Abstract
This study aimed to determine the proximate composition, gross energy, and amino acid amounts and mineral content in red mason bee Osmia bicornis (syn. Osmia rufa) cocoons to define their possible uses. This is the first study documenting the proximate composition and amounts of amino acids in Osmia bicornis cocoons. Like all other insect cocoons, red mason bee cocoons consist of silk. Samples were analyzed using AOAC standard methods. The dominant component of the examined cocoons was crude protein, which accounted for 52.91%. The examined cocoons were found to contain a considerable amount of crude fiber (30.69%)(consisting of NDF – 54.03% and ADF – 45.97%). Red mason bee cocoons consisted of 18 amino acids, including 39% of essential amino acids (EAA). The examined cocoons contained a significant amount of minerals. It appears from comparing the obtained results with other sources of dietary fiber in products of insect origin, that the Osmia bicornis cocoon differs in its composition. However, this fact may indicate its unique properties.
Introduction
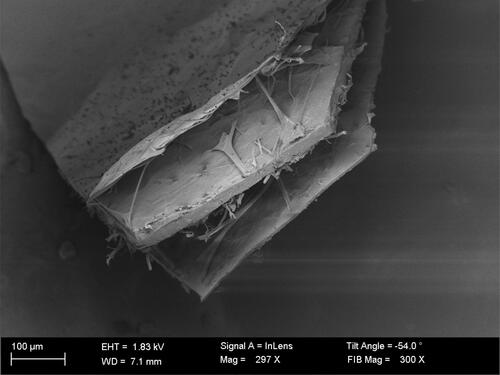
The red mason bee is a common species of solitary bee in the Megachilidae family found in Europe and North Africa (Giejdasz & Wilkaniec, Citation2002). Breeding of this insect has been popularized to cover the demand of pollinators (Biliński & Teper, Citation2004; Krunic et al., Citation1995). The cocoons produced by red mason bee larvae after the bees emerge are waste. The cocoon has an oval shape, diameter 5–7 mm, and length 9–15 mm. It is dark brown and its walls are made of three layers (). The extreme inside and outside layers are thin and light, while the middle one is thick (Banaszak & Romasenko, Citation1998). Like all other insect cocoons, red mason bee cocoons consist of silk. This is a substance that is secreted by some species of arthropods and swordfish (Fisher & Robertson, Citation1999; Sutherland et al., Citation2006; Citation2012; Walker et al., Citation2012). Silk has no clearly defined chemical composition or physical properties. This substance is described as a semicrystalline polymer, which mainly consists of fiber-forming structural proteins (Candelas et al., Citation1990; Dyakonov et al., Citation2012; Grześkowiak & Łochyńska, Citation2017; Vollrath, Citation2000). The best known in terms of properties are the types of silk fiber produced by the mulberry silkworm (Bombyx mori) and the spider Nephila clavipes (Shin et al., Citation2010). This study aimed to determine the proximate composition, gross energy, amino acid composition, and content of selected elements (i.e., silver (Ag), copper (Cu), manganese (Mn), iron (Fe), nickel (Ni), lead (Pb), zinc (Zn), cadmium (Cd), calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K)) in red mason bee (Osmia bicornis) cocoons to defined possible uses. The chemical composition of this material has, so far, not been analyzed. No research has been done on the structure and properties of Osmia bicornis cocoons and there are also no reports about the possible uses for this type of silk.
Materials and methods
Samples
The cocoons were collected from 35 different places in Poland where red mason bees were breeding, according to the method of Wójtowski and Wilkaniec (Citation1978). After the bees hatched, the cocoons were collected and stored in desiccators. . The pooled sample size was 2200 cocoons, i.e., 40 g. Before analysis, the pooled sample was homogenized.
Proximate composition and energy value analysis
The pooled cocoon sample was analyzed for moisture, crude protein, crude fat, crude fiber (NDF and ADF), and gross energy by using AOAC (Association of Official Analytical Chemists) standard methods. Moisture was determined after drying the cocoon sample at 105 °C to a constant weight. To measure the amount of crude protein in the sample, the Kjeldahl method (Kjeltec™ 2300Analyzer Unit (Foss, Hilleroed, Denmark)) was used. Crude protein content was calculated by multiplying the percentage of nitrogen by 6.25. Crude fat was measured after extraction in petroleum ether. The fiber was measured by using Van Soest fiber analysis (Fibertec™ E. Foss, Hilleroed, Denmark). The energy value was determined by using a bomb calorimeter. All analyses were performed in triplicate.
Amino acid composition analysis
For amino acid analysis, the pooled cocoon sample was hydrolyzed with 6 mol x L-1 HCl and determined using a AAA 500 amino acid analyzer (Ingos, Praha, Czech Republic). For tryptophan content measurement, the sample was hydrolyzed in 4 mol x L-1 LiOH and then the content of tryptophan was determined by using a Specol 11 (Carl Zeis Jena, Oberkochen, Germany) spectrophotometer.
Mineral content
Analysis of metal concentration was done by mineralizing the pooled cocoon sample. Mineralization was performed in a Microwave Reaction System MULTIWAVE PRO by ANTON PAARGmbH (Graz, Austria). Quantitative determination of selected elements (potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), silver (Ag), copper (Cu), manganese (Mn), iron (Fe), nickel (Ni), lead (Pb), zinc (Zn), and cadmium (Cd))was performed by flame atomic absorption spectroscopy (FAAS) Spectr AA-110/220 unit(Varian, Inc., California, USA).
Statistical analysis
All results are expressed as mean ± standard deviation (SD) of the triplicate determinations. All statistical analysis was performed using RStudio-1.1.463 (R Core Team, Citation2018). The statistical significance of the data was determined by the Kruskal-Wallis test (a significance level of α = 0.05 was used).
Results
The dominant component of the examined cocoons was crude protein, accounting for 52.91% (). Red mason bee cocoons were found to contain a considerable amount of crude fiber (30.69%); the ADF (acid detergent fiber) fraction consisted of 54.03% crude fiber, with the NDF (neutral detergent fiber) fraction consisting of 45.97%. Red mason bee cocoons consisted of 18 amino acids, including 39% of essential amino acids (EAA) (). The most abundant amino acid was alanine (18.67%) followed by glutamic acid (15.59%). Red mason bee cocoons were observed to contain a fairly high level of minerals (). Among the macro elements, the highest content was shown for potassium, while the lowest content was for sodium. The differences between these elements’ content were statistically significant. Among the microelements, the highest content was shown for manganese, while the lowest content was for lead. The differences between these elements’ content were statistically significant.
Table 1. Proximate composition and gross energy analysis of red mason bee (Osmia bicornisL.) cocoons.
Table 2. The amino acid content of red mason bee (Osmia bicornisL.) cocoons.
Table 3. Mineral content of red mason bee (Osmia bicornisL.) cocoons.
Discussion
The present study is the first performed on the chemical composition of red mason bee cocoons. Due to this, the proximate composition of the mentioned material was compared to products obtained from silkworm cocoons and chitin and to orange fiber, which are all used in nutrition. The dominant component of the examined cocoons was crude protein which accounted for 52.91%, a lower amount compared to the 75–90% of the protein in silkworm cocoons (Dyakonov et al., Citation2012). Red mason bee cocoons were found to contain a considerable amount of crude fiber (NDF and ADF). No research was conducted on the amount of crude fiber in the cocoons of other insects. Commercially available sericin, in the form of powder, contained 12% of moisture and 81.25% of protein. These are higher values than in the examined cocoons(respectively, 5.74% and 52.91%). Sericin hydrolysate contained 4.96% of moisture, which is comparable to the amount shown to be in red mason bee cocoons. This hydrolysate was found to have a much higher amount of protein (93.06%) compared to the examined cocoons (Vaithanomsat & Kitpreechavanich, Citation2008). These differences in composition have been attributed to the fact that each species of insect produces a unique type of silk (Weisman et al., Citation2008). Chitosan derived from shrimps contained 5.24% of moisture and a similar result was obtained for red mason bee cocoons (5.74%).The protein and fiber in chitosan were determined in much smaller amounts than in the examined cocoons (6.16% of protein and 8.76% of fiber in chitosan compared to 52.91% of protein and 30.69% of fiber in the cocoons). The lower protein content of chitosan results from the deproteinization of chitin (Isa et al., Citation2012). Orange fiber contains 7.9% moisture, 8.93% protein, 1.85% lipids, and 63.6% total dietary fiber (de Moraes Crizel et al., Citation2013). The proximate composition analysis showed that the red mason bee cocoons had a comparable amount of moisture (5.74%), a higher amount of protein (52.91%), and fat (3.99%), and a lower amount of dietary fiber (30.69%).The amino acid composition of silk fibers produced by arthropods has a significant impact on the physical properties of silk (Lucas et al., Citation1958). The amounts of amino acids in arthropods, cocoons, or silk in previous research were principally determined for sericin and fibroin (which are the main proteins of the silk produced by silkworms) but defined less frequently for spidroin (which is a silk protein produced by spiders) or for silk produced by wild moths and crickets. Alanine was the second main component of the silk produced by the mulberry silkworm (30% of all amino acids) (Zhou et al., Citation2000). In the silk proteins secreted by crickets from the Gryllacrididae family and spiders, 19% of all the amino acids are composed of alanine (Candelas et al., Citation1990; Walker et al., Citation2012). Regarding glutamic acid, it is present only in silk produced by wild moths and varies from 4 to 6% of all the amino acids in its composition (Rajput & Singh, Citation2015). Aspartic acid, in our own research, accounted for 8.32% of amino acids in the red mason bee cocoons, but did not occur in fibroin (Rajput & Singh, Citation2015) or was found only in small amounts (Dyakonov et al., Citation2012). In the composition of sericin, aspartic acid was the second main amino acid (16.8%). Leucine was 7.96% of the amino acids in the red mason bee cocoons and is present in fibroin at 1.5% (Rajput & Singh, Citation2015). Serine was 7.87% of all the amino acids in red mason bee cocoons. It was the dominant (32%) amino acid of sericin, or according to other sources, the second largest (16–20%) (Gupta et al., Citation2014; Rajput & Singh, Citation2015). Serine was also 17% of the amino acids of silk proteins secreted by crickets from the Gryllacrididae family and 11.4% of the amino acids of fibroin, and 31% of the amino acids of the leading spider silk thread (Okada et al., Citation2008; Walker et al., Citation2012; Zhou et al., Citation2000). Glycine was 7.57% of all the amino acids in the examined cocoons. This amino acid was determined as the main amino acid in the silk produced by silkworms and varies from 43 to 45% (Zhou et al., Citation2000). Lysine was found to be 7.03% in the studied cocoons and was the main component of sericin (Rajput & Singh, Citation2015). Valine was 5.07% of the amino acids in red mason bee cocoons, in fibroin it oscillated around 2.7%, while it was only found in sericin in small amounts (Dyakonov et al., Citation2012; Rajput & Singh, Citation2015). According to our own research, arginine accounted for 4.51% of the amino acids found in the cocoons of the red mason bee. Arginine was 0.59% of the amino acids in fibroin, it was not found in silkworm sericin, but it appeared at 5–15% in the sericin produced by wild moths. Threonine was 4–6% of sericin (Rajput & Singh, Citation2015) and 3.9% of the amino acids in the examined cocoons of red mason bees. Histidine marked at 2.76% in red mason bee cocoons appeared in trace amounts in fibroin (0.1%) and from 4–6% in sericin. Proline accounted for 1.44% of the amino acids in the tested cocoons, in fibroin, it oscillated around1%. Tyrosine, which occurred in the cocoons of red mason bees at 1.07%, constituted 11.2% of fibroin and 4–6% of sericin produced by wild butterflies. 0.63% of the amino acids in the tested material was phenylalanine, which in fibroin was at a level of 1.5%. Isoleucine, methionine, tryptophan, and cysteine were not found in other silk fibers, but they were found at 3.88, 1.72, 1.30, and 0.71% of all amino acids in the cocoons of red mason bees (Dyakonov et al., Citation2012; Gupta et al., Citation2014; Rajput & Singh, Citation2015). All the differences in composition result from the fact that the amounts of amino acids in silk fibers produced by arthropods are very diverse (Lucas et al., Citation1958).The average content of all the determined macroelements (potassium, sodium, calcium, magnesium) and microelements (manganese, nickel, iron, zinc, silver, and copper) in a dose of 10 g of cocoons fulfills or does not exceed the norm for daily nutritional requirements and their levels in food products (Flyvholm et al., Citation1984; Jarosz et al., Citation2017; Ratte, Citation1999). The amount of lead does not exceed the norm included in Commission Regulation (EC) On 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. The results showed that the examined material is rich in protein (53.89%) and crude fiber (30.69%) (consisting of NDF – 54.03% and ADF – 45.97%). As the cocoons have a considerable fiber fraction, after further research they can be used as an alternative source of dietary fiber in human nutrition. Cocoons in the form of powder could find applications in bakery products. The inclusion of additives such as fruit peels, rinds, coffee, silverskin, seeds, or chitosan in bakery products improved the daily dietary fiber intake and thus reduced the risk of constipation, colon cancer, overweight, gallstone formation, atherosclerosis, and hypercholesterolemia (Al-Sayed & Ahmed, Citation2013; Ausar et al., Citation2003; Huang et al., Citation2016; Li & Komarek, Citation2017; Pourfarzad et al., Citation2013; Salgado et al., Citation2011).
Conclusions
The red mason bee cocoons, while not presently having any commercial value, can be a source of useful dietary fiber and, after further research, can find food applications. It could be an effective way of using waste from breeding Osmia bicornis. Furthermore, deriving products from the cocoons does not affect the red mason bees in any way, because this occurs after the bees have emerged. Demand for Osmia bicornis cocoons could contribute to the increased breeding of this useful insect. It appears from the comparison of the obtained results with other sources of dietary fiber in products of insect origin, that the cocoon of Osmia bicornis differs in its composition. However, this fact may indicate its unique properties and does not exclude the possibility of using the examined cocoons, for example, as dietary fiber in human and animal nutrition.
Disclosure statement
The authors declare they have no potential conflict of interest in relation to the study in this paper.
Additional information
Funding
References
- Al-Sayed, H. M. A., & Ahmed, A. R. (2013). Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Annals of Agricultural Sciences, 58(1), 83–95. https://doi.org/10.1016/j.aoas.2013.01.012
- Ausar, S. F., Morcillo, M., León, A. E., Ribotta, P. D., Masih, R., Vilaro Mainero, M., Amigone, J. L., Rubin, G., Lescano, C., Castagna, L. F., Beltramo, D. M., Diaz, G., & Bianco, I. D. (2003). Improvement of HDL- and LDL-cholesterol levels in diabetic subjects by feeding bread containing chitosan. Journal of Medicinal Food, 6(4), 397–399. https://doi.org/10.1089/109662003772519985
- Banaszak, J., & Romasenko, L. (1998). Megachilid Bees of Europe (1st ed., pp. 13–16). Wydawnictwo Uczelniane WSP w Bydgoszczy.
- Biliński, M., & Teper, D. (2004). Rearing and utilization of the red mason bee – Osmia rufa L. (Hymenoptera. Megachilidae) for orchard pollination. Journal of Apicultural Science, 48, 69–74.
- Candelas, G. C., Arroyo, G., Carrasco, C., & Dompenciel, R. (1990). Spider silkglands contain a tissue-specific alanine trna that accumulates in vitro in response to the stimulus for silk protein synthesis. Developmental Biology, 140(1), 215–220. https://doi.org/10.1016/0012-1606(90)90069-u
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 set Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance), Vol. 364.
- de Moraes Crizel, T., Jablonski, A., de Oliveira Rios, A., Rech, R., & Flôres, S. H. (2013). Dietary fiber from orange byproducts as a potential fat replacer. LWT - Food Science and Technology, 53(1), 9–14. https://doi.org/10.1016/j.lwt.2013.02.002
- Dyakonov, T., Yang, C. H., Bush, D., Gosangari, S., Majuru., S., & Fatmi, A. (2012). Design and characterization of a silk-fibroin-based drug delivery platform using naproxen as a model drug. Journal of Drug Delivery, 2012, 490514. https://doi.org/10.1155/2012/490514
- Fisher, B. L., & Robertson, H. G. (1999). Silk production by adult workers of the ant melissotarsusemeryi (Hymenoptera. Formicidae) in South African fynbos. Insect Sociaux, 46, 8–83. https://doi.org/10.1007/s000400050116.
- Flyvholm, M. A., Nielsen, G. D., & Andersen, A. (1984). Nickel content of food and estimation of dietary intake. Z Lebensm Unters Forsch, 179(6), 427–431. https://doi.org/10.1007/BF01043419
- Giejdasz, K., & Wilkaniec, Z. (2002). Individual development of the red mason bee (Osmia rufa L. Megachilidae) under natural and laboratory conditions. Journal of Apicultural Science, 46, 51–57.
- Grześkowiak, J., & Łochyńska, M. (2017). Silk worm (Bombyx mori) – familiar insect with unfamiliar potencial. WiadomościZootechniczne, 1, 101–105.
- Gupta, D., Agrawal, A., & Rangi, A. (2014). Extraction and characterization of silk sericin. Indian Journal of Fibre& Textile Research, 39, 364–372.
- Huang, G., Guo, Q., Wang, C., Ding, H. H., & Cui, S. W. (2016). Fenugreek fibre in bread: Effects on dough development and bread quality. LWT - Food Science and Technology, 71, 274–280. https://doi.org/10.1016/j.lwt.2016.03.040
- Isa, M. T., Ameh, A. O., Tijjani, M., & Adama, K. K. (2012). Extraction and characterization of chitin and chitosan from Nigerian shrimps. International Journal of Biological and Chemical Sciences, 6(1). https://doi.org/10.4314/ijbcs.v6i1.40
- Jarosz, M., Rychlik, E., Stoś, K., Wierzejska, R., Wojtasik, A., Charzewska, J., Mojska, H., Szponar, L., Sajór, I., & &Kłosiewicz-Latoszek, L. (2017). Nutritional standards for the population of Poland. Instytut Żywnościi Żywienia, pp. 289.
- Krunic, M., Pinzauti, M., Felicioli, A., & Stanisavljevic, L. (1995). Further observations on Osmia cornutalatr. and O. rufa l. as alternative fruit pollinators. Domestication and Utilization. Archives of Biological Sciences, 47(1), 59–66.
- Li, Y. O., & Komarek, A. R. (2017). Dietary fibre basics: Health, nutrition, analysis, and applications. Food Quality and Safety, 1(1), 47–59. https://doi.org/10.1093/fqsafe/fyx007
- Lucas, F., Shaw, J. T., & Smith, S. G. (1958). The silk fibroins. Advances in Protein Chemistry, 13, 107–242. https://doi.org/10.1016/s0065-3233(08)60599-9
- Okada, S., Weisman, S., Trueman, H. E., Mudie, S. T., Haritos, V. S., & Sutherland, T. D. (2008). An Australian webspinner species makes the finest known insect silk fibers. International Journal of Biological Macromolecules, 43(3), 271–275. https://doi.org/10.1016/j.ijbiomac.2008.06.007
- Pourfarzad, A., Mahdavian-Mehr, H., & Sedaghat, N. (2013). Coffee silver skin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT - Food Science and Technology, 50(2), 599–606. https://doi.org/10.1016/j.lwt.2012.08.001
- R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/
- Rajput, S. K., & Singh, M. K. (2015). Sericin – A unique biomaterial. International Journal of Polymer and Textile Engineering, 2, 29–35.
- Ratte, H. T. (1999). Bioaccumulation and toxicity of silver compounds: A review. Environmental Toxicology and Chemistry, 18(1), 89–108. https://doi.org/10.1002/etc.5620180112
- Salgado, J. M., Rodrigues, B. S., Donado-Pestana, C. M., dos Santos Dias, C. T., & Morzelle, M. C. (2011). Cupuassu (Theobroma Grandiflorum) peel as potential source of dietary fiber and phytochemicals in whole-bread preparations. Plant Foods for Human Nutrition (Dordrecht, Netherlands), 66(4), 384–390. https://doi.org/10.1007/s11130-011-0254-0
- Shin, S., Yeon, S., Park, D., Oh, J., Kang, H., Kim, S., Joo, S. S., Lim, W.-T., Lee, J.-Y., Choi, K.-C., Kim, K. Y., Kim, S. U., Kim, J.-C., & Kim, Y.-B. (2010). Silk amino acids improve physical stamina and male reproductive function of mice. Biological & Pharmaceutical Bulletin, 33(2), 273–278. https://doi.org/10.1248/bpb.33.273
- Sutherland, T. D., Campbell, P. M., Weisman, S., Trueman, H. E., Sriskantha, A., Wanjura, W. J., & Haritos, V. S. (2006). A highly divergent gene cluster in honey bees encodes a novel silk family. Genome Research, 16(11), 1414–1421. https://doi.org/10.1101/gr.5052606
- Sutherland, T. D., Weisman, S., Walker, A. A., & Mudie, S. T. (2012). Invited review the coiled coil silk of bees, ants, and hornets. Biopolymers, 97(6), 446–454. https://doi.org/10.1002/bip.21702
- Vaithanomsat, P., & Kitpreechavanich, V. (2008). Sericin separation from silk degumming wastewater. Separation and Purification Technology, 59(2), 129–133. https://doi.org/10.1016/j.seppur.2007.05.039
- Vollrath, F. (2000). Strength and structure of spiders’ silks. Journal of Biotechnology, 74(2), 67–83. https://doi.org/10.1016/S1389-0352(00)00006-4
- Walker, A. A., Weisman, S., Church, J. S., Merritt, D. J., Mudie, S. T., & Sutherland, T. D. (2012). Silk from crickets: A new twist on spinning. PLoS One, 7(2), e30408. https://doi.org/10.1371/journal.pone.0030408
- Weisman, S., Trueman, H. E., Mudie, S. T., Church, J. S., Sutherland, T. D., & Haritos, V. S. (2008). An unlikely silk: The composite material of green lacewing cocoons. Biomacromolecules, 9(11), 3065–3069. https://doi.org/10.1021/bm8005853
- Wójtowski, F., & Wilkaniec, Z. (1978). Breeding and use of solitary bees settled in artificial nest. AR w Poznaniu Zakład Upowszechniania Postępu w Rolnictwie.
- Zhou, C. Z., Confalonieri, F., Medina, N., Zivanovic, Y., Esnault, C., Yang, T., Jacquet, M., Janin, J., Duguet, M., Perasso, R., & Li, Z. G. (2000). Fine organization of bombyx mori fibroin heavy chain gene. Nucleic Acids Research, 28(12), 2413–2419. https://doi.org/10.1093/nar/28.12.2413