Abstract
There is clear evidence for global and unsustainable high losses of the managed Western honey bee, Apis mellifera, colonies raising major concerns about the sustainability of pollination services and food security. Here, we address a conflicting selection scenario in workers to better understand life-history trade-offs underlying colony losses. Due to developmental plasticity, annual fluctuations in selection pressures result in long-living winter bees and short-living summer bees as adaptive responses to lack of food and ceased brood rearing in winter, or ample food and rapid brood turnover in spring and summer. Since trait evolution is governed by a balance of conflicting fitness advantages, trade-offs between longevity and other traits are inevitable (e.g. immune defense or detoxification). As worker longevity is essential to ensure colony functionality, these trade-offs render long-living workers more susceptible to the same stressors compared to short-lived ones. It seems as if trade-offs in workers play a previously overlooked key role, and the incorporation of long-living workers in research efforts is long overdue if our aim is to effectively mitigate declines and losses of social insect colonies globally. Indeed, managed honey bee colony losses in temperate regions predominantly occur throughout winter, but research efforts are almost exclusively conducted with short-lived summer bees, thereby sustaining the danger of false negative results. Thus, we should not shy away from meeting the respective logistic challenges. Studies of conflicting selection on workers will be informative about mechanisms underlying life history trade-offs, thereby fostering conservation efforts in the social insects.
For over a decade, there is clear evidence for high global losses of managed Western honey bee, Apis mellifera, colonies (Neumann & Carreck, Citation2010), raising major concerns about the sustainability of pollination services and human food security (Potts et al., Citation2016). However, there is still no sustainable solution at hand, probably because the underlying evolutionary scenario remains elusive and due to the common, but inadequate, focus on short-living summer bees in research. The majority of stressors identified to affect honey bees have been studied in short-living summer bees, even though conflicting selection on workers increases vulnerability in hibernating colonies, i.e. in long-living winter bees. Here, we address for the first time a conflicting selection scenario in honey bee workers to promote a focus on long-living winter bees required to understand life-history trade-offs underlying colony losses. The deceptive focus on summer bees has precluded current research efforts to develop the urgently required protection measures and may therefore constitute one main cause for the ongoing, yet possibly avoidable colony losses.
Due to developmental plasticity in honey bee workers, annual fluctuations in selection pressures result in long-living winter and short-living summer bees (eight months vs. three weeks; Riley, Citation1895) in all honey bee colonies in the temperate regions (Winston, Citation1987). Indeed, these identical looking, but physiologically different bees arise as adaptive responses to a lack of foraging ability and ceased brood rearing for instance during winter, as well as ample food and rapid brood turnover in spring and summer (Knoll et al., Citation2020). Accordingly, these bees differ in many traits, e.g. immune responses (Steinmann et al., Citation2015). Since the evolution of traits is governed by a balance of conflicting fitness advantages (Schluter et al., Citation1991), trade-offs between longevity and other costly traits are inevitable and well known (Flatt & Heyland, Citation2011; Sheldon & Verhulst, Citation1996). In insects, including social insects, such trade-offs have been reported repeatedly, e.g. between detoxification and longevity (Flatt & Heyland, Citation2011) or immune defense and longevity (e.g. in bumble bees (Moret & Schmid-Hempel, Citation2000), in crickets (Jacot et al., Citation2004), in flies (Libert et al., Citation2006), in meal worm beetles (Krams et al., Citation2013). A previous study measuring the cost of immune response in bumble bee workers, Bombus terrestris, revealed a 50 to 70% reduction in longevity in the artificially immune induced individuals compared to the control workers (Moret & Schmid-Hempel, Citation2000). Such drastic reductions in longevity undermine the immense costs of potential trade-offs.
In social bees, it is essential that sufficient individual survival is key to ensure the minimum number of workers required to maintain colony functionality over winter, e.g. for thermoregulation (Filipovic-Moskovljevic, Citation1972). Therefore, these trade-offs between longevity and other traits almost certainly render winter bees more susceptible to the same stressors compared to summer bees (). Indeed, winter bees are more susceptible to ectoparasitic mites Varroa destructor and the insecticide thiamethoxam when combined compared to summer bees (Straub et al., Citation2019). It appears as if such trade-offs in workers play a previously overlooked key role in our understanding of honey bee health in general and colony losses in particular. Phenotypic and transcriptomic comparisons between summer and winter bees will be required to better understand the mechanisms underlying this development plasticity and respective life-history trade-offs.
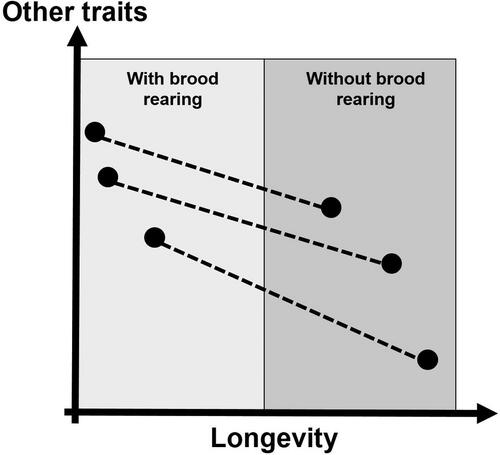
Figure 1. Life history trade-offs between longevity and other traits in social insect workers arising from conflicting selection between periods with brood rearing and without. When colonies rear brood (light grey box), selection on worker longevity is relaxed and other costly traits can be highly expressed if required (e.g. immune defense or detoxification). However, when colonies do not rear brood (darker grey box), worker longevity is under strong selection to maintain the minimum number of workers necessary for colony functionality. Therefore, expression of other traits is compromised. If workers are challenged in such periods without brood rearing colony death is consequently more likely to occur (black dots = phenotypic trait expression, dashed lines = differential trait expression due to conflicting selection).
In light of the consensus that honey bee colony losses in temperate regions predominantly occur throughout winter, it appears inadequate that research efforts are almost exclusively conducted with summer bees rather than winter bees. This is most likely due to the logistic constraints, e.g. reduced availability of winter bees and restrictions in the measurability of parameters. This reflects a major flaw as it ignores the long known differences between summer and winter bees resulting from the conflicting selection. Based on the above reasoning, we here raise caution that the common focus on summer bees sustains the considerable danger of false negative results, i.e. the measured impact of a stressor on summer bees is likely underestimating the actual effects on winter bees and ultimately the entire colony. Thus, we should not shy away from meeting the respective logistic challenges and thereby strengthen the focus on incorporating winter bees if our aim is to mitigate winter colony losses in honey bees. One feasible and promising approach appears to be the season-independent production of artificial winter bees (Fluri et al., Citation1982).
In conclusion, an extensive incorporation of long-living workers in research efforts is long overdue if our aim is to effectively mitigate losses of managed honey bee colonies globally. The evolutionary scenario outlined here may hold true for any period without brood rearing - irrespective of seasonality, degree of sociality and/or insect genera/species. Studies of conflicting selection on workers therefore will be informative about mechanisms underlying life history trade-offs and conservation efforts in the social insects.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Filipovic-Moskovljevic, V. (1972). Social minimum required for particular functions of worker bees (Apis mellifica L.). Acta Veterinaria-Beograd, 22, 167–176.
- Flatt, T., & Heyland, A. (Eds.). (2011). Mechanisms of life history evolution: The genetics and physiology of life history traits and trade-offs. Oxford Univ. Press.
- Fluri, P., Lüscher, M., Wille, H., & Gerig, L. (1982). Changes in weight of the hypopharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. Journal of Insect Physiology, 28(1), 61–68. https://doi.org/https://doi.org/10.1016/0022-1910(82)90023-3
- Jacot, A., Scheuber, H., & Brinkhof, M. W. G. (2004). Costs of an induced immune response on sexual display and longevity in field crickets. Evolution; International Journal of Organic Evolution, 58(10), 2280–2286.
- Knoll, S., Pinna, W., Varcasia, A., Scala, A., & Cappai, M. G. (2020). The honey bee (Apis Mellifera L., 1758) and the seasonal adaptation of productions. Highlights on summer to winter transition and back to summer metabolic activity. A review. Livestock Science, 235, 104011. https://doi.org/https://doi.org/10.1016/j.livsci.2020.104011
- Krams, I., Daukšte, J., Kivleniece, I., Kaasik, A., Krama, T., Freeberg, T. M., & Rantala, M. J. (2013). Trade-off between cellular immunity and life span in meal-worm beetles Tenebrio molitor. Current Zoology, 59(3), 340–346. https://doi.org/https://doi.org/10.1093/czoolo/59.3.340
- Libert, S., Chao, Y., Chu, X., & Pletcher, S. D. (2006). Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell, 5(6), 533–543. https://doi.org/https://doi.org/10.1111/j.1474-9726.2006.00251.x
- Moret, Y., & Schmid-Hempel, P. (2000). Survival for immunity: the price of immune system activation for bumblebee workers. Science, 290(5494), 1166–1168. https://doi.org/https://doi.org/10.1126/science.290.5494.1166
- Neumann, P., & Carreck, N. (2010). Honey bee colony losses. Journal of Apicultural Research, 49(1), 1–6. https://doi.org/https://doi.org/10.3896/IBRA.1.49.1.01
- Potts, S. G., Imperatriz-Fonseca, V., Ngo, H. T., Aizen, M. A., Biesmeijer, J. C., Breeze, T. D., Dicks, L. V., Garibaldi, L. A., Hill, R., Settele, J., & Vanbergen, A. J. (2016). Safeguarding pollinators and their values to human well-being. Nature, 540(7632), 220–229. https://doi.org/https://doi.org/10.1038/nature20588
- Riley, C. V. (1895). Longevity in insects. Proceedings of the Entomological Society of Washington, 3, 108–125.
- Schluter, D., Price, T. D., & Rowe, L. (1991). Conflicting selection pressures and life history trade-offs. Proceedings of the Royal Society B: Biological Sciences, 246, 11–17.
- Sheldon, B. C., & Verhulst, S. (1996). Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology & Evolution, 11(8), 317–321. https://doi.org/https://doi.org/10.1016/0169-5347(96)10039-2
- Steinmann, N., Corona, M., Neumann, P., & Dainat, B. (2015). Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS ONE, 10(6), e0129956. https://doi.org/https://doi.org/10.1371/journal.pone.0129956
- Straub, L., Williams, G. R., Vidondo, B., Khongphinitbunjong, K., Retschnig, G., Schneeberger, A., Chantawannakul, P., Dietemann, V., & Neumann, P. (2019). Neonicotinoids and ectoparasitic mites synergistically impact honeybees. Scientific Reports, 9(1), 8159.
- Winston, M. L. (1987). The biology of temperate and tropical honey bees. In The biology of the honey bee (pp. 214–224). Harvard University Press.
