Abstract
The accelerating decline of pollinator populations has become increasingly concerning in the last two decades. Honey bees are economically important pollinators and a model species to evaluate pollinator health. The decline in queen quality is one of the frequently reported causes associated with honey bee losses worldwide. Histopathology is an essential tool used for diagnostics and research in mammalian species and may provide insight into the histomorphological basis of a decline in queen quality. Thus, the purpose of this study was to summarize the previously described normal morphology of the entire reproductive tract of mated honey bee queens and to illustrate it by high quality photomicrographs. Accordingly, reproductive tracts of one-year old mated honey bee queens were processed for histology, serially sectioned, stained with hematoxylin and eosin, and used for the capture of high quality histological photomicrographs of the entire reproductive tract. This study illustrates the microscopic morphology of the queen reproductive tract which may facilitate further investigations of the cause and pathogenesis of the recent decline in reproductive fitness of honey bees using histopathology.
Introduction
Histology is an essential tool that facilitates accurate evaluation of the microscopic tissue structures and allows for a basic assessment of organ function. In vertebrate species, the study of microscopic morphologic alterations (histopathology) is the basis for the diagnosis of disease or evaluation of host response to noxious stimuli. It is used extensively for diagnostics, research, and regulatory purposes (risk and safety assessment) (Bernet et al., Citation1999; Brown et al., Citation2016). However, histopathology has not been developed and utilized to the same extent in insects as it has been in vertebrate species.
Despite the growing public awareness of pollinators’ importance, the beginning of the 21st century has been marked by compelling evidence of the decline in pollinators and dependent plants (Burkle et al., Citation2013; Morse & Calderone, Citation2000; Potts et al., Citation2010; Vanbergen & Initiative, Citation2013). Honey bees are the most researched pollinators; the decline in honey bee populations and deterioration of their health has been linked to a number of causes, including pesticide exposure (Sánchez-Bayo et al., Citation2016; Wood et al., Citation2018), climate (Beyer et al., Citation2018), infectious and parasitic diseases (Grozinger & Flenniken, Citation2019; Higes et al., Citation2008; Martín-Hernández et al., Citation2011), agricultural intensification (Ricketts et al., Citation2008; Winfree et al., Citation2009), and loss of genetic diversity (Forfert et al., Citation2017). Rigorous field and laboratory protocols have been established to study declines in honey bees. However, the results are often controversial and inconsistent between field and laboratory-based studies, leaving room for improvement of standard honey bee research techniques.
Queen failure and poor queen quality are consistently among the top three reported causes of honey bee colony losses (Amiri et al., Citation2017). The decreased quality of queens has been associated with the use of pesticides (Haarmann et al., Citation2002; Wu-Smart & Spivak, Citation2016), nosemosis (Alaux et al., Citation2011; Simeunovic et al., Citation2014; Traver & Fell, Citation2012), varroosis, and viral infections (Gauthier et al., Citation2011); however, the cause and pathogenesis remain unclear. Histopathological evaluation of the honey bee reproductive tract in addition to the current standard methods used for assessment of reproductive fitness (e.g. queen morphometric measures, ovariole count, sperm evaluation, brood pattern) may enhance our understanding of disease development, treatment, and prevention.
Previously, normal macroscopic and microscopic anatomy of the entire reproductive tract of mated honey bee queens was described and illustrated by diagrams (Snodgrass, Citation1985), and certain segments of the reproductive tract were well-characterized by electron microscopy (Biliński & Jaglarz, Citation1999; Cruz-Landim & Patrício, Citation2010; Gutzeit et al., Citation1993; Patrício & Cruz-Landim, Citation2002, Citation2008) or light microscopy (Camargo & Mello, Citation1970; Gutzeit et al., Citation1993; Stell, Citation2012). However, neither of these individual studies encompasses the entire reproductive tract in sufficient detail to provide a comprehensive reference for histopathology. Therefore, to facilitate the future histopathological evaluation of abnormalities in the honey bee reproductive tract, a comprehensive study with high quality photomicrographs illustrating the normal reproductive tract of mated honey bee queens is needed.
The purpose of this study was to i) perform serial histological sections of the abdomen of mated honey bee queens, ii) illustrate the entire reproductive tract of a mated honey bee queen with high quality photomicrographs, and iii) using these photomicrographs as a reference, summarize previously published descriptions of normal queen morphology.
Materials and methods
Honey bee queen origin and dissection
All queens in this study originated from healthy honey bee colonies managed for honey production in a research apiary located at the Goodale Research and Teaching Farm, University of Saskatchewan (52°01'50.6"N 106°32'26.6"W) during summer 2015. The research apiary was surrounded by commercial beekeeping operations, pastures, and agricultural crops, including alfalfa, canola, and cereals treated with pesticides according to standard agricultural practices. All colonies received standard spring and fall treatments of amitraz and oxytetracycline according to label instructions. The research apiary was established predominantly from local stock, with a minor contribution of New Zealand packaged bees that were cross-bred and represent a genetic mixture most closely resembling Apis mellifera carnica. All of the studied queens were naturally mated during early summer 2015 and collected in 10% formalin at the end of summer. To avoid artifact introduced by sectioning through the hard cuticle, the exoskeleton was removed from the abdomen prior to sectioning. The 7th sternum was left intact, as it is involved in forming part of the reproductive tract.
Tissue processing
The reproductive tracts, together with associated abdominal organs, were processed according to the standard mammalian procedure (Mepham, Citation1991) and embedded in Paraplast Plus (Leica Biosystems, Richmond, IL, USA) in 3 positions: cranial, lateral, and ventral to obtain sections in 3 different planes, namely: transverse, sagittal and dorso-ventral. Five µm serial sections were collected every 100 µm for histological evaluation. The slides were dried for 1 hour at 65 °C and stained using routine Harris’ hematoxylin and eosin (H&E) staining protocol (Luna, Citation1968); briefly: xylene 2 min., xylene 2 min., 100% ethyl alcohol 2 min., 100% ethyl alcohol 2 min., 90% ethyl alcohol 2 min., 70% ethyl alcohol 2 min., running 40 °C water 1 min., Surgipath Harris’s hematoxylin 560 (Leica Biosystems, Richmond, IL, USA) 5 min., running 40 °C water 1 min., 0.3% acid alcohol 15 sec., running 40 °C water 1 min., Surgipath blue buffer 8 (Leica Biosystems, Richmond, IL, USA) 1 min., running 40 °C water 3 min., 80% ethyl alcohol 1 min., Surgipath alcoholic eosin 515 Y (1%) (Leica Biosystems, Richmond, IL, USA) 30 sec., 90% ethyl alcohol 1 min., 90% ethyl alcohol 1 min., 100% ethyl alcohol 15 sec., 100% ethyl alcohol 15 sec., 100% ethyl alcohol 15 sec., xylene 2 min., xylene 1 min., xylene 1 min. The slides were then cover slipped in a fume hood using Micromount (Leica Biosystems, Richmond, IL, USA. We deliberately chose hematoxylin and eosin staining protocol for tissue evaluation because it is the most widely used stain in vertebrate histopathology for diagnostics, research, and regulatory risk assessment purposes (Fischer et al., Citation2008).
Photomicrographs
For macroscopic images, queens were dissected under a stereomicroscope Olympus SZ61 and photographed with Olympus DP71 microscope digital camera (Olympus, Japan). Each specimen was photographed at 7-12 consecutive field depths. The digital images were overlaid with Helicon Focus 6.7.1. (Helicon Soft Ltd., Ukraine) to obtain a full-depth, in-focus image. Histological images were captured using an Olympus BX51 microscope and Olympus DP71 microscope digital camera using various magnifications (reference bars indicated in figure legends).
Terminology and description of macroscopic and microscopic anatomy
The terminology and description of macroscopic and microscopic anatomy used in this manuscript are based on multiple sources of previously published literature referenced in the discussion section.
Results
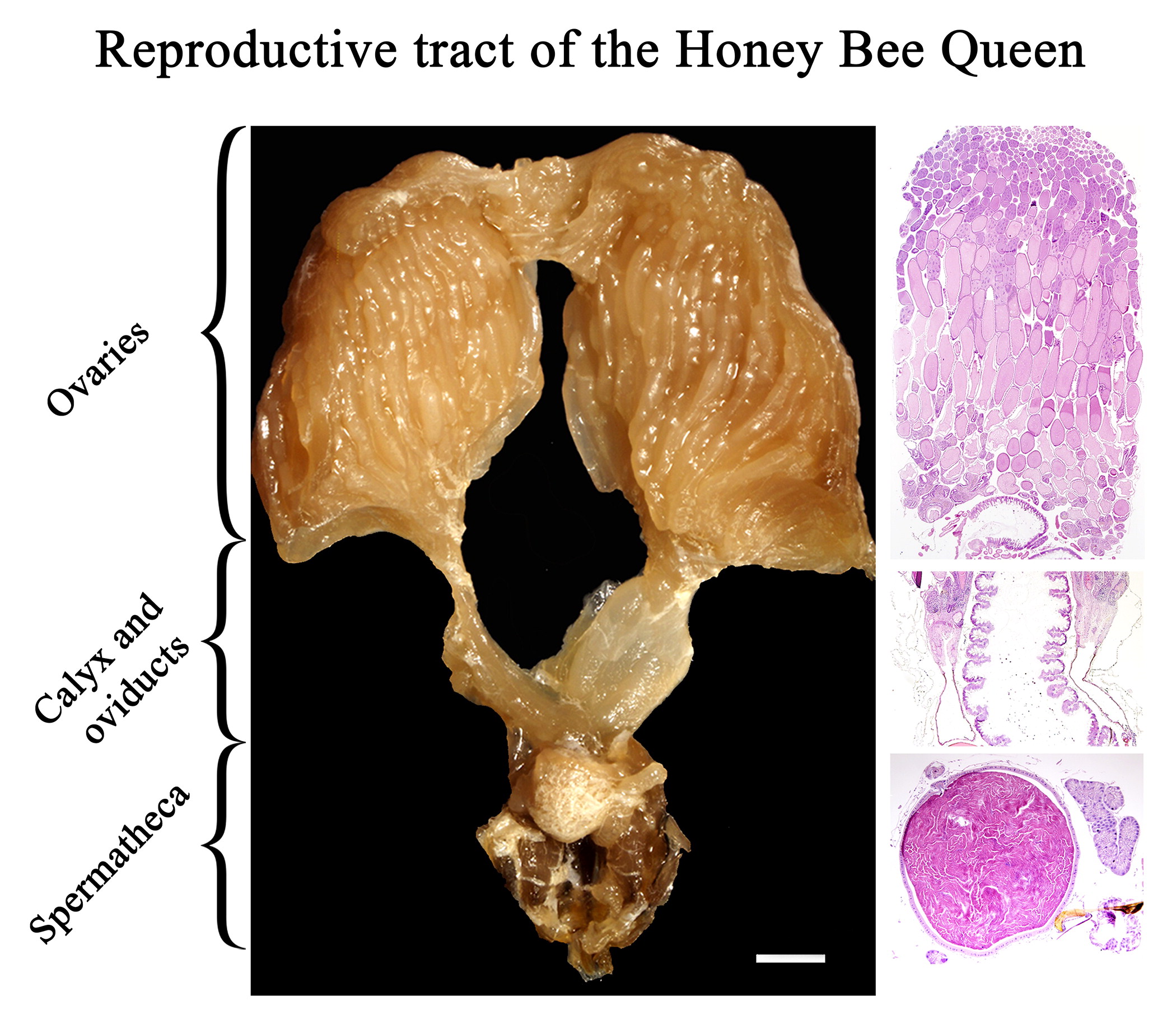
In this study, we reproduced the histological morphology of the entire reproductive system of mated honey bee queens and illustrated it with high quality photomicrographs. The reproductive system of the honey bee queen consists of paired ovaries () connected by calices () to paired lateral oviducts (), which merge to form a median oviduct () connected dorsally to the spermatheca () and caudally to the terminal portion of the reproductive tract (i.e. genital chamber and bursa copulatrix with lateral pouches) (). The spermatheca () is connected to the median oviduct () via the spermathecal duct (). Paired spermathecal glands () are connected to the spermatheca via the common duct (). The sperm pump is shown in .
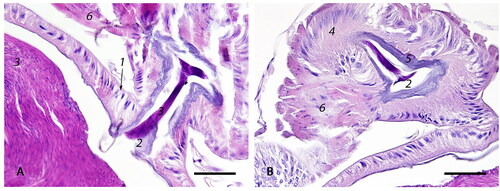
Figure 1. Macroscopic image of the reproductive tract of formalin fixed honey bee queen: (A) Upper reproductive tract including ovaries (1), calyx (2), lateral oviducts (3) (the right oviduct is distended with eggs), median oviduct (4), and spermatheca covered by tracheal network (5) (bar = 1mm); (B) Middle region of the reproductive tract including lateral oviducts (3), median oviduct (4), spermatheca (5), spermathecal glands (6), common duct (7), spermathecal duct (8), sperm pump (9); (bar = 0.5 mm).
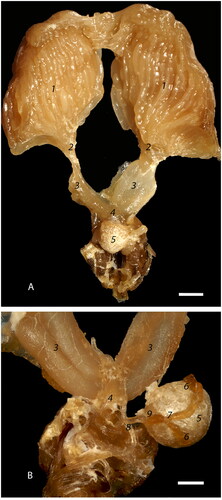
Figure 2. Photomicrographs of the ovary segments: (A) Low magnification view of the ovarioles within the ovaries showing sequential linear maturation of the oocytes (1), accompanied by trophocytes (2) (bar = 1mm); (B) The most cranial portion of an ovariole is the terminal filament (solid bar) composed of somatic and undifferentiated stem cells embedded between discoid cells (3) within an epithelial sheath (4). Germarium (dashed bar) includes cystocyte rosettes (5) which develop into a single oocyte and accompanying trophocytes (bar = 20µm); (C) Cranial portion of vitellarium showing the distinct arrangement of oocytes (1) into a single row, separated by nurse cell chambers (2). There is a distinct germinal vesicle within each oocyte (3) (bar = 100 µm).
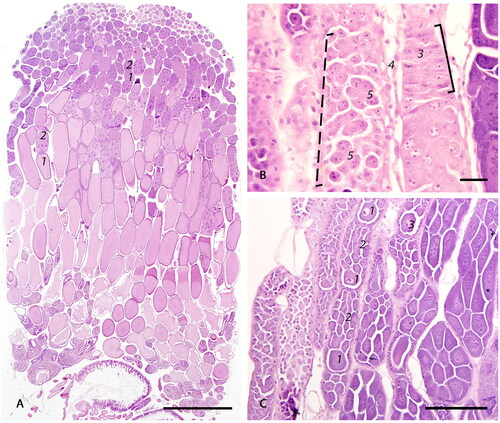
Figure 3. Photomicrographs of the follicles: (A) The oocyte with accompanying trophocytes form a follicle (black outline). The oocyte (1) is surrounded by a layer of single cuboidal epithelium (2). Trophic stalk (3) connects the oocyte with the trophocytes (4). The trophocyte chamber is surrounded by a thin layer of attenuated epithelial cells (5). Intertrophocitic median follicular cells (6) are located between trophocytes. The size of trophocytes increases with oocyte maturation and they eventually undergo degeneration (7) (bar = 50µm); (B) Blunted (8) and attenuated (9) follicular epithelium surrounding oocytes (1) in the middle and caudal sections of vitellarium (bar = 20µm).
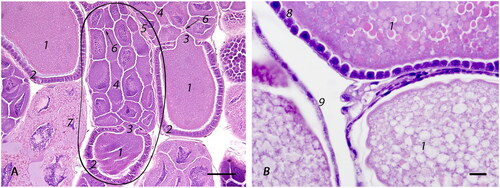
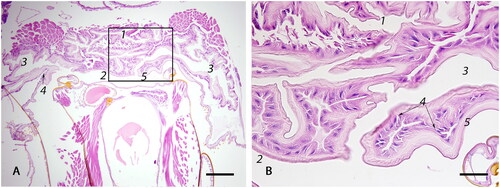
Figure 4. Photomicrographs of the cranial tubular region of the reproductive tract: (A) Low magnification view of the calyx (1) transition into lateral oviducts (2) with centrally located ventriculus (3) (bar = 500µm); (B) Calyx is formed by an irregularly thickened wall, lined by simple to bi-layered, slender, tall, columnar, densely-packed epithelium (4) on an eosinophilic basement membrane (5) and contains apical, optically vacant vacuoles (6) (bar = 50µm); (C) Cross section of a distended lateral oviduct with eggs (8): distention is facilitated by longitudinal folds (7) lined by attenuated epithelium (bar = 100µm); (D) Longitudinal section of a lateral oviduct lined by cuboidal epithelium (9) on an eosinophilic basement membrane (5) with an underlying, single layer of longitudinal muscle cells (10). The epithelium is covered by a chitinous, strongly basophilic intima that has numerous cteniform spines (11) (bar = 20µm).
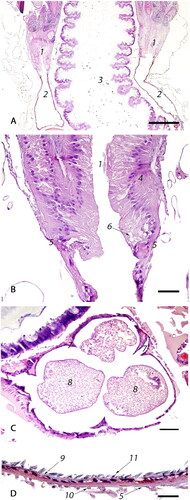
Figure 5. Photomicrographs of (A) the median oviduct (1) which is lined by simple cuboidal epithelium (2) and is externally surrounded by a well-developed outer sphincter muscle (3) that forms two lateral folds (4) of the median oviduct by protruding inwards; egg (5); (bar = 100µm); (B) The valve fold (circled) is a deep, transverse, epithelio-muscular inward projection, lined by simple cuboidal epithelium (6) that forms multiple invaginations (7), covered by a thin intima (8). The core of the stalk is composed of loosely arranged musculature (9) (bar = 100µm).
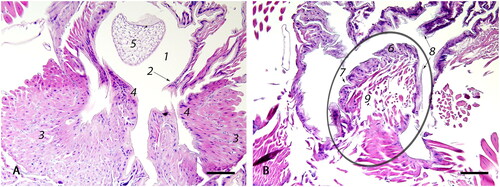
Figure 6. Photomicrographs of the spermatheca with its glands and ducts: (A) Low magnification image of the spermatheca (1) containing sperm (2); spermathecal glands (3) and spermathecal duct (4) (bar = 200µm); (B) The spermatheca is lined by simple cuboidal epithelium (5) on a thin, anucleate, basophilic basement membrane (6) with an overlying thin, basophilic cuticle (7). The spermatheca is surrounded by tracheal network (8). The fecundated spermatheca is filled with tightly packed spermatozoa (2) (bar = 20µm); (C) The spermathecal glands are lined by an external glandular epithelial layer (9), formed by tall, columnar epithelium that has large, basal nuclei and foamy cytoplasm with at least one, distinct, apical, intracytoplasmic vacuole (10), on a basophilic membrane formed by attenuated epithelium (11). The inner intima (12) is formed by smaller, tall columnar cells with basal nuclei and multiple intracytoplasmic canaliculi (13). The glandular lumen is covered by amorphous amphophilic material (14) (bar = 20µm).
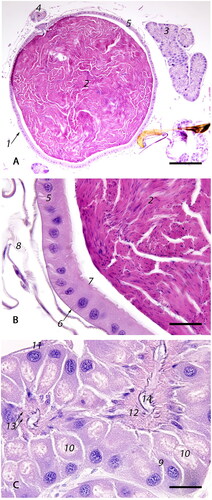
Figure 7. Spermathecal duct: (A) The orifice connecting the spermatheca and the spermathecal duct lined by high columnar, slender epithelial cells (1) covered by basophilic cuticle (2); sperm (3) (bar = 50µm); (B) The spermathecal duct is lined by tall columnar epithelium with basal nuclei (4), covered by a thick layer of basophilic cuticle (5). At the base of the duct, there is a set of circular and longitudinal muscles called the sperm pump (6) (bar = 50µm).
Histological morphology of the reproductive system of the mated honey bee queen
Ovaries
Each ovary ( and 2A–C) consists of densely packed clusters of ovarioles () which are thin chains of eggs that widen posterior, representing sequential, linear maturation of the oocytes () and trophocytes (). Macroscopically, an ovary is structurally reminiscent of a bunch of bananas, where each banana represents a separate ovariole. Each ovariole has four distinct regions which are: a short terminal filament, a germarium of intermediate length, and a long vitellarium which ends with the ovariole pedicle, all encased within an epithelial sheath (). The terminal filament (solid bar) is composed of undifferentiated stem cells embedded between discoid cells; the stem cells have large nuclei and poorly stained cytoplasm (). The germarium (dashed bar) contains germline cells (cystocytes) clustered into rosette-like structures (). In the distal portion of the germarium, the oocytes () become arranged into a single row, separated by nurse cell chambers (), which constitutes the transition to the vitellarium. Somatic cells are haphazardly arranged in the proximal germarium but form a distinct follicular epithelial lining around an oocyte and its corresponding trophocytes in the vitellarium ( and ).
In the centro-caudal portion of the ovariole, the ovum is almost completely covered by simple cuboidal somatic epithelium with a central nucleus, densely stippled chromatin, and eosinophilic cytoplasm (), leaving only a narrow opening on the anterior side, the trophic stalk () which allows access of nutrients from the trophocytes (). There is a distinct germinal vesicle within individual oocytes (). At the late stages of vitellogenesis, the epithelium of the ovum is blunted (cuboidal) () and subsequently becomes attenuated ().
Figure 8. Lower reproductive tract: (A) The genital chamber (1) is a continuation of the median oviduct posterior to the valve fold; through a cleft-like opening, it transitions into the bursa copulatrix (2) with bilateral bursal pouches (3) (bar = 200µm); (B) Enlarged squared area in A: genital chamber (1), bursa copulatrix (2), and bursal pouch (3) lined by cuboidal epithelium (4) with prominent cuticle (5) (bar = 50µm).
The trophocytes, or nurse cells, are large cells with dense, granular, eosinophilic cytoplasm and a large, pale, polymorphic nucleus with finely to coarsely stippled chromatin (). As they move posterior along the ovariole, the trophocytes increase in size and eventually undergo degeneration, characterized by cytoplasmic eosinophilic granularity with pallor (rarefaction), dispersal of the coarsely stippled nuclear chromatin, and eventual effacement of the nuclear membrane and cellular borders (). Small median follicular cells, previously described as intertrophocytic median follicular cells, are occasionally observed in between trophocytes within the nurse chamber ().
Calyx
The calyx () is a funnel-like structure on the caudal aspect of the ovaries that facilitates the transfer of eggs from ovarioles to the lateral oviduct ( and ). The calyx is composed of simple to the bi-layered, tall, columnar, densely packed epithelium () along an eosinophilic basement membrane (). The epithelial cells have a centrally located nucleus and an eosinophilic cytoplasm that contains round, optically vacant vacuoles () that increase in size posterior and expand the cytoplasm.
Lateral oviducts
The lateral oviducts () extend bilaterally from the calyx and unite ventrally toward the spermatheca (), to form the median oviduct ( and ). The oviducts are lined by cuboidal epithelium () on an eosinophilic basement membrane () with a thin, outer layer of longitudinal muscle (). The epithelial cells have eosinophilic cytoplasm with a well-defined, apical to central nucleus. The luminal side of the epithelial lining is covered by a thin, chitinous, strongly basophilic intima that has numerous cteniform (comb-like) spines pointing caudally (). The lining epithelium is attenuated when the oviduct lumen is distended by an egg ().
Median oviduct and valve fold
The median oviduct () is a short, muscular tubule that joins both lateral oviducts. It is posterior defined by the valve fold ( circled), which also constitutes the transition point of the median oviduct into the genital chamber. On the ventral surface, the median oviduct has two lateral folds () connected to sphincter muscles (). It is lined by epithelium similar to that found in the lateral oviducts, but instead of cteniform spines, it is covered by a thin, pale, amphophilic intima () that thickens posterior along the genital tract ().
The valve fold is a deep transverse epithelio-muscular inward projection on the ventral aspect of the posterior median oviduct ( circled). The stalk of the valve fold is composed of loosely arranged muscle () and is covered by a single layer of cuboidal to columnar epithelium () with multiple invaginations (). The epithelium of the valve fold is covered by a thin, amphophilic intima, similar to the median oviduct ().
Spermatheca, spermathecal duct and glands
The spermatheca ( and 6A-1) contains densely packed spermatozoa () in the mated queen and is connected to the adjacent median oviduct via the spermathecal duct ( and 6A-4). The spermatheca is lined by simple cuboidal epithelium () covered by a thin cuticle (). The spermatheca is surrounded by the well-developed tracheal network () which is intimately associated with the basement membrane () of the spermathecal epithelium () to facilitate gas exchange. The orifice connecting the spermatheca with the spermathecal duct () is lined by tall columnar, slender epithelial cells with basal nuclei ( and ). The lumen of the duct is covered by a thick layer of basophilic cuticle (). At the base of the duct, where it is still in contact with spermatheca, there is a set of circular and longitudinally arranged muscles called the sperm pump ().
Two long, convoluted, symmetrical spermathecal glands ( and ) are connected to the spermatheca via the common duct (). The spermathecal glands are lined by two epithelial layers – an external glandular layer () and an internal intima (). The external layer is composed of the tall columnar glandular epithelium () that has large, basal, densely basophilic nuclei and foamy cytoplasm with at least one large, distinct, pale eosinophilic, apical intracytoplasmic vacuole (). The external epithelium is surrounded by a thin, basophilic basement membrane formed by attenuated epithelial cells (). The internal intima is composed of much smaller cells () with ill-defined cell borders; small, round, basal nuclei; and multiple, optically vacant, linear, intracytoplasmic canaliculi directed toward the glandular lumen (). The glandular lumen is covered by amorphous amphophilic material ().
Genital chamber, bursa copulatrix, and bursal pouches
The median oviduct continues posterior to the valve fold to become the genital chamber () which is limited posterior by a cleft-like widening called the bursa copulatrix () with bilateral connections to two bursal pouches (). The genital chamber, bursa copulatrix, and bursal pouches are lined by a single layer of epithelium () covered by a cuticle () which has a similar histological structure to the median oviduct (). The most caudal portion of the reproductive tract is formed dorsally by the ventral part of the sting and ventrally by the 7th sternum.
Discussion
In this study, we reproduced the histomorphology of the entire reproductive system of mated honey bee queens and illustrated it by high quality photomicrographs. These photomicrographs augment and enhance previous diagrams and descriptions of macroscopic and microscopic queen reproductive anatomy illustrated by Snodgrass (Citation1985) and Camargo and Mello (Citation1970); they described and illustrated by diagrams the reproductive system of the honey bee queen in the order from cranial to caudal consisting of paired ovaries, paired lateral oviducts, a median oviduct with a valve fold, a genital chamber (vagina), a bursa copulatrix with lateral pouches, the sperm recepticulum: the spermatheca with its glands and a spermathecal duct.
Ovaries
The ovaries form a paired organ that takes up about 2/3 of the abdominal cavity in the honey bee queen and are located latero-ventral to the upper digestive tract (Camargo & Mello, Citation1970; Snodgrass, Citation1985). The ovaries are composed of ovarioles divided into four regions (i.e. terminal filament, germarium, vitellarium, and ovariole pedicle) based on morphological differentiation (Patrício & Cruz-Landim, Citation2002, Citation2008). The stem cells (terminal filament) differentiate into germinal cells forming rosette-like clusters (germarium); subsequently, each rosette produces a single oocyte and accompanying trophocytes which together comprise a follicle (vitellarium) (Martins et al., Citation2011; Patrício & Cruz-Landim, Citation2002, Citation2008; Snodgrass, Citation1985; Tanaka & Hartfelder, Citation2004), as illustrated in the present study ().
The ovary of the honey bee queen is polytrophic (multiple trophocytes per follicle) and meroistic (produce nutritive trophocytes and ova). Within each follicle, the trophocytes (nurse cells) () are attached by intercellular bridges and connected to the oocyte by the trophic stalk (a narrow opening) () which allows the transport of nutrients, RNA, and ribonucleoproteins from the trophocytes to the ovum during maturation () as described in previous studies (Berger & da Cruz-Landim, Citation2009; Biliński & Jaglarz, Citation1999; Cruz-Landim & Patrício, Citation2010; Snodgrass, Citation1985; Tanaka et al., Citation2006). After transferring their cytoplasmic contents to the developing ovum, the trophocytes undergo programmed cell death (apoptosis) () as found in earlier studies (Mpakou et al., Citation2006; Patrício & Cruz-Landim, Citation2008). Thus, the oocytes in the proximal vitellarium are small, followed by an organized cluster of trophocytes ( circle), whereas the oocytes in the distal vitellarium are greater in size, followed by apoptotic/degenerating trophocytes (), as described by Snodgrass, Citation1985. Ramamurty (Citation1977) suggested that degenerated trophocytes are phagocytosed by the intertrophocytic median follicular cells ().
During the late stages of vitellogenesis, the epithelium surrounding the ovum progressively attenuates and produces chorion and vitelline membrane (). At the end of the vitellogenesis, the trophic stalk (), forms an elevated, sieve-like micropyle (pore) in the ovum, through which the sperm enters during fertilization in the median oviduct (Landim & Yabuki, Citation1995; Nelson, Citation1915). When a mature ovum progresses from an ovariole to the calyx during ovulation, the ovum epithelium collapses and forms a corpus luteum (Patrício & Cruz-Landim, Citation2002), which later undergoes degeneration and resorption (Ramamurty, Citation1977). The corpus luteum is an unorganized mass of cells that obstructs the ovariole pedicle, thus temporarily preventing subsequent ovulation by the same ovariole (Patrício & Cruz-Landim, Citation2008). A mature ovum can be ovulated approximately every 3-5 hours from each ovariole (Ramamurty, Citation1977).
Calyx and lateral oviducts
After ovulation, the egg passes from the calyx () into a lateral oviduct () which has multiple accordion-like, longitudinal folds which allow expansion of the oviduct lumen for the passing egg (Laidlaw, Citation1944; Mackensen & Tucker, Citation1970) as illustrated in and . One of the distinctive features of the lateral oviduct is the presence of cteniform spines in the epithelial intima () which have been described in earlier studies (Berger-Twelbeck & Dorn, Citation1994; Gerber et al., Citation1978; Laidlaw, Citation1944). These spines are more numerous and pronounced in the anterior portion of the oviducts adjacent to the calyx and are absent in the median oviduct. The spines are believed to have a role in the unidirectional progression of the egg from the lateral to the median oviduct by preventing its cranial movement during muscle contraction (Gerber et al., Citation1978).
Median oviduct and the valve fold
Fertilization of an egg takes place in the median oviduct. It is believed that the valve fold (), together with the median oviduct sphincter (), slow down the egg when it passes through the median oviduct and push the micropylar end of the egg against the opening to the spermathecal duct to facilitate fertilization (Colin et al., Citation1999). The valve fold is also thought to regulate sperm outflow from the spermathecal duct during fertilization (Camargo & Mello, Citation1970).
Spermatheca, spermathecal duct and glands
The spermatheca ( and 6A-1), spermathecal duct ( and 6A-4), and the spermathecal glands ( and 6A-3) are accessory organs of the reproductive system of the honey bee queen studied in closer detail by Kapil (Citation1962). The spermatheca is located craniodorsally over the median oviduct at the level of the 5th sternum. It is a round, 1.2-1.3 mm in diameter, sperm recepticulum (Tarpy et al., Citation2011). The spermathecal glands () secrete proteins into the spermatheca via the common duct () to replace the proteinaceous fluid lost from the spermatheca with the sperm during fertilization (Klenk et al., Citation2004). At the base of the spermathecal duct, the muscular sperm pump () provides a constant sperm volume necessary for the fertilization of a single egg (Baer et al., Citation2016; Bresslau, Citation1905).
Genital chamber, bursa copulatrix, and bursal pouches
The posterior portion of the genital tract comprises the genital chamber, bursa copulatrix, and bursal pouches. Several papers refer to the genital chamber as the “vagina” (Kapil, Citation1962; Laidlaw, Citation1944; Snodgrass, Citation1985). However, Camargo and Mello (Citation1970) contend that this term is not appropriate as the genital chamber does not receive the drone’s endophallus during mating, except on rare occasions, and therefore this term should not be borrowed from mammalian anatomy. During copulation, the chitinous plates of the drone endophallus attach to the bursal pouches (Camargo & Mello, Citation1970; Woyke, Citation2011) and sperm is ejaculated into the lateral oviducts where it then travels backward via the spermathecal duct to be stored in the spermatheca within the next several hours. The valve fold is thought to regulate the backflow of sperm into the spermatheca by preventing its retrograde flow into the posterior genital chamber (Laidlaw, Citation1944). However, since sperm consistently escape backward into the bursa copulatrix after mating, the efficacy of regulation of sperm flow by the valve fold has been questioned by Camargo and Mello (Citation1970).
Use of light and electron microscopy in honey bee research
Histological evaluation of microscopic tissue samples is a universal gold standard technique used for studying normal tissue structures, detection and diagnosis of disease, and toxicologic risk assessment in vertebrate species (Crissman et al., Citation2004; Fiette & Slaoui, Citation2011). Historically, some of the first histological studies were used to understand and describe insect respiration in silkworms by Marcello Malpighi (1628–1694) who is now known as the “father of microscopic anatomy” (DiDio, Citation1995; West, Citation2013). However, unlike research in vertebrate species, insect studies today rarely use histological tissue evaluation as a research tool.
Nevertheless, in recent years histological and ultra structural morphologic alterations have been successfully used to study honey bee diseases and toxicities. For example, Koziy et al. (Citation2019) investigated the histological appearance of brood-food glands in nurse bees affected with deformed wing virus – a nearly ubiquitous honey bee disease worldwide. In this study, both the hypopharyngeal and mandibular glands of affected bees were markedly hypoplastic and contained a greater number of apoptotic cells (Koziy et al., Citation2019). These findings contribute to further understanding of deformed wing virus pathogenesis. Similarly, Zaluski et al. (Citation2017) described histopathology of morphologic changes in mandibular (reduced height of secretory cells) and hypopharyngeal (reduced acini size) glands of worker bees exposed to pyraclostrobin and fipronil. The authors concluded that these changes to the glandular morphology may negatively affect overall colony development, maintenance, and survival (Zaluski et al., Citation2017). Histological examination of the brain (de Almeida Rossi et al., Citation2013; Tomé et al., Citation2012) and the hepato-nephrotic system (Domingues et al., Citation2017) of honey bees has also enhanced the current body of literature on the pathogenesis and effect of certain pesticides on honey bee health revealing an array of degenerative sublethal changes in response to insecticide treatment. Using histological tissue evaluation has enabled the above researchers to detect and describe minute changes in bees in response to studied stimuli. Their discoveries now help us better understand the pathogenesis of disease and some toxicity.
Light microscopic and ultrastructural evaluation of ovaries but no other structures of the honey bee queen reproductive tract, were used in several studies. These studies focus on understanding both physiologic and pathologic processes in the ovary in response to various stimuli. For instance, ultrastructural evaluation of the ovary showed that delayed mating increases apoptotic and necrotic death of the ovarian germ line and somatic interstitial cells (Berger & da Cruz-Landim, Citation2009). Histology was used for more precise and reproducible estimation of ovariole numbers in honey bee queens (Jackson et al., Citation2011), which has since become one of the standard methods in honey bee research (Carreck et al., Citation2013). Additionally, histology was used in studies by Fyg in 1964, who described the rare direct infections of the queen reproductive system with yeast-like microorganisms (H-melanosis) and bacteria (B-melanosis) and characterized vitellarium degeneration in queens as an indirect effects of Nosema spp infection. These studies further emphasize the importance and benefit of thorough histopathological examination of the honey bee queen reproductive system.
The importance and novelty of the current study is that it provides high quality, detailed photomicrographs with a thorough sequential description of the hematoxylin and eosin stained histological structures of the entire reproductive tract of mated honey bee queen. We deliberately chose hematoxylin and eosin staining protocol for tissue evaluation because it is the most widely used stain in vertebrate histopathology for diagnostics, research, and regulatory risk assessment purposes and it allows detailed evaluation of microscopic changes in cells and tissues (Fischer et al., Citation2008).
By synthesizing descriptions and diagrams provided in earlier studies, this manuscript summarizes in detail the normal structure, nomenclature, and function of the microscopic structures of the queen’s reproductive tract and provides a visual example of each structure. Histological evaluation of reproductive histopathology is an essential step in toxicological risk assessment in vertebrate species, especially so in pharmaceutical drug development (Halpern et al., 2016) as well as in theriogenology research. Accordingly, the comprehensive characterization and illustration of normal light microscopic morphology of the entire reproductive tract of the honey bee queen performed in the current study is anticipated to serve as a reference for the future histopathological investigations of reproductive problems affecting honey bee queens.
Conclusions
The current study summarized the normal histological structure and functions of the entire honey bee queen reproductive tract and illustrated it with high-quality photomicrographs. It is anticipated that this study will facilitate further investigations of the pathogenesis of the recent decline in reproductive fitness of honey bees using histopathology.
Authors contribution
IK and ES designed experiment; IK, SW, RK, and ES participated in the design and interpretation of the data; IK performed experiments and analysis; IK and ES wrote the manuscript. All authors participated in the revision of the manuscript, read and approved the final manuscript.
Acknowledgements
We thank Claire Janse van Rensburg, Dr. Igor Moshynskyy, Dr. Sophie Derveau, Dr. Maud Ferrari, and Dr. Ahmad Al-Dissi for their contribution and constructive feedback. We also thank Madeleine Friesen and Phil Dillman for field and histology assistance, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alaux, C., Folschweiller, M., McDonnell, C., Beslay, D., Cousin, M., Dussaubat, C., Brunet, J.-L., & Conte, Y. L. (2011). Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). Journal of Invertebrate Pathology, 106(3), 380–385. https://doi.org/https://doi.org/10.1016/j.jip.2010.12.005
- Amiri, E., Strand, M. K., Rueppell, O., & Tarpy, D. R. (2017). Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects, 8(2), 48. https://doi.org/https://doi.org/10.3390/insects8020048
- Baer, B., Collins, J., Maalaps, K., Boer, S. P. A. & den, (2016). Sperm use economy of honeybee (Apis mellifera) queens. Ecology and Evolution, 6(9), 2877–2885. https://doi.org/https://doi.org/10.1002/ece3.2075
- Berger, B., & da Cruz-Landim, C. (2009). Ultrastructural analysis of the effect of mating delay on cell death in the ovaries of virgin honey bee (Apis mellifera L.) queens. Journal of Apicultural Research, 48(1), 60–66. https://doi.org/https://doi.org/10.3896/IBRA.1.48.1.12
- Berger-Twelbeck, P., & Dorn, A. (1994). Larval-adult oviduct transformation in an insect (Oncopeltus fasciatus): Cytological study. Tissue and Cell, 26(5), 785–795. https://doi.org/https://doi.org/10.1016/0040-8166(94)90061-2
- Bernet, D., Schmidt, H., Meier, W., Burkhardt‐Holm, P., & Wahli, T. (1999). Histopathology in fish: Proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases, 22(1), 25–34. https://doi.org/https://doi.org/10.1046/j.1365-2761.1999.00134.x
- Beyer, M., Junk, J., Eickermann, M., Clermont, A., Kraus, F., Georges, C., Reichart, A., & Hoffmann, L. (2018). Winter honey bee colony losses, Varroa destructor control strategies, and the role of weather conditions: Results from a survey among beekeepers. Research in Veterinary Science, 118, 52–60. https://doi.org/https://doi.org/10.1016/j.rvsc.2018.01.012
- Biliński, S. M., & Jaglarz, M. K. (1999). Organization and possible functions of microtubule cytoskeleton in hymenopteran nurse cells. Cell Motility and the Cytoskeleton, 43(3), 213–220. https://doi.org/https://doi.org/10.1002/(SICI)1097-0169(1999)43:3 < 213::AID-CM4 > 3.0.CO;2-I
- Bresslau, E. (1905). Der samenblasengang der bienenkönigin (Studien über den geschlechtsapparat und die fortpflanzung der bienen. I).
- Brown, A. P., Drew, P., Knight, B., Marc, P., Troth, S., Wuersch, K., & Zandee, J. (2016). Graphical display of histopathology data from toxicology studies for drug discovery and development: An industry perspective. Regulatory Toxicology and Pharmacology : RTP, 82, 167–172. https://doi.org/https://doi.org/10.1016/j.yrtph.2016.10.009
- Burkle, L. A., Marlin, J. C., & Knight, T. M. (2013). Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science (New York, N.Y.), 339(6127), 1611–1615. https://doi.org/https://doi.org/10.1126/science.1232728
- Camargo, J., & Mello, M. (1970). Anatomy and histology of the genital tract, spermatheca, spermathecal duct and glands of Apis Mellifica queens (Hymenoptera: Apidae). Apidologie, 1(4), 351–373. https://doi.org/https://doi.org/10.1051/apido:19700401
- Carreck, N. L., Andree, M., Brent, C. S., Cox-Foster, D., Dade, H. A., Ellis, J. D., Hatjina, F., Englesdorp, D. & van, (2013). Standard methods for Apis mellifera anatomy and dissection. Journal of Apicultural Research, 52(4), 1–40. https://doi.org/https://doi.org/10.3896/IBRA.1.52.4.03
- Colin, M. E., Ball, B. V., & Kilani, M. (1999, May 19–30). Bee disease diagnosis. In Options Méditerranéennes. Série B : Etudes et Recherches (CIHEAM). Presented at the Course on Bee Disease Diagnosis. CIHEAM-IAMZ.
- Crissman, J. W., Goodman, D. G., Hildebrandt, P. K., Maronpot, R. R., Prater, D. A., Riley, J. H., Seaman, W. J., & Thake, D. C. (2004). Best practices guideline: Toxicologic histopathology. Toxicologic Pathology, 32(1), 126–131. https://doi.org/https://doi.org/10.1080/01926230490268756
- Cruz-Landim, C., & Patrício, K. (2010). Nuclear activity in Apis mellifera L. (Hymenoptera, Apidae) queen ovary cells demonstrated by silver nitrate impregnation and ultrastructure. Brazilian Journal of Biology = Revista Brasleira de Biologia, 70(4), 1069–1073. https://doi.org/https://doi.org/10.1590/s1519-69842010000500023
- de Almeida Rossi, C., Roat, T. C., Tavares, D. A., Cintra-Socolowski, P., & Malaspina, O. (2013). Brain morphophysiology of Africanized bee Apis mellifera exposed to sublethal doses of imidacloprid. Archives of Environmental Contamination and Toxicology, 65(2), 234–243. https://doi.org/https://doi.org/10.1007/s00244-013-9897-1
- DiDio, L. J. (1995). Marcello Malpighi: The father of microscopic anatomy. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia, 100, 3–9.
- Domingues, C. E. C., Abdalla, F. C., Balsamo, P. J., Pereira, B. V. R., Hausen, M. d A., Costa, M. J., & Silva-Zacarin, E. C. M. (2017). Thiamethoxam and picoxystrobin reduce the survival and overload the hepato-nephrocitic system of the Africanized honeybee. Chemosphere, 186, 994–1005. https://doi.org/https://doi.org/10.1016/j.chemosphere.2017.07.133
- Fiette, L., & Slaoui, M. (2011). Necropsy and sampling procedures in rodents. In J.-C. Gautier (Ed.), Drug safety evaluation: Methods and protocols, methods in molecular biology (pp. 39–67). Humana Press. https://doi.org/https://doi.org/10.1007/978-1-60761-849-2_3
- Fischer, A. H., Jacobson, K. A., Rose, J., & Zeller, R. (2008). Hematoxylin and eosin staining of tissue and cell sections. CSH Protocols, 2008, pdb.prot4986. https://doi.org/https://doi.org/10.1101/pdb.prot4986
- Forfert, N., Troxler, A., Retschnig, G., Gauthier, L., Straub, L., Moritz, R. F. A., Neumann, P., & Williams, G. R. (2017). Neonicotinoid pesticides can reduce honeybee colony genetic diversity. Plos One, 12(10), e0186109. https://doi.org/https://doi.org/10.1371/journal.pone.0186109
- Gauthier, L., Ravallec, M., Tournaire, M., Cousserans, F., Bergoin, M., Dainat, B., Miranda, J. R. & de, (2011). Viruses associated with ovarian degeneration in Apis mellifera L. queens. Plos One, 6(1), e16217. https://doi.org/https://doi.org/10.1371/journal.pone.0016217
- Gerber, G. H., Neill, G. B., & Westdal, P. H. (1978). The anatomy and histology of the internal reproductive organs of the sunflower beetle, Zygogramma exclamationis (Coleoptera: Chrysomelidae). Canadian Journal of Zoology, 56(12), 2542–2553. https://doi.org/https://doi.org/10.1139/z78-342
- Grozinger, C. M., & Flenniken, M. L. (2019). Bee viruses: Ecology, pathogenicity, and impacts. Annual Review of Entomology, 64, 205–226. https://doi.org/https://doi.org/10.1146/annurev-ento-011118-111942
- Gutzeit, H. O., Zissler, D., & Fleig, R. (1993). Oogenesis in the honeybee Apis mellifera: Cytological observations on the formation and differentiation of previtellogenic ovarian follicles. Roux’s. Roux's Archives of Developmental Biology : The Official Organ of the EDBO, 202(3), 181–191. https://doi.org/https://doi.org/10.1007/BF00365309
- Haarmann, T., Spivak, M., Weaver, D., Weaver, B., & Glenn, T. (2002). Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. Journal of Economic Entomology, 95(1), 28–35. https://doi.org/https://doi.org/10.1603/0022-0493-95.1.28
- Halpern, W. G., Ameri, M., Bowman, C. J., Elwell, M. R., Mirsky, M. L., Oliver, J., Regan, K. S., Remick, A. K., Sutherland, V. L., Thompson, K. E., Tremblay, C., Yoshida, M., & Tomlinson, L. (2016). Scientific and regulatory policy committee points to consider review: Inclusion of reproductive and pathology end points for assessment of reproductive and developmental toxicity in pharmaceutical drug development. Toxicologic Pathology, 44(6), 789–809. https://doi.org/https://doi.org/10.1177/0192623316650052
- Higes, M., Martín‐Hernández, R., Botías, C., Bailón, E. G., González, ‐Porto, A. V., Barrios, L., Nozal, M. J., del, Bernal, J. L., Jiménez, J. J., Palencia, P. G., & Meana, A. (2008). How natural infection by Nosema ceranae causes honeybee colony collapse. Environmental Microbiology, 10(10), 2659–2669. https://doi.org/https://doi.org/10.1111/j.1462-2920.2008.01687.x
- Jackson, J. T., Tarpy, D. R., & Fahrbach, S. E. (2011). Histological estimates of ovariole number in honey bee queens, Apis mellifera, reveal lack of correlation with other queen quality measures. Journal of Insect Science (Online), 11, 82. https://doi.org/https://doi.org/10.1673/031.011.8201
- Kapil, R. P. (1962). Anatomy and histology of the female reproductive system ofApis indica F. (Hymenoptera, Apidae). Insectes Sociaux, 9(2), 145–163. https://doi.org/https://doi.org/10.1007/BF02224261
- Klenk, M., Koeniger, G., Koeniger, N., & Fasold, H. (2004). Proteins in spermathecal gland secretion and spermathecal fluid and the properties of a 29 kDa protein in queens of Apis mellifera. Apidologie, 35(4), 371–381. https://doi.org/https://doi.org/10.1051/apido:2004029
- Koziy, R. V., Wood, S. C., Kozii, I. V., van Rensburg, C. J., Moshynskyy, I., Dvylyuk, I., & Simko, E. (2019). Deformed wing virus infection in honey bees (Apis mellifera L.). Veterinary Pathology, 56(4), 636–641. https://doi.org/https://doi.org/10.1177/0300985819834617
- Laidlaw, H. H. (1944). Artificial insemination of the queen bee (Apis mellifera L.): Morphological basis and results. Journal of Morphology, 74(3), 429–465. https://doi.org/https://doi.org/10.1002/jmor.1050740307
- Landim, C. D. C., & Yabuki, A. T. (1995). Fine-structure and morphogenesis of the micropyle apparatus in bees eggs. Biocell, 9(2), 125–132.
- Luna, L. G. (1968). Manual of histologic staining methods of the Armed Forces Institute of Pathology. Pathology, 3(3), 249.
- Mackensen, O., & Tucker, K. W. (1970). Instrumental insemination of queen bees. U.S. Agricultural Research Service.
- Martín-Hernández, R., Botías, C., Barrios, L., Martínez-Salvador, A., Meana, A., Mayack, C., & Higes, M. (2011). Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitology Research, 109(3), 605–612. https://doi.org/https://doi.org/10.1007/s00436-011-2292-9
- Martins, J. R., Anhezini, L., Dallacqua, R. P., Simões, Z. L. P., & Bitondi, M. M. G. (2011). A honey bee hexamerin, HEX 70a, is likely to play an intranuclear role in developing and mature ovarioles and testioles. Plos One, 6(12), e29006. https://doi.org/https://doi.org/10.1371/journal.pone.0029006
- Mepham, B. L. (1991). Theory and practice of histological techniques, 3rd ed. J. D. Bancroft, A. Stevens (Eds). Churchill Livingstone, Edinburgh, 1990. No. of pages: 740. Price: £55. ISBN: 0 443 03559 8. The Journal of Pathology, 164(3), 281–281. https://doi.org/https://doi.org/10.1002/path.1711640316
- Morse, R. A., & Calderone, N. W. (2000). The value of honey bees as pollinators of U.S. crops in 2000. Bee culture, 128(3), 1–15.
- Mpakou, V. E., Nezis, I. P., Stravopodis, D. J., Margaritis, L. H., & Papassideri, I. S. (2006). Programmed cell death of the ovarian nurse cells during oogenesis of the silkmoth Bombyx mori. Development, Growth & Differentiation, 48(7), 419–428. https://doi.org/https://doi.org/10.1111/j.1440-169X.2006.00878.x
- Nelson, J. A. (1915). The embryology of the honey bee. Princeton University Press.
- Patrício, K., & Cruz-Landim, C. (2002). Mating influence in the ovary differentiation in adult queens of Apis mellifera L. (Hymenoptera, Apidae). Brazilian Journal of Biology = Revista Brasleira de Biologia, 62(4A), 641–649. https://doi.org/https://doi.org/10.1590/s1519-69842002000400012
- Patrício, K., & Cruz-Landim, C. D. (2008). Morphological aspects of cell reabsorption in laying queens and workers of Apis mellifera (Hymenoptera, Apidae). Iheringia. Série Zoologia, 98(4), 421–424. https://doi.org/https://doi.org/10.1590/S0073-47212008000400001
- Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., & Kunin, W. E. (2010). Global pollinator declines: Trends, impacts and drivers. Trends in Ecology & Evolution, 25(6), 345–353. https://doi.org/https://doi.org/10.1016/j.tree.2010.01.007
- Ramamurty, P. S. (1977). Unusual intertrophocytic position of follicle cells in the nurse chamber of the honeybee queen ovary (Apis mellifica). Apidologie, 8(2), 205–216. https://doi.org/https://doi.org/10.1051/apido:19770208
- Ricketts, T. H., Regetz, J., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., Bogdanski, A., Gemmill-Herren, B., Greenleaf, S. S., Klein, A. M., Mayfield, M. M., Morandin, L. A., Ochieng', A., Potts, S. G., & Viana, B. F. (2008). Landscape effects on crop pollination services: Are there general patterns? Ecology Letters, 11(5), 499–515. https://doi.org/https://doi.org/10.1111/j.1461-0248.2008.01157.x
- Sánchez-Bayo, F., Goulson, D., Pennacchio, F., Nazzi, F., Goka, K., & Desneux, N. (2016). Are bee diseases linked to pesticides? A brief review. Environment International, 89–90, 7–11. https://doi.org/https://doi.org/10.1016/j.envint.2016.01.009
- Simeunovic, P., Stevanovic, J., Cirkovic, D., Radojicic, S., Lakic, N., Stanisic, L., & Stanimirovic, Z. (2014). Nosema ceranae and queen age influence the reproduction and productivity of the honey bee colony. Journal of Apicultural Research, 53(5), 545–554. https://doi.org/https://doi.org/10.3896/IBRA.1.53.5.09
- Snodgrass, R. E. (1985). Anatomy of the honey bee. Cornell University Press.
- Stell, I. M. (2012). Understanding bee anatomy: A full colour guide. The Catford Press.
- Tanaka, É. D., Capella, I. C. S., & Hartfelder, K. (2006). Cell death in the germline – mechanisms and consequences for reproductive plasticity in social bees. Journal of Morphological Sciences, 23(1), 13.
- Tanaka, E. D., & Hartfelder, K. (2004). The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes. Arthropod Structure & Development, 33(4), 431–442. https://doi.org/https://doi.org/10.1016/j.asd.2004.06.006
- Tarpy, D. R., Keller, J. J., Caren, J. R., & Delaney, D. A. (2011). Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insectes Sociaux, 58(4), 569–574. https://doi.org/https://doi.org/10.1007/s00040-011-0180-z
- Tomé, H. V. V., Martins, G. F., Lima, M. A. P., Campos, L. A. O., & Guedes, R. N. C. (2012). Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS One, 7(6), e38406. https://doi.org/https://doi.org/10.1371/journal.pone.0038406
- Traver, B. E., & Fell, R. D. (2012). Low natural levels of Nosema ceranae in Apis mellifera queens. Journal of Invertebrate Pathology, 110(3), 408–410. https://doi.org/https://doi.org/10.1016/j.jip.2012.04.001
- Vanbergen, A. J., & Initiative., T. I. P. (2013). Threats to an ecosystem service: Pressures on pollinators. Frontiers in Ecology and the Environment, 11(5), 251–259. https://doi.org/https://doi.org/10.1890/120126
- West, J. B. (2013). Marcello Malpighi and the discovery of the pulmonary capillaries and alveoli. American Journal of Physiology. Lung Cellular and Molecular Physiology, 304(6), L383–L390. https://doi.org/https://doi.org/10.1152/ajplung.00016.2013
- Winfree, R., Aguilar, R., Vázquez, D. P., LeBuhn, G., & Aizen, M. A. (2009). A meta-analysis of bees' responses to anthropogenic disturbance. Ecology, 90(8), 2068–2076. https://doi.org/https://doi.org/10.1890/08-1245.1
- Wood, S. C., Kozii, I. V., Koziy, R. V., Epp, T., & Simko, E. (2018). Comparative chronic toxicity of three neonicotinoids on New Zealand packaged honey bees. Plos One, 13(1), e0190517. https://doi.org/https://doi.org/10.1371/journal.pone.0190517
- Woyke, J. (2011). The mating sign of queen bees originates from two drones and the process of multiple mating in honey bees. Journal of Apicultural Research, 50(4), 272–283. https://doi.org/https://doi.org/10.3896/IBRA.1.50.4.04
- Wu-Smart, J., & Spivak, M. (2016). Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Scientific Reports, 6, 32108. https://doi.org/https://doi.org/10.1038/srep32108
- Zaluski, R., Justulin, L. A., & de Oliveira Orsi, R. (2017). Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Scientific Reports, 7(1), 15217. https://doi.org/https://doi.org/10.1038/s41598-017-15581-5
