Abstract
This nation-wide survey including 382 Swedish apiaries is the first to document base-line information of the prevalence and distribution of the ectoparasitic mite Varroa destructor, the mite-associated viruses Deformed wing virus and Acute bee paralysis virus, and the bacterial honey bee pathogens Paenibacillus larvae and Melissococcus plutonius in the country. Varroa and associated viruses were not detected in the northern regions of the country. The bacterium P. larvae was detected in 6% of the investigated apiaries and it was absent in more than half of the counties, M. plutonius was detected in two apiaries in one county. Other results from our study include questionnaire responses, in which beekeepers reported total winter colony losses of 6.4%. Fifty-three percent of the beekeepers reported to have purchased queens the year preceding this study, and 40.1% moved colonies to the apiary. Queens were imported from European countries and the USA. The movements of bees were one of the factors strongly associated with the prevalence of the disease-causing organisms surveyed and colony losses. The majority of the participating beekeepers were not aware of any disease related signs in their apiaries despite positive laboratory findings. This highlights the importance of further outreach efforts to increase the beekeepers' awareness of diseases and disease management. The results provide a disease baseline for improvements of the surveillance system.
Introduction
Managed honey bees (Apis mellifera) perform critical pollination services to several agricultural crops. The economic value of honey bee pollination is estimated to several billion dollars; hence, the health of honey bees is an ongoing concern. Although the numbers of managed honey bee colonies worldwide are steadily increasing (Moritz & Erler, Citation2016), it is not enough to meet the increasing demand for pollination in agriculture (Aizen & Harder, Citation2009). Recent large-scale losses of managed honey bee colonies in some parts of the world and the decline of wild pollinators have raised awareness and concern of the lack of pollinators (Burkle et al., Citation2013). The cause of the reoccurring regional losses in honey bee colony numbers is suggested to be multifactorial; e.g. starvation, climate, pesticides, parasites, and pathogens (Genersch, Citation2010b; Goulson et al., Citation2015).
The Varroa mite, Varroa destructor, is considered to be the main threat to honey bees worldwide (Genersch, Citation2010b; Le Conte et al., Citation2010) and an untreated Varroa infested honey bee colony is expected to collapse within 2-3 years after the first introduction of the mite (Amdam et al., Citation2004). The Varroa mite does not only have a direct impact on honey bee health by feeding on fat body tissue (Ramsey et al., Citation2019), but they also have an indirect impact as a vector for honey bee viruses (Martin, Citation2001; Mondet et al., Citation2014). The mite was originally confined to the Eastern honey bee, Apis cerana, where a stable host-parasite relationship exists due to a long period of coevolution (Oldroyd, Citation1999). After a shift from the native host to the Western honey bee, A. mellifera, in the last century, the mite dispersed around the globe. Reports of Varroa in Europe date back to the 1970s and the first reported Swedish findings were from the island of Gotland in 1987 and on the mainland, in Skåne, in 1991 (Fries, Citation1987; Fries et al., Citation1991). The regulations from the Swedish Board of Agriculture (SBA) have since then been aimed at limiting the spread of Varroa in the country and had until 2016 not been reported from the most northern parts of Sweden except for close to the Finnish border (Kristiansen, Citation2002). Two of the viruses associated with Varroa infestations (varroosis) are Acute bee paralysis virus (ABPV) and Deformed wing virus (DWV). Symptoms of ABPV infection are paralysis, trembling, inability to fly, gradual darkening and loss of hair from the thorax and abdomen, and premature death of individual bees associated with a sharp decline of adults in the colony (de Miranda et al., Citation2010). DWV mainly causes benign infections without any signs of disease when transmitted vertically (through drones and queens) or horizontally (through larval food). However, when Varroa mites are feeding and reproducing in the brood cells they transmit the virus to the brood (vectorial transmission) which leads to deformation of the wings, discoloration, short and bloated abdomens, and death. The bees die as pupae or shortly after emergence (de Miranda & Genersch, Citation2010).
Two of the most economically important honey bee diseases are the bacterial brood diseases American foulbrood (AFB) and European foulbrood (EFB) affecting apiculture worldwide. AFB is caused by the spore-forming bacterium Paenibacillus larvae and is not only lethal to individual larvae but to entire honey bee colonies (Genersch, Citation2010a). Honey bee larvae become infected by ingesting food contaminated with P. larvae spores, and the remains of dead infected larvae contain billions of infectious spores that can stay in the environment for decades and serve as sources for new infections (Forsgren et al., Citation2008; Genersch, Citation2010a). The disease is epizootic and classified as a statutory notifiable disease in the European Union (Anonymous, Citation1992). In many European countries, Sweden included, the disease is controlled through the burning of colonies with disease signs combined with beekeeping management techniques to prevent the spread of the infectious agent. Current legislation does not allow European beekeepers to use antibiotics (Anonymous, Citation2010). Also, the other brood disease EFB, caused by the bacterium Melissococcus plutonius, is potentially lethal to honey bee colonies. As with AFB, the honey bee larvae become infected by ingesting contaminated food. The bacteria multiply in the midgut and the infected larvae usually die after four to five days (Forsgren, Citation2010; Genersch, Citation2010b).
In Sweden, every beekeeper has the responsibility to prevent the spread of bee diseases and is obliged to register the location of their apiaries. There is no national bee register, but the responsible authority (i.e. the Swedish Board of Agriculture) requires that the number of apiaries and colonies is reported by the beekeepers and recorded by their Country Administrative Board (CAB). The health of honey bees is controlled by local bee inspectors who perform visual inspections of colonies upon disease suspicion or when a beekeeper needs a permit issued by the bee inspector in order to move the bees out of restricted areas (due to AFB outbreaks) defined by the legal authorities. Bee inspectors can send samples to the National Reference Laboratory for Bee Health, NRL, at the Swedish University of Agricultural Sciences, SLU, where the diagnosis of honey bee diseases is performed.
Despite the clear benefits seen from comprehensive disease monitoring surveillance in other animal systems and the great importance of honey bees, there is little consistency in honey bee surveillance worldwide. Using surveillance studies to establish disease baselines for honey bees is an important first step towards detecting and mitigating emerging biotic threats (Traynor et al., Citation2016; vanEngelsdorp et al., Citation2014). The current honey bee disease surveillance mostly consists of apiary inspections and colony population monitoring (vanEngelsdorp et al., Citation2014). The surveillance of pathogens and parasites of honey bees in Sweden mainly relies on the passive surveillance done through diagnostics related to disease outbreaks as described above. The active surveillance of diseases, since many years reported for other animals, has not included honey bees. This study from 2016 is the first nationwide survey involving multiple honey bee diseases in Swedish apiaries. Our objectives when analyzing the collected data was to investigate (1) the prevalence and distribution of Varroa, two Varroa-associated viruses, ABPV and DWV, and two bacterial pathogens, P. larvae and M. plutonius in Swedish apiaries; (2) the beekeepers awareness of pathogens and diseases possibly affecting their beekeeping; (3) the potential risk factors for honey bee diseases and winter colony losses.
Materials and methods
Study design and selection of apiaries
The study was based on visits to a number of Swedish apiaries, including the sampling of honey bee colonies (A. mellifera) and completion of a questionnaire. In Sweden, registration of apiaries is mandatory, and each CAB keeps databases with information about beekeepers’ identities and their apiaries. For the purpose of this study, we contacted each CAB and asked for lists of registered apiaries. A random selection of apiaries from these lists was made for each county. The total number of selected apiaries was 385, and this sample size was partly based on sample size calculations for prevalence estimation (to estimate 50 ± 5% prevalence with a 95% confidence level) and detection of disease agents (to detect <2% prevalence with a 99% confidence level, also with imperfect tests), and on economic constraints. The number of selected apiaries from each county was set to correspond to the proportion of apiaries in the specific county, relative to the total number of apiaries in the country. The CABs were also asked to recommend local bee inspectors that perform their ordinary inspections and that potentially would be willing to perform the apiary visits, given the same financial compensation as for ordinary inspections. The bee inspectors were contacted and, once they agreed to participate, they received written instructions about what apiaries to visit and how to perform the sampling. The bee inspectors contacted the beekeepers to ask for their consent to participate in the study and to set a date for the visit. The visits were performed during the beekeeping season in 2016, from 4 March until 26 November. Every Swedish county (N = 21) was represented in our study population.
Sampling of honey bee colonies
Adult honey bees were collected from all apiaries in the study to determine the presence of the Varroa mite, the bacterial pathogens P. larvae and M. plutonius and the viral pathogens ABPV and DWV.
Five honey bee colonies separately distributed within each apiary were selected based on convenience and sampled by the bee inspector. All colonies were sampled if the apiary had less than five colonies. Approximately 300 adult bees were collected from each selected colony by stroking a small paper box across a brood frame. The samples were kept in a cooling box in the field and stored at −20 °C until analyzed.
Detection of varroa, P. larvae, M. plutonius, DWV and ABPV
To detect Varroa, bee samples were stirred for about 1 min in water with a detergent added using an electric household mixer. The bees were washed with a hand shower over a strainer to detect and count the Varroa mites (De Jong et al., Citation1982; Fries et al., Citation1991).
The samples were cultured for P. larvae according to Lindström and Fries (Citation2005). One hundred bees were crushed in a filter-grinding bag (Bioreba, Switzerland) with 20 mL of sterile water. The fluid produced was poured into a tube and centrifuged at 4000 X g for 10 min. After centrifugation, the supernatant was removed and the pellet re-suspended in 2 mL sterile 0.9% saline solution. The pellet was incubated in a water bath at 85 °C for 10 min to reduce contamination from non-spore forming bacteria before 10 µL was spread onto MYPGP-agar (Mueller-Hinton broth, Yeast extract, Potassium phosphate, Glucose, and Pyruvate) plates. The plates were incubated at 35 °C with 5% CO2 and after seven days, suspected P. larvae colonies were confirmed using real-time PCR. Briefly, a bacterial colony was suspended in nuclease-free water and used as template in the PCR reaction. Real-time PCR using BioRad CFX96 cycler using SsoFast EvaGreen SuperMix (Biorad, US) was performed according to the manufacturer’s recommendation and published primers (Martínez et al., Citation2010)
To determine the presence of M. plutonius, 100 adult bees were placed into a filter-grinding bag (Bioreba) with 20 mL of sterile water. The bees were crushed and 1 mL of the fluid produced was immediately subjected to DNA extraction using the QIAamp® genomic DNA isolation mini kit for Gram-positive bacteria (Qiagen, Germany). DNA was eluted with 100 µL elution buffer and stored at −20 °C until processed for molecular diagnostics. Quantitative real-time PCR using BioRad CFX96 cycler using SsoFast EvaGreen SuperMix (Biorad, US) was performed according to the manufacturer’s recommendation and using primers previously described (Roetschi et al., Citation2008).
To determine the quantity of ABPV and DWV, 30 bees were placed in a filter-grinding bag (Bioreba) with 5 mL nuclease-free water and frozen in liquid nitrogen. The frozen bees were crushed and 100 µL of the fluid was used for total RNA extraction using a Qiacube automated extraction robot (Qiagen) and the RNeasy manufacturer’s protocol for plant tissue. Eluted RNA was stored at −80 °C until further processed. Reverse transcription-quantitative PCR was run in the BioRad CFX96 cycler using iScript One-Step RT-PCR Kit with SYBR Green according to the manufacturer’s recommendation and primers used in Locke et al. (Citation2012).
Questionnaire
We designed a four-page combined submission form and questionnaire, with 18 questions related to; a) date of sampling, identities of bee inspector and beekeeper, and location of the apiary, b) number of apiaries and honey bee colonies owned by the beekeeper, c) bee health estimations including observations of clinical signs, mortality and potential disease control measures, and d) introduction of bees. The last page of the questionnaire allowed additional free-text comments. The questionnaire was set up as an online form using the Questback software (Questback AS, Oslo, Norway). The questionnaire was filled in by bee inspectors and beekeepers, in conjunction with the sampling, and sent to the laboratory together with the samples. The data from the paper version of the questionnaire was manually entered into the online version. A translated version can be found in the online supplementary material.
Statistical analysis
The information from the questionnaires and laboratory data were exported to a Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA). Data handling, descriptive analysis, mapping, and statistical modeling were performed in R statistical software version 3.5.0 (R Core Team, Citation2018). Model-building strategies followed techniques described by Dohoo et al., Citation2009 (Dohoo et al., Citation2009). Descriptive statistics of numeric variables included mean, medians, interquartile ranges, standard deviations, minimum and maximum values. We described categorical variables using frequency distribution tables. The prevalence of Varroa and the honey bee pathogens ABPV, DWV, P. larvae, and M. plutonius in each Swedish county was calculated by dividing the number of positive apiaries by the total number of apiaries sampled in the county. Prevalence was mapped for visualization.
Apiary level data was analyzed using univariable and multivariable logistic regression models with binomial outcomes. It was not possible to model for ABPV and M. plutonius due to the low number of positive findings. We built risk factor models for Varroa infestation, DWV infection level, and P. larvae infection. Threshold values for Varroa were set as infestation rates less than 3% versus an infestation rate equal to or higher than 3% in a honey bee colony. Decreased vigor and increased mortality of bee colonies are usually observed with the higher infestation rate (Barroso-Arévalo et al., Citation2019; Giacobino et al., Citation2017). For DWV, the outcome was the number of virus copies per bee in a honey bee colony, i.e. less than 107 virus copies per bee versus 107 virus copies or more per bee. A high amount of DWV is associated with disease signs in honey bees (Mockel et al., Citation2011; Zioni et al., Citation2011). The P. larvae outcome was the absence or presence of the pathogen. Additionally, we modelled risk factors for winter colony losses. The outcome variable for this model was based on the number of colonies lost in an apiary overwinter out of the total number of colonies before winter. The data sources for the explanatory variables of the models came mainly from the questionnaire responses. We obtained data on the number of honey bee colonies in the apiary, number of colonies managed by the beekeeper, observation of disease signs, Varroa treatment, purchase of queens, and colony movement. Due to the biological relevance of Varroa in the transmission of honey bee viruses and colony survival, the mites served as an explanatory variable when modeling for DWV and colony losses.
Regarding the model building process, we assessed the linearity of numeric explanatory variables by visually analyzing scatter plots between each of these variables and the log odds of the outcome. When lack of linearity was identified, we categorized these variables based on cut-points that created categories with an approximate number of observations. The categorical explanatory variables had more than 10% observations in each of its category. Prior to inclusion in multivariable models, univariable models were fit to separately test associations between the explanatory and outcome variables. If P < 0.2 in the likelihood ratio test, the explanatory variable was kept for the multivariable model. Potential collinearity problems were assessed testing associations between pairs of explanatory variables and, if biologically plausible, the Fisher’s exact tests were used to check these associations at a level of significance of 0.05. Significant results served as an exclusion criterion for one of the explanatory variables, in which the one that had a weaker univariable association (i.e. a higher P-value) with the outcome variable was not included the multivariable model. For multivariable modelling, we included the variables that passed the above criteria and possible interactions. The final multivariable model was selected using a backward stepwise elimination procedure and the Akaike information criterion. The variables kept in our models were significant at 5% level, assessed by the likelihood ratio test. Potential confounders in these models were assessed by individually excluding variables from the model and checking a change of at least 20% in the regression coefficients. The model fit was assessed using Hosmer-Lemeshow goodness of fit tests.
Results
Health status, characteristics, and management of apiaries
We received data from 382 out of 385 randomly selected apiaries distributed across all Swedish counties (see supplementary material ). There was insufficient sampling material from two apiaries to test for ABPV and DWV and for Varroa counts in six apiaries. We excluded questionnaire answers from six study apiaries due to inconsistent data. A summary of the answers from the questionnaire can be found in . The median number of honey bee colonies in the study apiaries was five, the interquartile range (IQR) = 3 − 7; the median number of apiaries per beekeeper was one (IQR = 1 − 2); and the median number of colonies per beekeeper was six (IQR = 3 − 10).
Figure 1. Prevalence of Varroa and honey bee pathogens in Sweden 2016. Acute bee paralysis virus (ABPV), Deformed wing virus (DWV), Melissococcus plutonius (MP), Paenibacillus larvae (PL), and Varroa destructor (Varroa).
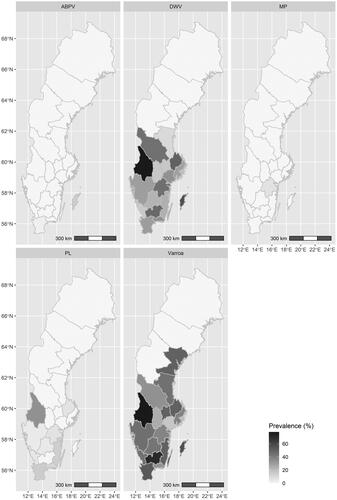
Table 1. Summary of questionnaire answers related to at least 2,185 colonies in Swedish apiaries surveyed in 2016. In 8 out of 376 study apiaries there was no information on the number of colonies.
An overview of the prevalence of Varroa and honey bee pathogens in Swedish apiaries per county is presented in . The disease agents with a higher overall prevalence were Varroa (57.4%; 216/376) and DWV (30.2%; 115/380). Varroa was absent in only three counties and the prevalence within apiaries in counties where the parasite was present varied between 16.7 and 77.8%. DWV was not detected in any of the investigated apiaries in the four most northern counties, whereas the prevalence ranged from 10 to 77.8% in apiaries in the other counties. ABPV was only detected in two apiaries in two counties. The bacterium P. larvae was present in 6% (23/382) of the investigated apiaries with the highest prevalence of 33.3% and with zero prevalence in more than half of the counties. M. plutonius was present in two apiaries from two related beekeepers in the same county.
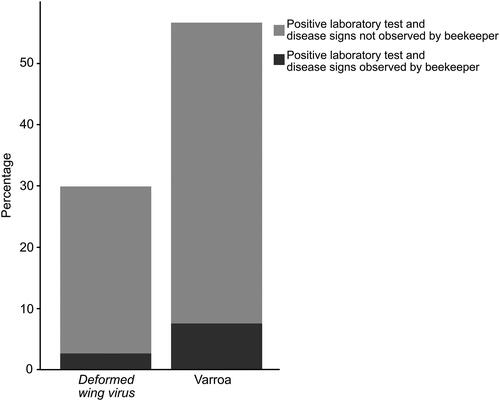
Fifty-three percent (172/323) of the beekeepers reported to have purchased queens the year preceding this study, and 40.1% (116/289) moved colonies to the apiary. In this period, the purchase of queens and movement of colonies between counties occurred in 19.8% (34/172) and 8.6% (10/116) of the apiaries respectively. One beekeeper unknowingly moved honey bee colonies from a P. larvae-infected apiary to an apiary in another county. We received reports of four cases of imports of queens from countries inside and outside Europe (Germany, Slovenia, and the United States) during the five years prior to our study. Most of the beekeepers had been treated against Varroa (88.9%; 330/371). Data on winter losses were reported from 139 apiaries, and the overall proportion of colonies lost over winter in these apiaries was 6.4% (42 out of 655 colonies). We found that the vast majority of beekeepers did not observe any disease related signs although the laboratory assays found the apiaries positive for the disease-causing organisms tested for ().
Risk factors for honey bee diseases and colony losses
The results from the risk factor regression models for Varroa, DWV, and P. larvae are summarized in . The odds of an apiary being highly infested by Varroa (i.e. ≥3 mites per 100 bees per colony) increased by 6% for each unit increase in the number of honey bee colonies. Compared to the apiaries with low levels of Varroa (i.e. <3 mites per 100 bees per colony) the high-level apiaries were 6 times more likely to contain elevated levels of DWV (i.e. ≥107 virus copies per bee). Apiaries that had received a honey bee colony from another apiary in the previous year had a three times higher chance of being positive for P. larvae than apiaries without any colony movement in the previous year. Considering the factors associated with high winter colony losses (), low levels of Varroa were the most crucial factor in reducing winter colony losses. The odds for colony losses also dropped if no movement of colonies between apiaries had occurred. With borderline significance, we observed a slight decrease in the odds for colony losses related to a higher number of honey bee colonies kept by beekeepers. There seemed to be confounding effects of the number of honey bee colonies per beekeeper with colony move and Varroa infestation levels in the colony loss model. Excluding this potential confounder from the model had little relevance for the biological interpretation of the associations between the explanatory variables and the outcome. The regression models were well fitted according to the Hosmer-Lemeshow statistics (0.2 < P ≤ 1).
Table 2. Results from the logistic regression models for risk factors associated with the outcomes high Varroa infestation (≥3 mites per 100 bees), high Deformed wing virus (DWV) virus loads (≥107 virus copies per bee), and Paenibacillus larvae infection.
Table 3. Results of a logistic regression model for risk factors associated with winter colony losses in Swedish apiaries.
The descriptive results for Varroa, DWV, and P. larvae, involving the studied risk factors and the causative agents associated with honey bee diseases are summarized in respectively. Putative risk factors for winter colony losses are summarized in Table S4.
Discussion
Sustainable disease surveillance systems are important as they quantify disease levels in different populations and regions and provide data to support the mitigation and prevention of important diseases (Lee et al., Citation2015). Losses of managed honey bee colonies are of great concern globally, not least in the USA where the organisation Bee Informed Partnership (BIP, beeinformed.org) has conducted a total of ten winter loss surveys since 2006-2007. They reported total winter colony losses during these years ranging from a low of 22% to a high of 36% and a total annual loss ranging from 34 to 45% (Kulhanek et al., Citation2017). Another of the many research initiatives to investigate winter losses of honey bee colonies is organized through COLOSS (= Prevention of honey bee COlony LOSSes, currently a non-profit organization). COLOSS use standardized methods for surveys of beekeepers to measure colony loss rates. The COLOSS survey from 2016 reported an overall European loss rate of 12.0% during winter 2015-2016, with marked differences among countries. The estimated overall winter loss rate in Sweden was 15.9% (Brodschneider et al., Citation2016). A local source of information about winter colony losses in Sweden is the information from the Swedish Beekeepers Association where data on winter colony losses have been recorded since 1920. The data show an overall winter colony loss rate of 13.1% over the century and 15.9% for winter 2015-2016. (Information from the Swedish Beekeepers Association compiled by the national bee health advisor Preben Kristiansen, pers. comm.). As methodologies, sampling and other factors differ between surveys, direct comparisons between the low overall winter colony loss rates in this study, 6.4%, should be made with caution. An under-estimation of this rate might have occurred due to the low response rate of the related question (139/376, 36%), especially if the non-respondents were beekeepers with large beekeeping operations.
Our data indicate that the odds for high Varroa numbers increase with increasing numbers of colonies in an apiary. This is in line with earlier studies (Frey & Rosenkranz, Citation2014; Seeley & Smith, Citation2015) showing that a high density of colonies leads to higher invasion rates and that crowding honey bee colonies in apiaries greatly increases their vulnerability to Varroa. Furthermore, the results reaffirm that low Varroa numbers are the most crucial factor for reducing winter colony losses (Chauzat et al., Citation2016) and that high numbers of Varroa are correlated to elevated levels of DWV (de Miranda & Genersch, Citation2010). A risk factor for spreading disease is the exchange and transfer of bees and beekeeping related materials. Half of the responding beekeepers in this study, 53.3%, reported having purchased queens in the previous year and 40.1% had moved colonies between apiaries and regions. One beekeeper moved bees from an apiary later diagnosed with P. larvae to another county, which clearly illustrates the risk of unknowingly spreading disease. A few queens were purchased from other European countries and one beekeeper reported illegal imports of queens from the USA.
After the introduction of Varroa in Sweden, the Swedish Board of Agriculture introduced regulations to prevent or at least slow down the spread of the mite in the country. This has not completely prevented the spread, but there are still regions in the north reported free of Varroa and the results from this survey reinforce earlier observations and reports. The Varroa mite acts as a biological vector for viruses like DWV and ABPV, and DWV was detected in all counties except four counties in the far north. The spread of DWV coincides with the presence of Varroa and follows the spread of the mite. The other Varroa associated virus, ABPV, was only detected in one apiary on the Baltic island Gotland and in another apiary in the most southern part of the country (Skåne). The reason for ABPV not being more widely spread may be because the virus is too virulent, i.e. kills its host too fast, to spread effectively (Sumpter & Martin, Citation2004; Traynor et al., Citation2020). This could explain why the less virulent virus DWV has such a high incidence while ABPV is so sparingly present. It is also worth noting that the counties where ABPV is detected, Gotland and Skåne, are the counties where Varroa was first introduced in the country (Fries, Citation1987; Fries et al., Citation1991). At that time (late 80 s, early 90 s), ABPV was the most dominant Varroa associated virus in Europe before it was replaced by DWV (de Miranda et al., Citation2010).
The reporting of AFB incidences in Sweden is based on the observation of disease symptoms reported by the bee inspectors to the legal authorities. If one considers the reported number of apiaries with colonies with signs of disease in relation to the estimated total number of apiaries in Sweden, the AFB prevalence in Swedish beekeeping has varied from 0.5% to 1% over the last decade (Data from the Swedish Board of Agriculture compiled by bee health advisor Preben Kristiansen, pers. comm.). There is, however, a reason to suspect an under-reporting of the disease due to factors such as the unrealistic compensation for affected beekeepers. Subclinical levels of P. larvae can be detected by microbiological culture or molecular methods from samples of honey, adult bees, and hive debris. In this study, we used microbiological cultivation of P. larvae from samples of adult bees, a method proven to be well correlated with signs of disease in the honey bee colony (Forsgren & Laugen, Citation2014; Nordström et al., Citation2002), to screen for subclinical levels of the bacterium. Even though only young honey bee larvae develop disease, adult bees are carriers of the infectious agent (Lindström, Citation2008). We investigated the subclinical presence of the bacterium in a selection of the country's apiaries and in most of the examined apiaries (94%), the bacteria could not be detected. This is an important argument in discussions between beekeepers and regulatory authorities about simplifying the regulations on the management and movement of bee colonies. It is important to highlight that there are many apiaries in areas free of this pathogen and that status is worth preserving. The causative agent of EFB, M. plutonius, was detected in bee samples from only two apiaries. Historically, EFB has been considered less serious than AFB, but reports of more virulent strains of the bacterium and more serious disease outbreaks have become increasingly common in recent years (Grossar et al., Citation2020). In 2010, Norway had an outbreak of EFB after a long time of no disease that led to extensive investigations and sanitation (Grossar et al., Citation2020). This supports the relevance of continuous EFB monitoring for the prevention of outbreaks of this disease in Sweden.
The majority of the beekeepers participating in this study were not aware of any disease or any disease related signs in their colonies despite positive laboratory findings, and one beekeeper unknowingly moved colonies from an apiary where P. larvae was present. This reaffirms results from other studies and highlights the importance of improved beekeeper training to promote good beekeeping practices (Jacques et al., Citation2017).
Conclusions
Active surveillance programs provide superior insight on the prevalence, incidence, and geographic distribution of disease agents and their epidemic potential over passive surveillance based on symptoms and self-reporting. Passive surveillance based on self-reporting of disease by beekeepers is furthermore affected by training, beekeeping practice, social and economic factors leading towards an under-reporting of the true incidence. Improved knowledge of diseases (i.e. disease signs, consequences, and regulatory control measures) supplemented by realistic compensation for affected beekeepers might help mitigate the under-reporting of, for example, AFB to the legal authorities. More accurate data on prevalence, incidence, and distribution of diseases at national and international levels, to which this study contributes, would provide the authorities with a superior foundation for improving the legal statue and guidelines for disease prevention and control.
Questionnaire
Download PDF (40.8 KB)Tables S1-S4
Download MS Word (37.4 KB)Figure S1
Download Zip (441.5 KB)Acknowledgments
The authors would like to thank all beekeepers and bee inspectors for their participation in this study. We thank Cecilia Persson and Emilia Semberg for excellent technical assistance. We are also grateful to Preben Kristiansen for supporting sample selection and contribute information.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
, and the Questionnaire are available via the ‘Supplementary’ tab on the article’s online page (http://dx.doi.org/10.1080/00218839.2021.1902679).
Additional information
Funding
References
- Aizen, M. A., & Harder, L. D. (2009). The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Current Biology : CB, 19(11), 915–918. https://doi.org/https://doi.org/10.1016/j.cub.2009.03.071
- Amdam, G. V., Hartfelder, K., Norberg, K., Hagen, A., & Omholt, S. W. (2004). Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during overwintering? Journal of Economic Entomology, 97(3), 741–747. https://doi.org/https://doi.org/10.1093/jee/97.3.741
- Anonymous. (1992). Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A (I) to Directive 90/425/EEC. Official Journal of the European Communities, L268, 54–72.
- Anonymous. (2010). Commission regulation (EU) No 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuff of animal origin. Official Journal of the European Union, L15, 1–72.
- Barroso-Arévalo, S., Fernández-Carrión, E., Goyache, J., Molero, F., Puerta, F., & Sánchez-Vizcaíno, J. M. (2019). High load of deformed wing virus and varroa destructor infestation are related to weakness of honey bee colonies in Southern Spain. Frontiers in Microbiology, 10, 1331. https://doi.org/https://doi.org/10.3389/fmicb.2019.01331
- Brodschneider, R., Gray, A., van der Zee, R., Adjlane, N., Brusbardis, V., Charrière, J.-D., Chlebo, R., Coffey, M. F., Crailsheim, K., Dahle, B., Danihlík, J., Danneels, E., de Graaf, D. C., Dražić, M. M., Fedoriak, M., Forsythe, I., Golubovski, M., Gregorc, A., Grzęda, U., … Woehl, S. (2016). Preliminary analysis of loss rates of honey bee colonies during winter 2015/16 from the COLOSS survey. Journal of Apicultural Research, 55(5), 375–378. https://doi.org/https://doi.org/10.1080/00218839.2016.1260240
- Burkle, L. A., Marlin, J. C., & Knight, T. M. (2013). Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science (New York, N.Y.), 339(6127), 1611–1615. https://doi.org/https://doi.org/10.1126/science.1232728
- Chauzat, M.-P., Jacques, A., Laurent, M., Bougeard, S., Hendrikx, P., & Ribière-Chabert, M. (2016). Risk indicators affecting honeybee colony survival in Europe: One year of surveillance. Apidologie, 47(3), 348–378. https://doi.org/https://doi.org/10.1007/s13592-016-0440-z
- De Jong, D., De Andrea Roma, D., & Gonçalves, L. S. (1982). A comparative analysis of shaking solutions for the detection of varroa jacobsoni on adult honeybees. Apidologie, 13(3), 297–306. https://doi.org/https://doi.org/10.1051/apido:19820308
- de Miranda, J. R., & Genersch, E. (2010). Deformed wing virus. Journal of Invertebrate Pathology, 103, S48–S61. https://doi.org/https://doi.org/10.1016/j.jip.2009.06.012
- de Miranda, J. R., Cordoni, G., & Budge, G. (2010). The Acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex. Journal of Invertebrate Pathology, 103, S30–S47. https://doi.org/https://doi.org/10.1016/j.jip.2009.06.014
- Dohoo, I., Martin, W., & Stryhn, H. (2009). Veterinary epidemiologic research (2nd ed., 3rd Print). VER Inc.
- Forsgren, E. (2010). European foulbrood in honey bees. Journal of Invertebrate Pathology, 103, S5–S9. https://doi.org/https://doi.org/10.1016/j.jip.2009.06.016
- Forsgren, E., & Laugen, A. T. (2014). Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie, 45(1), 10–20. https://doi.org/https://doi.org/10.1007/s13592-013-0225-6
- Forsgren, E., Stevanovic, J., & Fries, I. (2008). Variability in germination and in temperature and storage resistance among Paenibacillus larvae genotypes. Veterinary Microbiology, 129(3–4), 342–349. https://doi.org/https://doi.org/10.1016/j.vetmic.2007.12.001
- Frey, E., & Rosenkranz, P. (2014). Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. Journal of Economic Entomology, 107(2), 508–515. https://doi.org/https://doi.org/10.1603/ec13381
- Fries, I. (1987). Diagnostik av kvalstret Varroa jacobsoni. Bitidningen, 86, 335–342.
- Fries, I., Aarhus, A., Hansen, H., & Korpela, S. (1991). Comparison of diagnostic methods for detection of low infestation levels ofVarroa jacobsoni in honey-bee (Apis mellifera) colonies. Experimental and Applied Acarology, 10(3–4), 279–287. https://doi.org/https://doi.org/10.1007/BF01198656
- Genersch, E. (2010a). American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. Journal of Invertebrate Pathology, 103, S10–S19. https://doi.org/https://doi.org/10.1016/j.jip.2009.06.015
- Genersch, E. (2010b). Honey bee pathology: Current threats to honey bees and beekeeping. Applied Microbiology and Biotechnology, 87(1), 87–97. https://doi.org/https://doi.org/10.1007/s00253-010-2573-8
- Giacobino, A., Pacini, A., Molineri, A., Bulacio Cagnolo, N., Merke, J., Orellano, E., Bertozzi, E., Masciangelo, G., Pietronave, H., & Signorini, M. (2017). Environment or beekeeping management: What explains better the prevalence of honey bee colonies with high levels of Varroa destructor? Research in Veterinary Science, 112, 1–6. https://doi.org/https://doi.org/10.1016/j.rvsc.2017.01.001
- Goulson, D., Nicholls, E., Botias, C., & Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science (New York, N.Y.), 347(6229), 1255957–1255957. https://doi.org/https://doi.org/10.1126/science.1255957
- Grossar, D., Kilchenmann, V., Forsgren, E., Charrière, J.-D., Gauthier, L., Chapuisat, M., & Dietemann, V. (2020). Putative determinants of virulence in Melissococcus plutonius, the bacterial agent causing European foulbrood in honey bees. Virulence, 11(1), 554–567. https://doi.org/https://doi.org/10.1080/21505594.2020.1768338
- Jacques, A., Laurent, M., Ribière-Chabert, M., Saussac, M., Bougeard, S., Budge, G. E., Hendrikx, P., & Chauzat, M.-P. (2017). A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. Plos One, 12(3), e0172591. https://doi.org/https://doi.org/10.1371/journal.pone.0172591
- Kristiansen, P. (2002). Varroa i Norrbotten. Bitidningen, 101–109, 19.
- Kulhanek, K., Steinhauer, N., Rennich, K., Caron, D. M., Sagili, R. R., Pettis, J. S., Ellis, J. D., Wilson, M. E., Wilkes, J. T., Tarpy, D. R., Rose, R., Lee, K., Rangel, J., & vanEngelsdorp, D. (2017). A national survey of managed honey bee 2015–2016 annual colony losses in the USA. Journal of Apicultural Research, 56(4), 328–340. https://doi.org/https://doi.org/10.1080/00218839.2017.1344496
- Le Conte, Y., Ellis, M., & Ritter, W. (2010). Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie, 41(3), 353–363. https://doi.org/https://doi.org/10.1051/apido/2010017
- Lee, K. V., Steinhauer, N., Rennich, K., Wilson, M. E., Tarpy, D. R., Caron, D. M., Rose, R., Delaplane, K. S., Baylis, K., Lengerich, E. J., Pettis, J., Skinner, J. A., Wilkes, J. T., Sagili, R., & vanEngelsdorp, D. (2015). A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie, 46(3), 292–305. https://doi.org/https://doi.org/10.1007/s13592-015-0356-z
- Lindström, A. (2008). Distribution of Paenibacillus larvae spores among adult honey bees (Apis mellifera) and the relationship with clinical symptoms of American foulbrood. Microbial Ecology, 56(2), 253–259. https://doi.org/https://doi.org/10.1007/s00248-007-9342-y
- Lindström, A., & Fries, I. (2005). Sampling of adult bees for detection of American foulbrood (Paenibacillus larvae subsp. larvae) spores in honey bee (Apis mellifera) colonies. Journal of Apicultural Research, 44(2), 82–86. https://doi.org/https://doi.org/10.1080/00218839.2005.11101154
- Locke, B., Forsgren, E., Fries, I., & de Miranda, J. R. (2012). Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control. Applied and Environmental Microbiology, 78(1), 227–235. https://doi.org/https://doi.org/10.1128/AEM.06094-11
- Martin, S. J. (2001). The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. Journal of Applied Ecology, 38(5), 1082–1093. https://doi.org/https://doi.org/10.1046/j.1365-2664.2001.00662.x
- Martínez, J., Simon, V., Gonzalez, B., & Conget, P. (2010). A real-time PCR-based strategy for the detection of Paenibacillus larvae vegetative cells and spores to improve the diagnosis and the screening of American foulbrood: Sensitive detection of Paenibacillus larvae in honey. Letters in Applied Microbiology, 50(6), 603–610. https://doi.org/https://doi.org/10.1111/j.1472-765X.2010.02840.x
- Mockel, N., Gisder, S., & Genersch, E. (2011). Horizontal transmission of deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. The Journal of General Virology, 92(Pt 2), 370–377. https://doi.org/https://doi.org/10.1099/vir.0.025940-0
- Mondet, F., Miranda, J. R., de Kretzschmar, A., Conte, Y. L., & Mercer, A. R. (2014). On the front line: Quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathogens, 10(8), e1004323 https://doi.org/https://doi.org/10.1371/journal.ppat.1004323
- Moritz, R. F. A., & Erler, S. (2016). Lost colonies found in a data mine: Global honey trade but not pests or pesticides as a major cause of regional honeybee colony declines. Agriculture Ecosystems and Environment, 216, 44–50. https://doi.org/https://doi.org/10.1016/j.agee.2015.09.027
- Nordström, S., & Fries, I. (1995). A comparison of media and cultural conditions for identification of Bacillus larvae in honey. Journal of Apicultural Research, 34(2), 97–103. https://doi.org/https://doi.org/10.1080/00218839.1995.11100894
- Nordström, S., Forsgren, E., & Fries, I. (2002). Comparative diagnosis of American foulbrood using samples of adult honey bees and honey. Journal of Apicultural Science,46, 5–12.
- Oldroyd, B. P. (1999). Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends in Ecology & Evolution, 14(8), 312–315. https://doi.org/https://doi.org/10.1016/S0169-5347(99)01613-4
- Ramsey, S. D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J. D., Cohen, A., Lim, D., Joklik, J., Cicero, J. M., Ellis, J. D., Hawthorne, D., & vanEngelsdorp, D. (2019). Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proceedings of the National Academy of Sciences of the United States of America, 116(5), 1792–1801. https://doi.org/https://doi.org/10.1073/pnas.1818371116
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/.
- Roetschi, A., Berthoud, H., Kuhn, R., & Imdorf, A. (2008). Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie, 39(3), 362–371. https://doi.org/https://doi.org/10.1051/apido:200819
- Seeley, T. D., & Smith, M. L. (2015). Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie, 46(6), 716–727. https://doi.org/https://doi.org/10.1007/s13592-015-0361-2
- Sumpter, D. J. T., & Martin, S. J. (2004). The dynamics of virus epidemics in Varroa -infested honey bee colonies. Journal of Animal Ecology, 73(1), 51–63. https://doi.org/https://doi.org/10.1111/j.1365-2656.2004.00776.x
- Traynor, K. S., Mondet, F., de Miranda, J. R., Techer, M., Kowallik, V., Oddie, M. A. Y., Chantawannakul, P., & McAfee, A. (2020). Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends Parasitol, 36(7), 592–606. https://doi.org/https://doi.org/10.1016/j.pt.2020.04.004
- Traynor, K. S., Rennich, K., Forsgren, E., Rose, R., Pettis, J., Kunkel, G., Madella, S., Evans, J., Lopez, D., & vanEngelsdorp, D. (2016). Multiyear survey targeting disease incidence in US honey bees. Apidologie, 47(3), 325–347. https://doi.org/https://doi.org/10.1007/s13592-016-0431-0
- vanEngelsdorp, D., Saegerman, C., Nguyen, B. K., & Pettis, J. (2014). Honey bee health surveillance. In W. Ritter (Ed.), Bee health and veterinarians (pp. 215–222). OIE World Organisation for Animal Health.
- Zioni, N., Soroker, V., & Chejanovsky, N. (2011). Replication of Varroa destructor virus 1 (VDV-1) and a Varroa destructor virus 1-deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology, 417(1), 106–112. https://doi.org/https://doi.org/10.1016/j.virol.2011.05.009