Abstract
Protein and phenolic compound content and antioxidant capacity were determined from honey bee collected unifloral pollen pellets from Ranunculus, Brassica, Filipendula, Rubus, Vicia, Trifolium, Epilobium, and Taraxacum species of boreal coniferous zone in Finland. The aim of the study was to investigate the relation between the chemical composition and the botanical origin of the pollen pellets. The protein content of pollen pellets ranged between 18.2 and 29.3%. One hundred and twenty phenolic components were detected from the samples and their total amount was highest in Brassica and the lowest in Taraxacum pollen pellets. Cinnamic acid content was high in Brassica and T. repens compared to other species. Most of the flavonoids in pollen pellets were flavonols, which were highest in Ranunculus, Filipendula and Epilobium originated pollen pellets. The pollen pellets of Ranunculus, Brassica, Filipendula, and T. repens had an antioxidant activity of over 80%. The lowest activities, about 40%, were measured in Taraxacum and Epilobium samples.
Graphical abstract
Introduction
Plants produce pollen grains for sexual reproduction and a great deal of plant species need some cooperation with animals in the pollination. Honey bees are regarded as excellent pollinators. During the foraging flight, pollen grains accumulate on the body hairs of the honey bees and thereby are transported from flower to flower, but also, as nectar moistened pollen pellets, to the hive for the primary food for broods and offspring (Thorp, Citation2000). Pollen is known to be a honey bee’s main source for protein, fats, mineral nutrients, and many other compounds (Keller et al., Citation2005). Honey bees can utilise a wide spectrum of plant species as a source of pollen, but during one foraging flight they visit only the flowers of one plant species. As a result of this, unique unifloral pollen pellets, containing pollen grains mainly of one plant species with a characteristic colour, size, shape, flavour, and chemical composition are loaded to the pollen baskets of a honey bee and transported to the hive (Di Paola-Naranjo et al., Citation2004). These pollen pellets can be collected by the beekeepers from the entrance of a hive.
Pollen grains are loaded with proteins, other nutrients, and necessary compounds that are needed for the germination and fertilisation process of pollen (e.g., Roulston et al., Citation2000). Phenolic compounds, like flavonoids, are common in the pollen grains of seed plants and they act there as colourants by making the pollen visible to pollinators and as protectants by preventing overheating and the penetration of mutagenic ultraviolet light (Lunau, Citation2000). Furthermore, it has been shownthat some flavonols are essential stimulants in the growth of pollen tubes (Vogt et al., Citation1995).
On the other hand, proteins and phenolic compounds of the pollen grains may be regarded as plants’ valuable reward for the pollinating insects, like honey bees. Much protein is needed for successful honey bee colony development and rearing the broods, especially in the spring (Herbert et al., Citation1977). However, honey bees are not able to choose pollen sources on the basis of protein content (Keller et al., Citation2005). It has been suggested that the odour of pollen, contributed by phenolic or volatile compounds may help the pollinators to find and choose the best kind of pollen (Arenas & Farina, Citation2012; Gronquist et al., Citation2001; Liu et al., Citation2006). Furthermore, Liu et al. (Citation2006) suggested that honey bees can detect and estimate the composition diversity of phenolic compounds in pollen and they tend to prefer floral species of the most suitable phenolic content for the nutrition of broods.
In this study, we analysed the chemical composition of unifloral pollen pellets, which were collected by honey bees from the boreal coniferous zone in Finland. The main interest was in the possible origin-specific differences in the chemical composition and nutritive value of the pollen pellets. Therefore, we determined the content of protein, the composition of individual phenolic compounds, and the antioxidant activity from the pellets. We hypothesise that (1) the protein and phenolic content and antioxidant capacity of the unifloral pollen pellets vary in relation to the botanical origin of the pollen, and (2) pollen pellets are composed of a mixture of multifunctional types of compounds, which may give a high nutritive and bioactive value to the pellets.
Materials and methods
Botanical identification of the pollen pellets
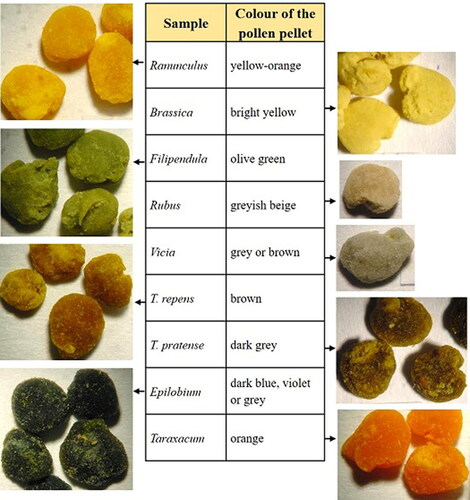
In summer 2013 honey bee-collected pollen pellets were received from the beekeepers of seven different locations in Finland (). All these beekeepers collect pollen for commercial purposes. The samples were stored at −18 °C until analysis. The botanical origin of the pollen pellets was identified by their colour, which can be used as a preliminary indication of species composition (Kirk, Citation1994), and further verified from the pellets with the light microscope (400 x magnification) by comparing the pollen grains with reference samples (Sawyer, Citation1981).
Table 1. Botanical origins, colours and collecting places of pollen pellet samples.
Crude protein analysis
For the evaluation of the total protein content in the pollen pellets, the nitrogen content of the pollen samples was analysed by ISO 13878-based method using a nitrogen analyser LECO® FP-528 (Leco Corporation Svenska AB, Uplands Väsby, Sweden). Two replicate sub-samples of dried and ground pollen pellet samples were used for the measurements. Protein content was calculated by multiplying nitrogen% by 6.25. Results are presented in percentage of dry weight.
HPLC-analysis of phenolic compounds and some amino acids
The extraction and analyses of phenolic compounds were conducted as described in Nybakken et al., Citation2012. In brief, 8 mg of grounded pollen powder was homogenised for 30 seconds at 5,500 rpm (Precellys®), four times with 800 µl of cold methanol (MeOH) and incubated in an ice bath for 15 minutes. After this, the MeOH-pollen extract was centrifuged at 13,000 rpm for three minutes (Eppendorf® Centrifuge 5415 R) and the supernatants were collected to glass vials. MeOH was evaporated to dryness (Eppendorf® concentrator) and dried samples stored in a freezer until HPLC analysis. Three subsamples were prepared and analysed from every pollen pellet sample.
For HPLC analyses, the dried phenolic fraction was suspended with 300 µl of methanol and 300 µl of Milli-Q water. The used HPLC device (1100 series, Agilent) consists of ALS autosampler (G1329A), a binary pump (G1312A), a vacuum degasser (G1322A), a diode array detector (G1315B), a column compartment (G1316A), and a C18 reverse-phase column (Zorbax SB-C18, 4.6 × 75 mm, particle size 3.5 μm, Agilent). The injection volume was 10 µl. Aquatic 1.5% tetrahydrofuran + 0.25% orthophosphoric acid solution (=A) and HPLC grade methanol (=B) were used as elution solvents. The samples were eluted with flow rate of 2 ml/min according to the following gradient: 0-5 min 100% A; 5-10 min 85% A, 15% B; 10-20 min 70% A, 30% B; 20-50 min 50% A, 50% B; 50% B; 50-55 min 100% B. The temperature of the column was +30 °C and of the injector +20 °C. The HPLC runs were monitored at 220 nm and 320 nm. Analysed phenolic compounds were quantified against commercial standards. The identification of the compounds was based on a comparison of retention times and spectral characteristics as described in Julkunen-Tiitto and Sorsa (Citation2001) or by using a UHPLC-quadrupole time-of-flight liquid chromatography/mass spectrometer as described in Nissinen et al. (Citation2016).
Analysis of the antioxidant activity
Antioxidant activity was determined from the MeOH-extracted HPLC samples by using a DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay with slight modifications according to Mărghitaş et al. (Citation2009). This assay is based on a single electron donation of antioxidants to neutralise DPPH radical. An aliquot of 400 µl of MeOH and 100 µl of the sample was mixed with 1000 µl of DPPH (75 mg/1 L). Samples were vortexed and kept for five minutes at room temperature before the discolouration of the DPPH is measured at 517 nm. The blank sample contained MeOH instead of a pollen sample. The percentage of inhibition at 517 nm was calculated as follows: Inhibition% = (1 – Absorbancesample/Absorbanceblank) x 100.
Statistics
Differences between botanical origins in phenolic profile, protein content, and antioxidant activity were investigated by univariate ANOVA using IBM SPSS statistics for Windows (Version 25.0.0.2). The botanical origin of the pellet was used as a fixed factor and the amount of protein content (log[1-x] modified to meet requirements of parametric tests), and antioxidant activity ([x]^2) were used as a dependent variable. Tukeýs HSD test was selected as post-hoc test. Multiple List Comparator (http://www.molbiotools.com) was used to identify the number of compounds shared by the pollen pellets of different botanical origins.
Microsoft Office Excel was used for evaluating the correlation between phenolic compounds and antioxidant activity.
Results
The botanical origin of the pollen pellets
The botanical origin of the pollen pellets was identified by light microscope and thereby the plant species were classified into nine different groups according to their colour: Ranunculus, Brassica, Filipendula, Rubus, Vicia, Trifolium repens, T. pratense, Epilobium and Taraxacum (). These genera were chosen for the chemical analyses, and they are also among those plant species, whose pollen can be found in the greatest quantity in Finnish honey samples (Salonen et al., Citation2009). For some species, the colour of pollen pellets was variable, such as in the pollen pellets from E. angustifolium where the colour varied from a deep, dark blue or violet to a dark grey, and the pollen pellets from F. ulmaria were coloured by several shades of green. The pollen pellets in the genera Trifolium were identified at species level due to their distinctly different colours, the pellets of T. repens and T. pratense being brown and dark grey, respectively.
The protein content in pollen pellets
The protein contents in the pollen pellets of different plant species are presented in . Protein content as a percentage of dry weight ranged from 18% (Taraxacum and Ranunculus) to 29% (Rubus and Brassica).
Table 2. Protein (% DW + s.e.) content in pollen pellet samples. Statistically significant differences at p < 0.05 (Tukey HSD) are marked with letters a-b.
Phenolic compounds in pollen pellets
Altogether, 120 different phenolic compounds were detected in the pollen pellets, not all fully identified (Tables S1 and S2). The composition of the compounds depended highly on the botanical origin of the pollen pellets, so only a few of the compounds were found in all the samples. The chemical structures and their amounts in phenolic groups are presented in and the amounts of individual compounds are presented in supplementary data.
Figure 1. A) Distribution of detected phenolics in different phenolic groups (mg g−1 FW) and B) total number of individual compounds and number of identical compound levels in the pollen pellets of different botanical origins.
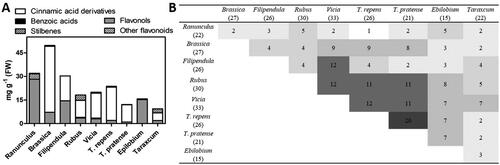
The phenolic acid composition in pollen pellets was wide and included almost 50 different compounds of caffeic, cinnamic, and p-hydroxycinnamic acids and two benzoic acid derivatives. However, the flavonoid composition in the pollen pellets was even wider, and most of the 56 different flavonoids detected were from the class of flavonols. Of the flavonols, twenty were identified as the derivatives of kaempferol, fifteen as derivatives of quercetin, and six as derivatives of myricetin. In addition, compounds from other flavonoid classes were found in minor quantities in some samples, such as eriodictyol, ampelopsin, luteolin, naringenin, and their derivatives. Fourteen compounds in samples were identified only at the group level as stilbenes (Table S1).
Antioxidant capacity
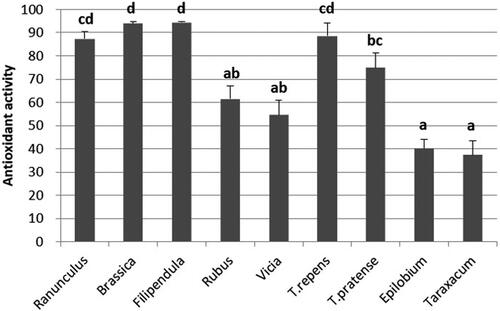
Antioxidant activities were different among the pollen pellet samples from different botanical origins (). Pollens of Ranunculus, Brassica, Filipendula, and T. repens had quite high antioxidant capacities as their inhibition percentages were over 80%. The lowest antioxidant activities were measured from Taraxacum and Epilobium samples, at 40% and 38%, respectively.
Figure 2. The percentage of inhibition of the pollen pellets extract in DPPH assay (mean ± s.e.). Statistically significant differences at p < 0.05 (Tukey HSD) are marked with letters a-d.
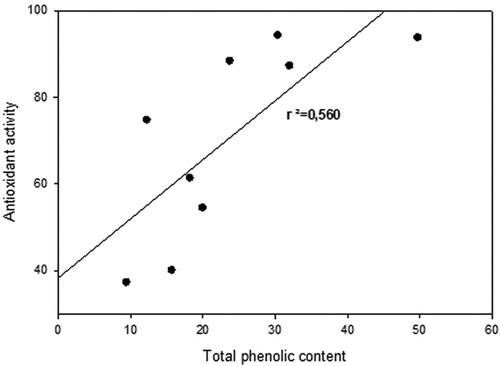
In this study, the antioxidant activity did not correlate totally with the total content of phenols in pellets (), nor with any of the phenolic compound groups (results not shown).
Discussion
The protein content in pollen pellets
The protein content in the pollen pellets varied widely according to plant species; 18% of the protein was found in Taraxacum and Ranunculus samples to 29% in Rubus and Brassica samples (). These values are close to the amounts found in Polish pollen pellets of these species (Szczesna, Citation2006). However, in the pollen pellets collected from Argentina, the measured protein contents have been lower e.g., for Taraxacum (13.3%), Brassica (21.3%), T. pratense (22.9%) (Forcone et al., Citation2011).
The diet of mixed pollens is suggested to be better for honey bees as it provides more extensively all the essential amino acids and nutrients (Alaux et al., Citation2010). In unifloral pollen pellets, some nutrients may be absent; for instance, the pollen pellets of Taraxacum lack several essential amino acids (Keller et al., Citation2005). Because the protein and amino acid content of the pollen that honey bees collect varies widely depending on the plant species and locations of collection, it means that also pollen’s nutritive value may vary greatly according to the origin (Roulston et al., Citation2000).
Phenolic compounds in pollen pellets
Flavonols and their glycoside derivatives are the most common compounds in pollen because they are essential in the plant fertilisation process as they are stimulants for the growth of the pollen tube (Markham & Campos, Citation1996). Kaempferol and quercetin and their derivatives have been recognised to be important compounds in inducing pollen germination (Vogt et al., Citation1995). Furthermore, it has been suggested that flavonoids may play a role in the restriction of the cross-pollination between plant species (Markham & Campos, Citation1996). However, the role of phenolic acids or stilbenes in pollen grain is still uncertain, as they do not tend to affect the germination process (Vogt et al., Citation1995). Both flavonoids and cinnamic acids are known to be effective antioxidants (e.g., Shahidi & Ambigaipalan, Citation2015), and thereby, they may also act as protectants of pollen against the oxidative action of intensive sunlight and ultraviolet radiation.
The total amount of different compounds was the lowest in Taraxacum and the highest in Brassica pollen pellets (9.39 and 49.68 mg g−1, respectively) (Table S1), but the amount was not in direct relation to the number of compounds found in pollen pellets. For example, in Taraxacum pollen pellets, the amount of the phenolics was about a quarter of the amount in T. repens, although the number of different compounds detected in their pollen pellets was equal ().
Cinnamic and hydroxycinnamic acids were the most abundant compounds in Brassica pollen pellets, comprising 84% of the total amount of the phenols, while in Epilobium the amount of phenolic acids was very low, and Ranunculus pollen had no phenolic acids at all. In Trifolium pollen pellets, all the cinnamic acid derivatives belong to caffeic acids derivatives and they composed about 90% of all the phenolics.
With regard to flavonoids, their number and the amount were high in Ranunculus, Epilobium and Filipendula, compared to the pollen pellets of other botanical origins. In the pollen pellets of Ranunculus and Epilobium over 97% of phenolics were composed of flavonoids, mainly different kaempferol derivatives. In Ranunculus, methylated kaempferol glycosides comprised 67% of the flavonoids, while in Epilobium, just one compound (kaempferol 3-rhamnoside) constituted 66% of the total phenol content in pollen pellets. Quercetin derivatives were most abundant in Filipendula pollen pellets, and the proportion of flavonoids to phenolic acids was almost equal (48% and 52%, respectively). Myricetin derivatives were found only in Taraxacum and Filipendula pollen pellets while kaempferol derivatives were found in all pollen groups. In Taraxacum, stilbenes and flavonoids comprised half of the amounts of phenols (27% and 20%, respectively) and phenolic acids the rest at 53%. The most variable composition of different kinds of phenols was found in the pollen pellets of Rubus and Vicia origins. The highest amounts of stilbenes were found in Rubus species pollen.
Moreover, it is obvious that pollen grains contain the same kind of phenolic composition as the herbal plant itself. For instance, kaempferol 3-O-rhamnoside, which was the main flavonol in the pollen pellets of Epilobium (Table S1), is also found in the nectar and honey collected from E. angustifolium flowers (Salonen, Citation2011). In accordance with the previous pollen pellet analyses, E. angustifolium plant extracts have contained phenolic compounds of different derivatives of flavonols (mainly quercetins and kampferols), phenolic acids, and ellagitannins (Schepetkin et al., Citation2016; Tóth et al., Citation2009). Also, the aerial plant-part extracts of Filipendula, T. pratense and T. repens have contained similar flavonols and phenolic acids as the pollen pellets in this study (Esmaeili et al., Citation2015; Katanić et al., Citation2015; Kicel & Wolbiś, Citation2013). In our study, the main flavonoid components in the pollen pellets of Filipendula were the two derivatives of quercetin and kaempferol (Table S1). Isorhamnetin and an unidentified quercetin derivative constituted 54% and kaempferol 3-rhamnoside and an unidentified kaempferol derivative constituted 31% of the amount of flavonoids.
Antioxidant capacity
Several compounds in pollen can act as antioxidants, which help to eliminate the dangerous free radicals by preventing the oxidation of molecules. It has been suggested that the composition of phenolics rather than their concentration is affecting the antioxidant activity of the pollen pellets (Almaraz-Abarca et al., Citation2004; Mărghitaş et al., Citation2009). The potency of a compound depends much on its chemical structure. Both hydroxybenzoic and hydroxycinnamic acids are known to be effective antioxidants due to the hydroxyl substituents in their aromatic rings (Natella et al., Citation1999). In all flavonoids the 3′, 4′-dihydroxy configurations in their B-ring possess antioxidant activity. According to Shahidi and Ambigaipalan (Citation2015), quercetin has shown the highest antioxidative activity against oxygen radicals among different flavonoids, while myricetin has been most efficient in protecting alpha-tocopherol from decomposition, suggesting that different flavonoids may have different tasks in antioxidation. Also, some stilbenes, especially the derivatives of resveratrol, have turned out to be as efficient as the well-known tocopherols and ascorbic acid in antioxidation. In this study, the phenolic composition in Epilobium pollen pellets was mainly comprised of one compound (kaempferol 3-rhamnoside, Table S1), and kaempferols tend not to be as powerful antioxidants as quercetin derivatives (Shahidi & Ambigaipalan, Citation2015). However, if kaempferols are abundant, as in Ranunculus in this study (Table S1), they represent high antioxidant activity.
Vicia and Rubus pollen pellets had almost the same antioxidant activities and the amount of total phenols, although the individual compounds in them are totally different, Rubus being rich in stilbenes and Vicia in phenolic acids. Also, the pollen pellets of Ranunculus (containing mainly methylated kaempferols), Brassica (containing mainly cinnamic and hydroxycinnamic acids), Filipendula (containing both quercetins and caffeic acids), and Trifolium (containing mainly caffeic acids) have large differences in their phenolic content (), and yet, their antioxidant activity is almost the same ().
In this study, the antioxidant activity did not correlate totally with the total content of phenols in pellets (r2 = 0.560; ), nor with any of the phenolic compound groups (results not shown). The same kind of observations has been made by other researchers as well (Campos et al., Citation2003; Mărghitaş et al., Citation2009). Antioxidant activity of the pollen pellets may also be caused by other compounds such as active biological antioxidants vitamins C and E, which are abundant in pollen grains (Bogdanov, Citation2017). Recent studies have also suggested that flavonols act synergistically with other cellular oxidants, e.g., alpha-tocopherol (e.g., Shahidi & Ambigaipalan, Citation2015).
DPPH antioxidant activity assay has many limitations. Nevertheless, it is one way of showing differences and diversity in the antioxidant capacity of biological materials. Other antioxidant capacity assays like ORAC or ABTS would have given more information when evaluating the antioxidant capacity of unifloral pollen pellets in this study.
Conclusions
The pollen pellets examined in this study were collected from the plant species in the boreal coniferous zone by honey bees. Both our hypotheses, that the protein and phenolic content and antioxidant capacity of these unifloral pollen pellets vary in relation to the botanical origin of the pollen, and that these pollen pellets are composed of a mixture of multifunctional types of compounds, which give a high nutritive and bioactive value to the pellets, were confirmed. Thus, bee collected pollen pellets might be considered as a source of functional food for humans as well. This could be a big marketing advantage for the Finnish beekeeping industry.
Tables S1 and S2
Download MS Word (51.7 KB)Acknowledgements
Special thanks to all beekeepers who collected pollen pellets, to students Anastasia Savolainen, Uula Vainio and Heidi Pulkkinen who sorted the pollen pellets, to Tarja Ollikka from the Finnish Beekeepers Association who verified the plant species identification of the pollen pellets and to Sinikka Sorsa who helped in protein analysis.
Disclosure statement
No conflicts of interest.
Supplementary material
Supplementary Tables S1 and S2 are available via the ‘Supplementary’ tab on the article’s online page (http://dx.doi.org/10.1080/00218839.2021.1902145).
Additional information
Funding
References
- Alaux, C., Ducloz, F., Crauser, D., & Le Conte, Y. (2010). Diet effects on honeybee immunocompetence. Biology Letters, 6(4), 562–565. https://doi.org/https://doi.org/10.1098/rsbl.2009.0986
- Almaraz-Abarca, N., Campos, M. G., Avila-Reyes, A., Naranjo-Jimenez, N., Herrera-Corral, J., & Gonzales-Valdez, L. S. (2004). Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Intersciencia, 29, 574–578.
- Arenas, A., & Farina, W. M. (2012). Learned olfactory cues affect pollen-foraging preferences in honeybees, Apis mellifera. Animal Behaviour, 83(4), 1023–1033. https://doi.org/https://doi.org/10.1016/j.anbehav.2012.01.026
- Bogdanov, S. (2017). The Bee Pollen Book. Retrieved January 6, 2018 from http://www.bee-hexagon.net/pollen/
- Campos, M. G., Webby, R., F., Markham, K. R., Mitchell, K. A., & Da Cunha, A. P. (2003). Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. Journal of Agricultural and Food Chemistry, 51(3), 742–745. https://doi.org/https://doi.org/10.1021/jf0206466
- Di Paolo-Naranjo, R., Sanchez-Sanchez, J., Gonzalez-Paramas, A. M., & Rivas-Gonzalo, J. C. (2004). Liquid chromatographic-mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. Journal of Chromatography A, 1054, 205–210.
- Esmaeili, A. K., Taha, R. T., Mohajer, S., & Banisalam, B. (2015). Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). BioMed Research International, 2015, 643285.
- Forcone, A., Aloisi, P. V., Ruppel, S., & Muñoz, M. (2011). Botanical composition and protein content of pollen collected by Apis mellifera L. in the north-west of Santa Cruz (Argentinean Patagonia). Grana, 50(1), 30–39. https://doi.org/https://doi.org/10.1080/00173134.2011.552191
- Gronquist, M., Bezzerides, A., Attygalle, A., Meinwald, J., Eisner, M., Eisner, T. (2001). Attractive and defensive functions of the ultraviolet pigments of a flower (Hypericum calycinum). Proceedings of the National Academy of Sciences of the United States of America, 98, 13745–13750.
- Herbert, E. W., Shimanuki, H., & Caron, D. (1977). Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie, 8(2), 141–146. https://doi.org/https://doi.org/10.1051/apido:19770204
- Julkunen-Tiitto, R., & Sorsa, S. (2001). Testing the effects of drying methods on willow flavonoids, tannins, and salicylates. Journal of Chemical Ecology, 27(4), 779–789. https://doi.org/https://doi.org/10.1023/a:1010358120482
- Katanić, J., Boroja, T., Stanković, N., Mihailović, v., Mladenović, M., Kreft, S., Miroslav, M., & Vrvić, M. M. (2015). Bioactivity, stability and phenolic characterization of Filipendula ulmaria (L.) Maxim. Food & Function, 6(4), 1164–1175. https://doi.org/https://doi.org/10.1039/c4fo01208a
- Keller, I., Fluri, P., & Imdorf, A. (2005). Pollen nutrition and colony development in honey bees – Part I. Bee World, 86(1), 3–10. https://doi.org/https://doi.org/10.1080/0005772X.2005.11099641
- Kicel, A., & Wolbiś, M. (2013). Phenolic content and DPPH radical scavenging activity of the flowers and leaves of Trifolium repens. Natural Product Communications, 8(1), 99–102.
- Kirk, W. D. (1994). A colour guide to pollen loads of the honey bee. International Bee Research Association.
- Liu, F.-L., Zhang, X.-W., Chai, J.-P., & Yang, D.-R. (2006). Pollen phenolics and regulation of pollen foraging in honeybee colony. Behavioral Ecology and Sociobiology, 59(4), 582–588. https://doi.org/https://doi.org/10.1007/s00265-005-0084-x
- Lunau, K. (2000). The ecology and evolution of visual pollen signals. Plant Systematics and Evolution, 222(1–4), 89–111. https://doi.org/https://doi.org/10.1007/BF00984097
- Mărghitaş, L. A., Stanciu, O. G., Dezmirean, D. S., Bobiş, O., Popescu, O., Bogdanov, S., & Campos, M. G. (2009). In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chemistry, 115(3), 878–883. https://doi.org/https://doi.org/10.1016/j.foodchem.2009.01.014
- Markham, K. R., & Campos, M. (1996). 7- and 8-o-methylherbacetin-3-o-sophorosides from bee pollens and some structure/activity observations. Phytochemistry, 43(4), 763–767. https://doi.org/https://doi.org/10.1016/0031-9422(96)00286-5
- Natella, F., Nardini, M., Di Felice, M., & Scaccini, C. (1999). Benzoic and cinnamic acid derivatives as antioxidants: Structure–activity relation. Journal of Agricultural and Food Chemistry, 47(4), 1453–1459. https://doi.org/https://doi.org/10.1021/jf980737w
- Nissinen, K., Nybakken, L., Virjamo, V., & Julkunen-Tiitto, R. (2016). Slow-growing Salix repens (Salicaceae) benefits from changing climate. Environmental and Experimental Botany, 12, 59–68.
- Nybakken, L., Hörkkä, R., & Julkunen-Tiitto, R. (2012). Combined enhancements of temperature and UVB influence growth and phenolics in clones of the sexually dimorphic Salix myrsinifolia. Physiologia Plantarum, 145(4), 551–564. https://doi.org/https://doi.org/10.1111/j.1399-3054.2011.01565.x
- Roulston, T. H., Cane, J. H., & Buchmann, S. L. (2000). What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecological Monographs, 70, 617–643.
- Schepetkin, I. A., Ramstead, A. G., Kirpotina, L. N., Voyich, J. M., Jutila, M. A., & Quinn, M. T. (2016). Therapeutic potential of polyphenols from Epilobium angustifolium (Fireweed). Phytotherapy Research: PTR, 30(8), 1287–1297. https://doi.org/https://doi.org/10.1002/ptr.5648
- Shahidi, F., & Ambigaipalan, P. (2015). Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects. Journal of Functional Foods, 18, 820–897. https://doi.org/https://doi.org/10.1016/j.jff.2015.06.018
- Salonen, A. (2011). Boreal unifloral honeys: Screening properties and composition. Publications of the University of Eastern Finland. Dissertations in Forestry and Natural Science, 51p.
- Salonen, A., Ollikka, T., Grönlund, E., Ruottinen, L., & Julkunen-Tiitto, R. (2009). Pollen analyses of honey from Finland. Grana, 48(4), 281–289. https://doi.org/https://doi.org/10.1080/00173130903363550
- Sawyer, R. (1981). Pollen identification for beekeepers. University College Cardiff Press.
- Szczesna, T. (2006). Protein content and amino acid composition of bee-collected pollen from selected botanical origin. Journal of Apicultural Science, 50, 81–90.
- Vogt, T., Wollenweber, E., & Taylor, L. P. (1995). The structural requirements of flavonols that induce pollen germination of conditionally male fertile petunia. Phytochemistry, 38(3), 589–592. https://doi.org/https://doi.org/10.1016/0031-9422(94)00703-V
- Thorp, R. W. (2000). The collection of pollen by bees. Plant Systematics and Evolution, 222(1-4), 211–223. https://doi.org/https://doi.org/10.1007/BF00984103
- Tóth, B. H., Blazics, B., & Kéry, A. (2009). Polyphenol composition and antioxidant capacity of Epilobium species. Journal of Pharmaceutical and Biomedical Analysis, 49(1), 26–31. https://doi.org/https://doi.org/10.1016/j.jpba.2008.09.047