a focus on small molecules, and on solid oral dose forms. With the trend towards more biopharmaceuticals and more drug device combinations, future research plans will include testing and refining the framework using case studies involving other industry segments e.g. macro-molecules, stratified medicines and extending the approach to health care contexts (e.g. medical devices and digital e-health care technologies) – all of which will require some additional AMTs in process analytics, diagnostics, and across the extended supply chain. These activities form part of an ongoing research agenda, as part of project ReMediES (Re-configuring Medicines E2E Supply), which explores
| • | Implications of digitalisation – from the perspective of AMT disruptions on traditional supply chains, solutions to customise and address customer needs, and the effect on downstream supply chains to patient delivery. | ||||
| • | Development of ‘smart’ packaging AMTs, for security and anti-counterfeiting. | ||||
| • | Implications, in terms of AMT challenges in formulation, and the wider supply chain. | ||||
| • | The impact of regulatory trends, together with affordability and access to care/markets. | ||||
Several diverse pharmaceutical products have also been identified as part of this on-going study, in order to provide the basis for exploring alternative product-process supply network options and value chain implications arising from a technology intervention. These case studies were chosen as they represent products at different stages of their product life cycle, have dissimilar product volume and pack complexity profiles, and varying transformation challenges if an alternative process, value network and/or business case is implemented. In turn, this will inform a classification system (in development) that will enable ease of comparability to identify and connect drug product, AMT, and device, where ‘combinations’ of technologies may well benefit from similar value network design and reconfiguration opportunities.
Funding
This research is supported by the Engineering and Physical Sciences Research Council (EPSRC) Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation [grant number EP/1033459/1], its industrial partners, and the UK Department of Business, Innovation and Skills (BIS) Advanced Manufacturing Supply Chain Initiative (AMSCI) funded project ‘ReMediES’ (Re-configuring Medicines E2E Supply) [grant number TS/ L006529/1].
Disclosure statement
No potential conflict of interest was reported by the authors. Interview data in this paper have been anonymised to protect confidentiality. Additional raw data related to this publication cannot be openly released, as the interviewees did not consent to open data sharing.
Notes
1. The CVR (content validity ratio) is a linear transformation of a proportional level of agreement on how many experts within a panel rate an item ‘essential’ calculated in the following way (Ayre and Scally Citation2014):
CVR = ne − (N/2)/(N/2), where CVR is the content validity ratio, ne is the number of panel members indicating an item ‘essential,’ and N is the number of panel members.
References
- Adner, R., and R. Kapoor. 2010. “Value Creation in Innovation Ecosystems: How the Structure of Technological Interdependence Affects Firm Performance in New Technology Generations.” Strategic Management Journal 31 (3): 306–333. doi:10.1002/smj.821.
- Ariss, S. S., T. S. Raghunathan, and A. Kunnathar. 2002. “Factors Affecting the Adoption of Advanced Manufacturing Technology in Small Firms.” SAM Advanced Management Journal 65 (2): 14–23.
- Avorn, J. 2014. “The $2.6 Billion Pill: Methodologic and Policy Considerations.” New England Journal of Medicine 372: 1877–1879. doi:10.1056/NEJMp1500848.
- Ayre, C., and A. J. Scally. 2014. “Critical Values for Lawshe’s Content Validity Ratio: Revisiting the Original Methods of Calculation.” Measurement and Evaluation in Counseling and Development 47 (1): 79–86. doi:10.1177/0748175613513808.
- Brown, C. J., Y. C. Lee, Z. K. Nagy, and X. Ni. 2014. “Evaluation of Crystallization Kinetics of Adipic Acid in an Oscillatory Baffled Crystallizer.” Crystal Engineering Communications 16 (34): 8008–8014. doi:10.1039/c4ce00192c.
- Callahan, C. J., and X.-W. Ni. 2014. “An Investigation into the Effect of Mixing on the Secondary Nucleation of Sodium Chlorate in a Stirred Tank and an Oscillatory Baffled Crystallizer.” Crystal Engineering Communications 16 (4): 690–697. doi:10.1039/c3ce41467a.
- Chan, F. T. S., M. H. Chan, H. Lau, and R. W. L. Ip. 2001. “Investment Appraisal Techniques for Advanced Manufacturing Technology (AMT): A Literature Review.” Integrated Manufacturing Systems 12 (1): 35–47. doi:10.1108/09576060110361528.
- CMAC. 2014. CMAC Annual Report. http://www.cmac.ac.uk/files/media/cmac_brochure_2014.pdf.
- Daly, R., T. S. Harrington, G. D. Martin, and I. M. Hutchings. 2015. “Inkjet Printing for Pharmaceutics - A Review of Research and Manufacturing.” International Journal of Pharmaceutics 494 (2): 554–567. doi:10.1016/j.ijpharm.2015.03.017.
- DiMasi, J. A. 2002. “The Value of Improving the Productivity of the Drug Development Process.” PharmacoEconomics 20 (Supplement 3): 1–10.10.2165/00019053-200220003-00001
- DiMasi, J. A., R. W. Hansen, and H. G. Grabowski. 2003. “The Price of Innovation: New Estimates of Drug Development Costs.” Journal of Health Economics 22: 151–185. doi:10.1016/S0167-6296(02)00126-1.
- DiMasi, J. A., H. G. Grabowski, and R. W. Hansen. 2016. “Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs.” Journal of Health Economics 47: 20–33. doi:10.1016/j.jhealeco.2016.01.012.
- Dixon, J., P. England, G. Lawton, P. Machin, and A. Palmer. 2010. “Medicines Discovery in the 21st Century: The Case for a Stakeholder Corporation.” Drug Discovery Today 15 (17–18): 700–703. doi:10.1016/j.drudis.2010.07.004.
- Edwards, P. J. 2009. “Value Networks Identify Innovation in 21st Century Pharmaceutical Research.” Drug Discovery Today 14 (1-2): 68–77. doi:10.1016/j.drudis.2008.09.015.
- Eisenhardt, K. M., and M. E. Graebner. 2007. “Theory Building From Cases: Opportunities and Challenges.” Academy of Management Journal 50 (1): 25–32. doi:10.5465/AMJ.2007.24160888.
- Erhard, A., J. Anscombe, G. Ortolani, T. Wintermantel, and E. de Bres-Riemslag. 2013. Building Value-based Healthcare Business Models. Munich: AT Kearney.
- Farooq, S., and C. O’Brien. 2012. “A Technology Selection Framework for Integrating Manufacturing within a Supply Chain.” International Journal of Production Research 50 (11): 2987–3010. doi:10.1080/00207543.2011.588265.
- Goyal, S., and S. Grover. 2012. “Advanced Manufacturing Technology Effectiveness: A Review of Literature and Some Issues.” Frontiers of Mechanical Engineering 7 (3): 256–267. doi:10.1007/s11465-012-0330-7.
- Gunawardana, K. D. 2006. “Introduction of Advanced Manufacturing Technology: A Literature Review.” Sabaragamuwa University Journal 6 (1): 116–134.
- Harrington, T. S., and J. S. Srai. 2012. “Defining Product-service Network Configurations and Location Roles: A Current and Future State Analysis Framework for International Engineering Operations.” International Journal of Product Development 17 (3/4): 228–253. doi:10.1504/IJPD.2012.052103.
- Harrington, T. S., and J. S. Srai. 2016. “Understanding Stages of Supply Network Emergence in Technology Commercialisation.” International Journal of Manufacturing Technology and Management. doi:10.1504/IJMTM.2016.10000001.
- Harrington, T. S., J. S. Srai, M. Kumar, and J. Wohlrab. 2016. “Identifying Design Criteria for Urban System ‘Last-mile’ Solutions – A Multi-stakeholder Perspective.” Production Planning and Control 27 (6): 456–476. doi:10.1080/09537287.2016.1147099.
- Hay, M., D. W. Thomas, J. L. Craighead, C. Economides, and J. Rosenthal. 2014. “Clinical Development Success Rates for Investigational Drugs.” Nature Biotechnology 32: 40–51. doi:10.1038/nbt.2786.
- Jacobides, M. G. 2005. “Industry Change Through Vertical Disintegration: How and Why Markets Emerged in Mortgage Banking.” Academy of Management Journal 48 (3): 465–498. doi:10.5465/AMJ.2005.17407912.
- Laird, T. 2013. “Special Feature Section: Polymorphism and Crystallisation.” Organic Process Research and Development 17: 443–444. doi:10.1021/op4000303.
- Leyshon, A. 2001. “Time–Space (and Digital) Compression: Software Formats, Musical Networks, and the Reorganisation of the Music Industry.” Environment and Planning A 33: 49–77. doi:10.1068/a3360.
- McGlone, T., N. Briggs, C. Clark, C. Brown, J. Sefcik, and A. J. Florence. 2015. “Oscillatory Flow Reactors (OFRs) for Continuous Manufacturing and Crystallization.” Organic Process Research and Development 19 (9): 1186–1202. doi:10.1021/acs.oprd.5b00225.
- Nair, A., and W. R. Boulton. 2008. “Innovation-oriented Operations Strategy Typology and Stage-based Model.” International Journal of Operations and Production Management 28 (8): 748–771. doi:10.1108/01443570810888599.
- Powell, K. A., A. N. Saleemi, C. D. Rielly, and Z. K. Nagy. 2015. “Periodic Steady-state Flow Crystallization of a Pharmaceutical Drug Using MSMPR Operation.” Chemical Engineering and Processing: Process Intensification 97: 195–212. doi:10.1016/j.cep.2015.01.002.
- Powell, K. A., A. N. Saleemi, C. D. Rielly, and Z. K. Nagy. 2016. “Monitoring Continuous Crystallization of Paracetamol in the Presence of an Additive Using an Integrated PAT Array and Multivariate Methods.” Organic Process Research and Development 20 (3): 626–636. doi:10.1021/acs.oprd.5b00373.
- RAE (Royal Academy of Engineering). 2012. Industrial Systems: Capturing Value through Manufacturing. London: RAE. ISBN 1-903496-69-1.
- Rantanen, J., and J. Khinast. 2015. “The Future of Pharmaceutical Manufacturing Sciences.” Journal of Pharmaceutical Sciences 104 (11): 3612–3638. doi:10.1002/jps.24594.
- Saberi, S., and R. M. Yusuff. 2012. “Neural Network Application in Predicting Advanced Manufacturing Technology Implementation Performance.” Neural Computing and Applications 21 (6): 1191–1204. doi:10.1007/s00521-010-0507-0.
- Sarasvathy, S. D., and N. Dew. 2005. “New Market Creation through Transformation.” Journal of Evolutionary Economics 15 (5): 533–565. doi:10.1007/s00191-005-0264-x.
- Sebastiao, H., and S. Golicic. 2008. “Supply Chain Strategy for Nascent Firms in Emerging Technology Markets.” Journal of Business Logistics 29 (1): 75–91. doi:10.1002/j.2158-1592.2008.tb00069.x.
- Shah, N. 2004. “Pharmaceutical Supply Chains: Key Issues and Strategies for Optimization.” Computers and Chemical Engineering 28: 929–41. doi:10.1016/j.compchemeng.2003.09.022.
- Srai, J. S., and L. S. Alinaghian. 2013. “Value Chain Reconfiguration in Highly Disaggregated Industrial Systems: Examining the Emergence of Health Care Diagnostics.” Global Strategy Journal 3: 88–108. doi:10.1111/j.2042-5805.2012.01047.x.
- Srai, J. S., and M. Gregory. 2008. “A Supply Network Configuration Perspective on International Supply Chain Development.” International Journal of Operations and Production Management 28 (5): 386–411. doi:10.1108/01443570810867178.
- Srai, J. S., C. Badman, M. Krumme, M. Futran, and C. Johnston. 2015. “Future Supply Chains Enabled by Continuous Processing—Opportunities Challenges May 20–21 2014 Continuous Manufacturing Symposium.” Journal of Pharmaceutical Sciences 104 (3): 840–849. doi:10.1002/jps.24343.
- Srai, J. S., T. S. Harrington, L. A. Alinaghian, and M. A. Phillips. 2015. “Evaluating the Potential for the Continuous Processing of Pharmaceutical Products – A Supply Network Perspective.” Chemical Engineering and Processing: Process Intensification 97: 248–258. doi:10.1016/j.cep.2015.07.018.
- Variankaval, N., A. S. Cote, and M. F. Doherty. 2008. “From Form to Function: Crystallization of Active Pharmaceutical Ingredients.” AIChE Journal 54 (7): 1682–1688. doi:10.1002/aic.11555.
- Voelpel, S. C., M. Leibold, and E. B. Tekie. 2004. “The Wheel of Business Model Reinvention: How to Reshape Your Business Model to Leapfrog Competitors.” Journal of Change Management 4 (3): 259–276. doi:10.1080/1469701042000212669.
- Yin, R. K. 2009. Case Study Research: Design and Methods. 4th ed. Thousand Oaks, CA: Sage.
- Zahra, S. A., and A. P. Nielsen. 2002. “Sources of Capabilities, Integration and Technology Commercialization.” Strategic Management Journal 23 (5): 377–398. doi:10.1002/smj.229.
- Zhang, Y., and M. Gregory. 2011. “Managing Global Network Operations along the Engineering Value Chain.” International Journal of Operations and Production Management 31 (7): 736–764. doi:10.1108/01443571111144832.
Appendix 1.
Six intervention points where research on the inkjet printing of pharmaceutical drug products will focus on (adapted from Daly et al., Citation2015)
Appendix 2.
Expert informants, by role, and their contribution to the 5 workshops
Workshop 1: Royal Society of Chemistry Symposium
Workshop 2: MIT Continuous Manufacturing Symposium
Workshop 3: Cambridge International Manufacturing Symposium
Workshop 4: Process technology applications and linkages
Workshop 5: Expert panel assessment
Appendix 3:
Four-stage framework, with case study example, to assess the feasibility of adopting continuous manufacturing technologies for the production of pharmaceutical drug products
Appendix 3 (continued)