Abstract
Spring imports of queen honey bees (Apis mellifera L.) are essential to replace winter colony losses in Canada, but contribute to the spread of treatment-resistant strains of pathogens and undesirable genetic traits. A possible alternative to these imports is the mass storage of queens during winter. By overwintering a strong colony (queen bank) containing large numbers of mated queens isolated in cages, beekeepers could acquire local queens early in the spring. In this study, we tested the efficacy of overwintering queen banks at two different queen densities (40 and 80). In the 40-queen banks (40 QB), 74.2% of queens survived the 6-month overwintering period, while 42.1% of queens survived in the 80-queen banks (80 QB). When compared to queens overwintered free in their colony, queens from bank colonies were smaller and lighter in early spring but had similar sperm viability and sperm count. Overwintering queens in banks did not have an impact on their acceptance in a nucleus colony but reduced their oviposition in the initial weeks following their introduction. After several days in nucleus colonies, queens from banks had regained a size and weight similar to that of queens overwintered normally, suggesting that they could perform well over a complete beekeeping season. This study achieved promising results and highlights the potential of this technique for the beekeeping industry in Canada and worldwide.
Introduction
The beekeeping industry is vital to the economy and the environment around the world, but faces several threats such as the spread of honey bee (Apis mellifera L.) parasites and diseases as well as the excessive use of pesticides on crops (Hristov et al., Citation2020; Hung et al., Citation2018). In Canada and regions with a similar climate, the winter period also results in the loss of many colonies. Despite beekeepers’ efforts, between 18 and 45% of colonies die each winter in Canada (Bixby et al., Citation2018). To replace these losses, beekeepers create new colonies in which they introduce a young, mated queen. Moreover, queens are often replaced every year or two to maintain hive productivity (Amiri et al., Citation2017; Szabo & Lefkovitch, Citation1991). Unfortunately, cold weather in early spring, combined with limited food resources in the surrounding environment, significantly limit the production of local queens and drones until late spring (Boes, Citation2010; Neumann et al., Citation1999; Woyke & Ruttner, Citation1958). To satisfy the spring demand for queens, Canadian beekeepers must import queens from other countries (Agriculture and Agri-Food Canada (AAC), Citation2021). Importing queens has some risks, however, such as the dissemination of treatment-resistant strains of pathogens or undesirable genotypes (Kono & Kohn, Citation2015; Mutinelli, Citation2011; Shimanuki & Knox, Citation1997). The industry’s dependence on imports also renders it vulnerable to changes in honey bee import regulations that may be adopted because of exotic pathogen exposure (Manitoba Beekeepers’ Association (MBA), Citation2015) or, more recently, the socio-economic impacts of the COVID-19 pandemic (Agriculture and Agri-Food Canada (AAC), Citation2021; Bixby et al., Citation2021).
A possible alternative to these imports is the mass storage and overwintering of queens in bank colonies (Levesque, Citation2022; Rousseau & Giovenazzo, Citation2021; Wyborn et al., Citation1993). Normally, queens spend the winter free in their colony. In Canada, hives are either covered with insulating material and overwintered outdoors or placed in an environmentally controlled room at about 5 °C. This ensures that bees consume the food supply at an optimal rate and thus increases their chances of surviving the winter (Desai & Currie, Citation2016). During this period, the queen ceases or reduces ovipositing and bees form a cluster around her to generate heat (Mattila et al., Citation2001; Melathopoulos, Citation2007; Merz et al., Citation1979; Seeley & Visscher, Citation1985).
Mass storage of queens during winter is a practice developed recently and is very different from standard overwintering of colonies. This method consists of storing several queens in a single colony, called a bank, during the winter period. The queens are reared in late summer, isolated individually in cages and grouped together on a modified frame. This holding frame is placed in the center of the bank colony and the worker bees care for the queens throughout their storage period by feeding them through the mesh of the cages (Harp, Citation1967; Levinsohn & Lensky, Citation1981; Rousseau & Giovenazzo, Citation2021). Typically, several queens cannot live in the same colony without fighting each other. However, when a physical barrier is maintained between them, as in queen banking, they can coexist for a prolonged period (Farrar, Citation1953; Reid, Citation1975).
To store queens for a full winter, several conditions must be met to ensure their survival. Bank colonies must be populous with a large proportion of young nurse bees to ensure that the queens are sufficiently fed and warmed (Dietz et al., Citation1983; Gençer, Citation2003; Wyborn et al., Citation1993). Indeed, low queen survival in banks has been associated with an insufficient bee population (Gençer, Citation2003). Queen banks must also be initially queenless or with a laying queen isolated from the stored queens, to avoid any aggression toward caged queens (Pettis et al., Citation1998; Robinson, Citation1984). Wyborn et al. (Citation1993) observed a 25% survival rate of queens after six months of storage when bank colonies were queenright, compared to 60% survival in queenless colonies. A similar trend was reported by Gençer (Citation2003), for a study that achieved no queen survival in queenright banks. Furthermore, the mesh of the queen bank cages must allow the workers to feed the queens while preventing the bees from attacking them by pulling their legs, antennae or wings (Free & Butler, Citation1958; Reid, Citation1975; Woyke, Citation1988). An external heat supply also contributes to the success of long-term queen storage, as it induces the cluster to expand and cover a larger area around the group of queens (Rousseau & Giovenazzo, Citation2021). In this way, the banked queens on the periphery have a better chance of being fed and warmed by the workers. If not warmed properly, the banked queens will suffer chill-coma and die (Free & Spencer-Booth, Citation1960). Overwintering banks of 40 queens in an environmentally controlled room set at 15 ± 1 °C was recently tested and showed that 85% of the banked queens survived for five months (Rousseau & Giovenazzo, Citation2021).
Before this technique can be used on a large scale, several other elements remain to be tested to develop a method that consistently achieves a high rate of queen survival. For example, the optimal number of queens that can be stored in a single bank remains unknown. A higher density of queens would be more profitable but could compromise queen survival since more queens would be located at the periphery of the holding frame (Abd Al-Fattah et al., Citation2016; Wyborn, Citation1991). In addition to ensuring queen survival, queen banking must not reduce their reproductive quality or performance in a colony. A high-quality queen is necessary for a colony to produce a large amount of honey, pollinate crops efficiently, resist diseases and survive through the winter (Döke et al., Citation2019; Free & Racey, Citation1968). The few studies available on this subject suggest that banking queens does not compromise their reproductive quality, acceptance in a colony or performance during the beekeeping season (Gençer, Citation2003; Wyborn et al., Citation1993).
The main objective of this research was therefore to test the efficacy of winter mass storage of honey bee queens at two densities inside bank colonies. To achieve this, queens were stored from October 2019 to April 2020. Their survival, reproductive quality and performance in a nucleus colony were measured in early spring. The first research hypothesis tested is that queen survival is influenced by the density of queens in banks, specifically that queens survive better when fewer queens are stored in a bank. The second hypothesis is that the mass storage of queens has no impact on their reproductive quality or on their performance in a nucleus colony.
Materials and methods
Queen rearing and experimental colonies
From August 6 to 27, 2019, 630 honey bee (A. mellifera) queens were produced by three local queen breeders: 30 by our research center (Centre de Recherche en Sciences Animales de Deschambault/CRSAD; http://crsad.qc.ca/, QC, Canada; 46° 67′50.6″ N, 71° 91′44.9″ O), 436 by Miels d’Anicet (https://mielsdanicet.com, QC, Canada; 46° 72′49.4″ N, 75° 43′57.2″ O) and 134 by Rayons de miel (https://rayons-de-miel.business.site/, QC, Canada; 46° 10′83.9″ N, 71° 39′78.2″ O). Queens from each breeder were sister queens from their European-derived stock. They were reared using the Doolittle method and mated in mating nucleus colonies (Büchler et al., Citation2013; Laidlaw & Eckert, Citation1962). Young laying queens were received at our research center on August 27, individually isolated in cages (California Mini Queen Cages, Mann Lake Ltd., Hackensack Minnesota USA #HD-398) and grouped in a cardboard box with attendant workers and sugar candy. They were kept in a dark room at 25 °C until distribution among the various colonies the next day.
In parallel with queen rearing, a total of 30 control colonies and 10 bank colonies were created from the livestock of our research center on August 27. The control colonies consisted of five frame nucleus colonies with four brood frames and one frame of honey and pollen. The bank colonies were created by merging two strong queenless colonies together. Colonies selected for this task had at least 16 brood frames covered with bees, to ensure a strong population of nurse bees throughout overwintering. The colonies were merged by placing them on top of each other and separating them with a minimally pierced sheet of newspaper. This limits the aggressive behavior of the workers towards the foreign colony by allowing the two colonies to mix gradually.
Distribution of queens in colonies and winter preparation
The day after the control and bank colonies were created, the 630 queens were distributed among them (). Ten queens from each of the three breeding centers (total of 30 queens) were introduced randomly, one into each of the 30 control nucleus colonies, by placing a cage containing a queen accompanied by four workers (Jz-Bz Queen Cage, #QC-1100, Propolis etc.) between the two central frames. One of these queens was rejected by the colony in the week following its introduction. The control group thus consisted of 29 nucleus colonies. The remaining 600 queens were randomly placed in 10 modified frames, each with a total of either 40 (40 QB) or 80 (80 QB) individually caged queens. Each frame was subsequently introduced into a bank colony, in the center of the upper brood chamber. The proportion of queens produced by each breeding center was respected for each frame and queens were given a number for future identification purposes.
Figure 1. Schematic representation of the distribution of the 630 queens among the control nucleus colonies (Control), the 40-queen bank colonies (40 QB) and the 80-queen bank colonies (80 QB) on August 28, 2019. In bank colonies, queens are isolated in cages (California Mini Queen Cages, Mann Lake Ltd., Hackensack Minnesota USA #HD-398) and distributed evenly on both sides of the modified holding frames. Each frame is inserted in the center of the upper brood chamber of the bank colony.
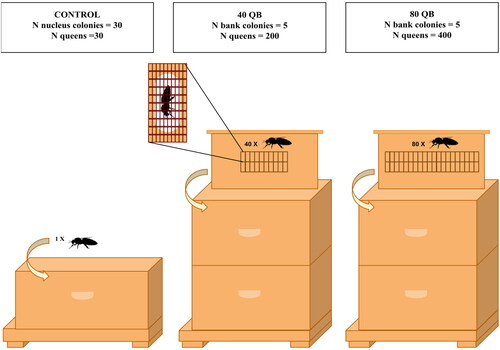
On September 5, data loggers (Onset MX2303A, Hoskin Scientific Ltée, Saint-Laurent, QC, Canada) were placed in the apiary, next to the banked queens, to record temperature and relative humidity during the storage period. All colonies were treated against Varroa destructor with Apivar® as prescribed per label on September 18 (2 strips/brood chamber, 4 strips per bank colony, 1 strip per control colony). At the same time, control and bank colonies were respectively fed 10 L and 32 L of 2:1 sucrose syrup using Miller top feeders (#FE-1100, Propolis etc.). No additional syrup was given throughout the entire protocol (September to April). On October 8, the bank colonies were inspected to assess queen survival before overwintering. All colonies were kept outdoors from the time they were created on August 27 until their transfer into the rooms in which they would be overwintering.
Colony overwintering
The control and bank colonies were placed in an environmentally controlled room on November 5 and October 10 respectively. Control colonies were brought into the environmentally controlled room in November since this represents the time when colonies are normally overwintered. Bank colonies were brought in earlier in order to minimize cluster formation and thus help ensure queen survival. The control colonies were overwintered at 5 ± 1 °C, as recommended by industry standards (Boucher et al., Citation2011), and queen bank colonies were overwintered at 15 ± 1 °C. The choice of this temperature was based on the results of our team’s previous research (Rousseau & Giovenazzo, Citation2021).
Queen survival, morphometrics and fertility
Survival of queens in control and bank colonies was verified in early spring, on April 16. The following week, queen morphometric measurements and fertility were estimated on a sample of randomly selected queens from each overwintering regimes (N = 10 queens per group/2 queens per bank colony).
To measure these traits, queens were firstly euthanized by CO2 overdose. Queens were examined to determine whether they had been injured by the workers’ mandibles during winter. Abdomen width and length were measured using a digital precision scale (Model 93882, Mitutoyo SR44) and body weight was evaluated using a precision scale (Model KHA 203, Kilotech Inc., Quebec City, QC, Canada). Both ovaries were also removed from the abdomen and weighed. To assess fertility, the spermatheca was removed from the abdomen, placed in a 1.5 milliliter (mL) Eppendorf with 1 mL of modified Kiev Buffer (Moritz, Citation1984; 0.3 g D + Glucose, 0.41 g potassium chloride, 0.21 g sodium bicarbonate, 2.43 g sodium citrate 2 hydrate in 100 mL of deionized water) and delicately opened with pliers to liberate sperm. Two dyes were added to this solution, SYBR-14 and propidium iodide (live/dead Sperm Viability Kit, L-7011; Life Technology Inc., Carlsbad, CA, USA). The solution was incubated for 15 minutes at 37 °C and centrifuged. The viability of spermatozoa was measured using a microscope equipped with a fluorescence filter (Zeiss Observer Z1 microscope) at 400× magnification. For each queen, four slides with 10 microliters (μL) of the sperm solution were prepared. Live and dead sperm were counted, up to a total of about 200 sperm per slide, then the mean was calculated for each queen. The total number of sperm present in the spermatheca was estimated using a hemacytometer (Cell-Vu Sperm Counting Chamber, Model DRM 600, Fisher Scientific Ltd.) and an optical microscope at 400× magnification. Four counts were performed for each queen, counting the number of sperm in a 1 mm2 square of the hemacytometer grid. For each queen, the mean was calculated and the total number of sperm cells in the queen’s spermatheca was estimated as described by Rousseau et al. (Citation2015).
Queen introduction in nucleus colonies
On May 13, 2020, 24 nucleus colonies were created from honey bee stock of our research station, each consisting of a brood frame, a food frame (syrup and pollen), an empty frame, approximately 1 kg of worker bees, a feeder frame filled with 1: 1 sucrose syrup and a pollen patty (Global Patties, Alberta, Canada). The next day, surviving queens from the control group were removed from their colony and placed in individual California Mini Queen cages (N = 8). Surviving caged queens from the queen banks were also removed from both bank groups (N = 8 per group). The same day, all queens were randomly distributed and introduced in one of the 24 nucleus colonies by placing the caged queens between the two center frames. Queen introduction success was confirmed by the presence of a laying queen after 12 days, on May 25. On the same date, the development of the nucleus colonies was assessed by measuring the brood area with a staggered grid (Delaplane et al., Citation2013). The brood population (eggs and larvae) was then estimated by converting the brood area to brood cells while considering the number of cells per square inch and the hexagonal shape of the cells (Giovenazzo & Dubreuil, Citation2011). All surviving queens were then euthanized to repeat measurement of the parameters evaluated for queens after overwintering.
Statistical analyses
Statistical analyses were carried out with R software (R Core Team, Citation2021, version 4.1.1) and the results were interpreted with a significance level of 0.05. The survival rates of queens in bank colonies of different densities were transformed using an angular transformation to meet the normality assumption and compared using repeated measures ANOVA.
Queen abdominal width, abdominal length, body weight, ovary weight, sperm viability and sperm count data, both after winter and after 12 days in a colony, were compared between the three groups using linear mixed effect models (lme function). For the ovary weight of queens after 12 days in a colony, values were transformed using a cubic transformation since model residuals did not follow a normal distribution. For all queen reproductive quality traits, queen provenance (different breeders) was included in the models. The original bank colony was also defined as a random effect since several queens were selected from each bank. For queen quality after 12 days in a colony, the strength of the nucleus colony before the introduction of the queens, estimated by the number of frames covered by bees, was also included in the models. When a significant difference was found, pairwise comparisons using adjusted Tukey tests were performed to see where the difference occurred (emmeans and pairs functions).
The success of queen introduction in the nucleus colonies was analyzed with a binomial-response generalized linear model with bias reduction (brglm function). The brood population between the various groups was compared using a linear mixed effect model. When necessary, adjusted Tukey tests were performed. Colony strength was included in the models and the original bank colony was defined as a random effect.
The correlations between all numeric variables (queen quality traits and brood population) were estimated separately on queens after they had overwintered and after 12 days in a colony and were measured with Spearman rank correlation tests. The effect of the experimental group on each variable was removed before performing the correlation tests.
Results
Queen survival
All 29 control queens survived from their introduction on August 27 to the end of overwintering on April 16 (N = 29). Since queen survival in bank colonies was assessed at two different times (October 8 and April 16), the queen survival rate in banks was calculated for two periods: from August 27 to October 8 (outdoor banking period) and from October to April 16 (overwintering period). For the outdoor banking period, queen survival was similar between the two groups of different queen densities (F = 1.54, p = 0.16). For the overwintering period, queen survival was significantly higher for the 40 QB than for the 80 QB (F = 3.78, p < 0.01) ().
Table 1. Queen survival in bank colonies at two different queen densities, 40 and 80 (N = 5 banks per group) from their creation until the end of the overwintering period (August 27, 2019 to April 16, 2020).
Temperature and relative humidity inside bank colonies
Between the installation of data loggers on September 5 and their entry in the environmentally controlled room on October 10, the temperature and relative humidity inside the hives fluctuated according to the outside conditions. After October 10, the temperature and relative humidity stabilized gradually in both groups. The two queen densities, 40 QB and 80 QB, maintained similar average daily temperatures, both starting at 26 °C and progressively rising to an average of 29.6 °C and 29.1 °C, respectively. Temperature remained stable between December 2019 and April 2020. Relative humidity gradually decreased from about 60% on October 10 to 35% for both experimental groups. For a detailed representation of temperature and relative humidity variations inside banks, see supplementary figure (Figure S1).
Queen morphometrics and fertility after winter
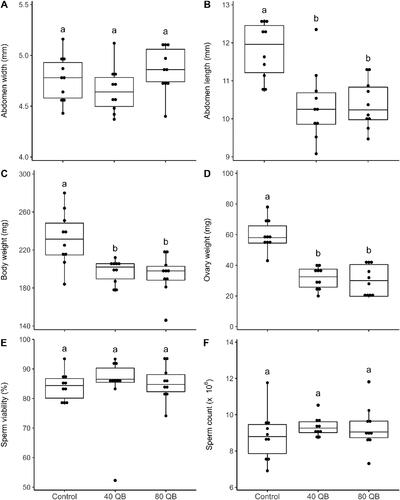
Immediately after assessing queen survival on April 16, several anatomical characteristics were measured on queens from each experimental group in order to estimate their reproductive quality. Abdominal width was not different between groups (F = 1.86, p = 0.19) (). The other three anatomical measures were significantly different between the control queens and those from the banks at two different queen densities: abdominal length, body weight as well as ovary weight (). Control queens had significantly longer abdomens, were heavier and had heavier ovaries compared to banked queens (respectively: F = 1.86, p = 0.19: F = 9.36, p < 0.01: F = 29.69, p < 0.0001). Abdominal length was positively correlated with abdominal width (rs = 0.54, p < 0.01) and body weight (rs = 0.48, p < 0.01). Body weight was also positively correlated with abdominal width (rs = 0.38, p = 0.04) and ovary weight (rs = 0.73, p < 0.0001). There was no difference in sperm viability (F = 1.60, p = 0.23) or sperm count (F = 0.60, p = 0.56) (). Sperm viability was between 80 and 90% for all groups, and sperm count was between 8 and 9 million sperm cells for all groups as well. For all traits measured, no significant difference was observed between the queens banked at two different densities. No injuries to the queens were observed.
Figure 2. Queen reproductive quality traits measured on April 16, 2020, after the 6-month overwintering period: abdomen width (mm) (A), abdomen length (mm) (B), body weight (mg) (C), ovary weight (mg) (D), sperm viability (%) (E) and sperm count (×10ε) (F) (mean ± SE) of control queens, queens from 40 QB and 80 QB (N = 10 per group). The scale of the y-axis of the A, B, C, E and F graphs does not start at zero in order to adequately show the values and their standard errors. Statistically different groups are represented by different letters above values (p ≤ 0.05).
Queen acceptance and performance in nucleus colonies
Twelve days after their introduction into 24 nucleus colonies, a total of 20 queens had been accepted: eight from control colonies, five from the 40 QB and seven from the 80 QB. Although a few queens had been rejected, the acceptance of queens was not significantly different between the control queens and those from the 40 QB (z ratio = 1.24, p = 0.43) or 80 QB (z ratio = 0.70, p = 0.76). All accepted queens were laying after 12 days and young uncapped larvae were present. However, the control queens produced significantly more brood (eggs and larvae) than those from the 40 QB (t ratio = 3.03, p = 0.02). Control queens produced an average of 2438 ± 309 (X ± SE) brood cells, while queens from 40 QB produced an average of 1031 ± 413 brood cells. No significant difference was observed between the brood population of control queens and that of the queens from 80 QB, which produced an average of 1668 ± 357 (t ratio = 1.73, p = 0.23).
Queen morphometrics and fertility after introduction in nucleus colonies
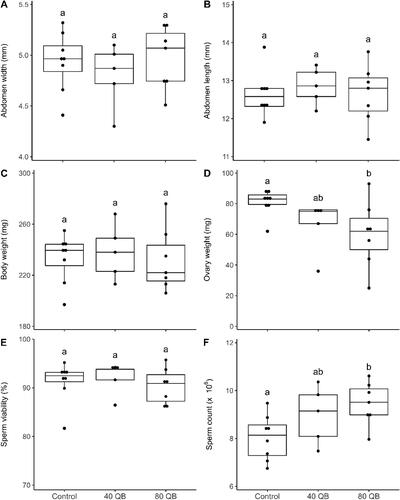
After 12 days in nucleus colonies, queen abdominal width (F = 0.47, p = 0.64), abdominal length (F = 0.16, p = 0.86) and body weight (F = 0.08, p = 0.93) were equivalent between all groups (). Ovary weight was significantly higher for control queens compared to queens from 80 QB (t ratio = 2.78, p = 0.04), but was equivalent to those from 40 QB (t ratio = 1.66, p = 0.25) (). Ovaries of control queens were approximately 14 mg heavier than queens from 80 QB. The abdominal length was again positively correlated with body weight (rs = 0.74, p < 0.001) and was correlated with ovary weight (rs = 0.69, p < 0.001). Body weight was also positively correlated with ovary weight (rs = 0.86, p < 0.0001). Sperm viability was above 91% for all groups of queens and was statistically equivalent between all overwintering regimes (F = 0.30, p = 0.75) (). The sperm count of control queens was significantly lower than that of the queens from 80 QB (t ratio = −2.78, p = 0.04), but was equivalent to that of queens from 40 QB (t ratio = −1.42, p = 0.36) (). No significant correlation was found between the brood population and any of the queen reproductive quality traits.
Figure 3. Queen reproductive quality traits measured on May 25, 2020, 12 days after their introduction in nucleus colonies: abdomen width (mm) (A), abdomen length (mm) (B), body weight (mg) (C), ovary weight (mg) (D), sperm viability (%) (E) and sperm count (x10ε) (F) (mean ± SE) of control queens (N = 8), queens from 40 QB (N = 5) and 80 QB (N = 7). The scale of the y-axis of the A, B, C, E and F graphs does not start at zero in order to adequately show the values and their standard errors. Statistically different groups are represented by different letters above values (p ≤ 0.05).
Discussion
In this research, we tested the efficacy of winter mass storage of queens in bank colonies at two densities. We measured queen survival rate, reproductive quality and performance in a colony. We also studied the correlations between morphology, fertility and performance of queens. Our results show that queen survival through the winter was significantly reduced at the higher queen density in bank colonies. Moreover, we observed that while storing queens in bank colonies reduced their weight and size, these physiological changes were temporary. Banking queens did not affect the content of their spermatheca nor their acceptance in nucleus colonies. However, stored queens produced less brood than control queens after the first 12 days following their introduction in nucleus colonies in spring. No correlation was found between queen reproductive quality traits and their performance in a nucleus colony.
Effect of queen density in bank colonies
The significantly higher winter survival rate of queens in the 40 QB suggests that queen density in banks has an impact on their long-term survival. For the 6-month winter period, queens from 40 QB attained a survival rate of 74.2%, while 42.1% of queens survived in 80 QB. This concurs with the results of other studies showing that stored queens located at the ends of the frame are abandoned more often by the workers than queens located in the center (Abd Al-Fattah et al., Citation2016; Gençer, Citation2003; Wyborn, Citation1991). For example, Sharaf (Citation2016) observed that queens stored in the center of the frame were heavier than those on the periphery, thus suggesting that queens in the middle received more care. Queens on the periphery are then less likely to be adequately fed and heated, which undermines their survival and reproductive quality. Since queens occupied a larger area of the frame at a higher density, more queens were present on the periphery of our 80-queen frames. Although an environmentally controlled room temperature of 15 °C limits cluster formation inside bank colonies, some clustering of bees can still be observed at this temperature (Sumpter & Broomhead, Citation2000). It is therefore possible that workers had difficulty covering and warming the entire 80-queen frame. The location of the surviving queens on the frames with 80 queens supports this theory since most of these queens were close to each other. This may suggest that workers could not cover the entire 80-queen frame and therefore cared for a group of queens that were near each other.
On average, for the 8-month storage period, the workers seem to be able to efficiently care for 28 queens in a 40-queen bank and 30 queens in an 80-queen bank. It would therefore be interesting to test a density of 30 queens, to see whether the queen survival rate would be higher. Also, since queen survival was similar between the two groups for the initial outdoor banking period (from August to October), assessing queen survival at different times during the winter would be relevant, to determine at what point queen survival in the 80 QB begins to differentiate from that of the 40 QB. This could provide information on how to improve our high-density bank system.
Although the queen survival rate was not the same for the two queen densities during winter, the temperature and relative humidity inside bank colonies of both groups were similar. All colonies were able to raise the temperature above ambient temperature. This likely reflects the fact that the bee population in both groups was approximately the same size. By vibrating their wing muscles, bees can raise the temperature and control the humidity inside their hive (Free & Spencer-Booth, Citation1960; Stabentheiner et al., Citation2003). Thus, the number of queens stored does not seem to have an impact on the temperature and relative humidity in a bank colony. It is important to note, however, that the data loggers were placed in the middle of the frame holding the queens, such that conditions may not have been the same at the hive periphery. It would therefore be interesting to install data loggers at several locations in the bank colonies, in order to test the hypothesis that queens at the periphery of the frames receive less care than those with a central position. Based on the results obtained in this study, 40 queens seem to be an appropriate number of queens to store in banks in order to achieve a high survival rate.
Influence of banking on queen reproductive quality
After overwintering, queen abdomen length, body weight and ovary weight revealed that queens overwintered in bank colonies were significantly smaller and lighter than control queens. This is consistent with the results of several studies that observed that confining queens in cages temporarily reduced their size and weight (Cobey, Citation2007; Sharaf, Citation2016; Szabo, Citation1975). Indeed, Szabo (Citation1975) noted a reduction in queen weight of 38 mg after a 3-day incubator confinement. Similarly, Sharaf (Citation2016) found that queens stored in a cage for 75 days were significantly lighter than queens isolated in a queen-excluder, where they could move freely on a frame. The confinement of the queens and the forced cessation of egg-laying thus seem to have a negative impact on their size and weight.
Unlike banked queens, control queens could move freely in their respective colonies. They resumed laying in late winter in order to generate new brood before spring (Mattila et al. Citation2001). Their ovaries were therefore active and thus heavier, which influenced queen weight and abdomen length. Indeed, the development of a queen’s ovaries varies with the intensity of her oviposition. In winter, when egg-laying temporarily ceases, her ovaries are less developed. This change is also accompanied by a significant decrease in fat-body protein (Shehata et al., Citation1981). It is thus normal for queens overwintered free in their colony to be larger and heavier than queens confined to a cage for eight months.
Even though our stored queens were smaller upon overwintering, their spermatheca content appears to have remained intact, since sperm viability and total sperm count were equivalent in all groups. This suggests that the reproductive potential of stored queens would be equivalent to standard overwintered queens and that they could perform similarly in a colony. It is also interesting to observe that the density of queens stored in bank colonies did not influence the quality of surviving queens, since all measured traits were equivalent in the two groups of banked queens. Developing an effective method of storing more queens in a colony while maintaining their quality would then be relevant since it would have a great economic advantage. We could, for example, try dividing 80 queens over two frames, so that fewer queens are on the periphery of the holding frames.
Queen quality assessment also revealed that abdomen size, body weight and ovary weight were often intercorrelated. This is to be expected, since the reproductive organs of a mated queen take up most of the space in her abdomen (Hatjina et al. 2014). Therefore, a queen with heavier ovaries usually has a longer abdomen and is heavier (Es’kov & Es’kova, Citation2013; Prešern & Smodiš Škerl, Citation2019). Several other studies found similar correlations (Amiri et al., Citation2017; Delaney et al., Citation2011; Woyke, Citation1971). These results indicate that a single measurement on the external anatomy of queens would be sufficient to estimate other morphological traits. This would reduce the handling required to assess queen reproductive quality.
Influence of banking on queen acceptance and performance
There was no difference in queen acceptance between the three groups. Similar results have been obtained in other studies, which showed that stored queens were readily accepted following their introduction and that their acceptance rate was the same as that of queens overwintered free in their colony (Gençer, Citation2003; Levinsohn & Lensky, Citation1981; Poole et al., Citation1973). In our study, although acceptance was statistically similar between groups, we observed that a few stored queens were rejected by their colony, while all control queens were accepted. This outcome could be due to the fact that control queens were transferred into new cages before being introduced into a new colony, having originally been free in their colony, while banked queens were kept in the same cage in which they overwintered. After winter, queen cages are sometimes soiled with bee droppings and likely permeated with odors and pheromones from other bees and queens of their bank colony. These factors may have contributed to the rejection of some stored queens since pheromones play a crucial role in the success of the introduction of a queen into a hive (Pettis et al., Citation1998; Yadava & Smith, Citation1971). It is also possible that the reduced size and weight of stored queens had a negative impact on their introduction since lighter queens generally have a lower acceptance rate. However, available studies correlating queen weight with colony acceptance do not involve queens that have been confined for a period of time. The literature on this subject rather concerns the weight of queens at emergence or after mating (Mattila et al., Citation2001; Nelson & Gary, Citation1983). Thus, it is unclear whether a reduction in queen weight due to confinement would affect their acceptance in a colony.
Research generally shows that queen performance is not affected by long-term storage (Gençer, Citation2003; Wyborn et al., Citation1993), paralleling findings on queen acceptance. Notably, Gençer (Citation2003) assessed queen performance for three months and observed no difference in the number of bee frames and brood area between colonies with queens overwintered in bank colonies and those with queens overwintered normally. Another study showed that queen performance was similar between the different overwintering regimes for two consecutive years (Wyborn et al., Citation1993). In our study, the brood population of control colonies was superior to that of colonies with banked queens after 12 days. As explained previously, queens overwintered free in their colony resume laying in late winter to increase the bee population. It is therefore possible that control queens began ovipositing immediately after being released from their cages. The stored queens were confined for eight months in their respective cages, and, once released, may have taken more time than the control queens to begin laying, since their ovaries had been inactive throughout that period. Since many studies have found no difference in the performance of queens under different overwintering methods, it is likely that the brood population would have evened out between groups over the course of the beekeeping season. Nevertheless, future studies should be undertaken to verify this assumption and improve our knowledge on long-term storage of queens.
Queen reproductive quality after introduction in nucleus colonies
After 12 days in nucleus colonies, accepted queens were removed from the colony to assess their reproductive quality. At this moment, abdomen size and body weight were equal between stored and control queens. The only parameter that was lower for stored queens was ovary weight, which had nonetheless increased compared to the measures taken on queens after winter. Their release from the cage and egg-laying seems to have generally reversed the physiological changes they underwent during storage in bank colonies. These results indicate that the size reduction observed in banked queens after winter is temporary and probably caused by their confinement in cages. As mentioned above, the cessation of oviposition in queens causes several physiological changes, such as a reduction in body weight and ovary size (Shehata et al., Citation1981). Confining queens in a cage for eight months forces cessation of egg-laying. As they resume laying, the morphology of the queens returns to normal. Following this logic, had the stored queens been left in their colony for a longer period, their ovaries might have reached weights comparable to those of the control group.
The sperm count was higher for queens from 80 QB than for control queens. Control queens may have depleted more sperm from their spermatheca after they resumed ovipositing in late winter. They had been laying eggs for several months before their introduction in a new nucleus colony in spring, unlike the banked queens which were confined in cages. However, further studies are needed to confirm these results.
We found no significant correlation between any of the queen quality traits and the brood population in nucleus colonies. Correlations might have been observed if queens had spent a complete beekeeping season in their colony. Indeed, available studies show that the performance of queens depends on their quality. Colonies with a high-quality queen generally have a higher bee population, produce more honey, have a higher pollination potential and resist diseases well (Akyol et al., Citation2008; Rangel et al., Citation2013).
Study limitations
Although the findings of this study are promising, care must be taken when extrapolating these data. Storing queens in bank colonies is a recent practice and results are not consistent across the available literature. The method used to store queens on a long-term basis differs from study to study, resulting in a great deal of variation in the data on survival rate of queens as well as the effect of storage on their quality. Much more information on this technique is required in order to improve it. Furthermore, although queen storage in bank colonies can be practiced anywhere in the world, it is important to consider that the manipulations in this study took place under the cold temperate climate of eastern Quebec.
Moreover, the number of colonies used in spring 2020 to assess queen acceptance and performance was low. This reduces the power of our statistical analyses and increases the margin of error. Queen performance in nucleus colonies was measured after a period of 12 days in order to verify acceptance and short-term reproduction capacity. Therefore, the results do not represent a complete Canadian beekeeping season and could have been different if the queens had been left in their colony until the end of summer. Correlations between queen quality traits and colony performance could also have appeared. It would therefore be relevant to test the performance of queens during a complete beekeeping season, by evaluating several parameters such as the brood and bee population in the hives, honey production, the incidence of diseases and parasites and winter survival. It would also be important to transfer the stored queens into new cages before their introduction, to limit the presence of foreign odors and pheromones. Other elements of the method that remain to be tested include the impact of supplying the banks with brood or young nurse bees during the winter period. It would also be interesting to assess the abundance of Varroa mites and Nosema (Nosema ceranae) spores in banks, to see whether queen survival and quality can be influenced by the health of the colony in which they are stored.
This study demonstrates the potential of queen mass storage for the Canadian beekeeping industry. Using this technique, beekeepers could breed queens when conditions are favorable and store them in bank colonies until they are needed. Beekeepers could then use these queens to compensate for their winter losses or simply to replace old or inefficient queens. Once commercialized, this method would significantly reduce bee imports and thus minimize the associated risks. Its use would also promote the self-sufficiency of the local beekeeping industry as well as the development of Canadian bee genetics adapted to local conditions.
Figure S1
Download MS Word (663.8 KB)Acknowledgements
The authors would like to thank the beekeeping and administrative team of the Centre de Recherche en Sciences Animales de Deschambault. A special thanks is given to Émile Houle who was involved in data collection and building the queen holding frames, and to queen breeders Anicet Desrochers and Maggie Lamothe-Boudreau for their contribution of mated queens. We also thank Gaétan Daigle for his guidance in data analysis, and Karen Grislis for revising the syntaxis and grammar of the paper.
Data availability statement
The data that support the findings of this study are available from the corresponding author, P.G., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abd Al-Fattah, M., E-D, H., Sharaf., & Y., Ibrahim. (2016). Factor affecting the quality of mated honey bee queens stored for different periods in queen-right bank colonies. Effect of cage level and position on holding frame. Journal of Apicultural Research, 55(4), 284–291. 10.1080/00218839.2016.1243296
- Agriculture and Agri-Food Canada (AAC). (2021). Statistical overview of the Canadian honey and bee industry. https://agriculture.canada.ca/sites/default/files/documents/2021-10/honey_report_2020-eng.pdf
- Akyol, E., Yeninar, H., & Kaftanoglu, O. (2008). Live weight of queen honey bees (Apis mellifera L.) predicts reproductive characteristics. Journal of the Kansas Entomological Society, 81(2), 92–100. https://doi.org/10.2317/JKES-705.13.1
- Amiri, E., Strand, M. K., Rueppell, O., & Tarpy, D. R. (2017). Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects, 8(2), 48. https://doi.org/10.3390/insects8020048
- Bixby, M., Guama, M. M., Hoover, S. E., Pernal, S. F. (2018). Canadian honey bee queen bee breeders’ reference guide. http://honeycouncil.ca/wp-content/uploads/2018/12/FinalQueenBreederReferenceGuide2018.pdf
- Bixby, M. E. F., Polinsky, M., Scarlett, R., Higo, H., Common, J., Hoover, S. E., Foster, L. J., Zayed, A., Cunningham, M., & Guarna, M. M. (2021). Impacts of COVID-19 on Canadian beekeeping: Survey results and a profitability analysis. Journal of Economic Entomology, 20, 1–10. https://doi.org/10.1093/jee/toab180
- Boes, K. E. (2010). Honeybee colony drone production and maintenance in accordance with environmental factors: An interplay of queen and worker decisions. Insectes Sociaux, 57(1), 1–9. https://doi.org/10.1007/s00040-009-0046-9
- Boucher, C., Desjardins, F., Giovenazzo, P., Marceau, J., Pettigrew, A., Tremblay, H., & Tremblay, N. (2011). Gestion Optimale du Rucher (2nd ed.). CRAAQ.
- Büchler, R., Andonov, S., Bienefeld, K., Costa, C., Hatjina, F., Kezic, N., Kryger, P., Spivak, M., Uzunov, A., & Wilde, J. (2013). Standard methods for rearing and selection of Apis mellifera queens. Journal of Apicultural Research, 52(1), 1–30. https://doi.org/10.3896/IBRA.1.52.1.07
- Cobey, S. W. (2007). Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance. Apidologie, 38(4), 390–410. https://doi.org/10.1051/apido:2007029
- Delaney, D. A., Keller, J. J., Caren, J. R., & Tarpy, D. R. (2011). The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie, 42(1), 1–13. https://doi.org/10.1051/apido/2010027
- Delaplane, K. S., Dag, A., Danka, R. G., Freitas, B. M., Garibaldi, L. A., Goodwin, R. M., & Hormaza, J. I. (2013). Standard methods for estimating strength parameters of Apis mellifera colonies. Journal of Apicultural Research, 52(4), 1–28. https://doi.org/10.3896/IBRA/1.52.1.03
- Desai, S. D., & Currie, R. W. (2016). Effects of wintering environment and parasite-pathogen interactions on honeybee colony loss 387 in North Temperate regions. PloS One, 11(7), e0159615. doi: 10.1371/journal.pone.0159615
- Dietz, A., Wilbanksx, T. W., & Wilbanks, W. G. (1983). Investigations on long-term queen storage in a confined system. Apiacta, 28, 67–70.
- Döke, M. A., McGrady, C. M., Otieno, M., Grozinger, C. M., & Frazier, M. (2019). Colony size, rather than geographic origin of stocks, predicts overwintering success in honey bees (Hymenoptera:Apidae) in the Northeastern United States. Journal of Economic Entomology, 112(2), 525–533. doi:10.1093/jee/toy377
- Es’kov, E. K., & Es’kova, M. D. (2013). Factors influencing wing size and body weight variation in the western honeybee. Russian Journal of Ecology, 44(5), 433–438. https://doi.org/10.1134/S1067413613050056
- Farrar, C. L. (1953). Two-queen colony management. Bee World, 34(10), 189–194. https://doi.org/10.1080/0005772X.1953.11094821
- Free, J. B., & Butler, C. G. (1958). The size of apertures through which worker honeybees will feed one another. Bee World, 39(2), 40–42. https://doi.org/10.1080/0005772X.1958.11095034
- Free, J. B., & Racey, P. A. (1968). The effect of the size of honeybee colonies on food consumption, brood rearing and the longevity of the bees during winter. Entomologia Experimentalis Et Applicata, 11(2), 241–249. https://doi.org/10.1111/j.1570-7458.1968.tb02048.x
- Free, J. B., & Spencer-Booth, Y. (1960). Chill-coma and cold death temperatures of Apis mellifera. Entomologia Experimentalis Et Applicata, 3(3), 222–230. https://doi.org/10.1111/j.1570-7458.1960.tb00451.x
- Gençer, H. (2003). Overwintering of honey bee queen en mass in reservoir colonies in a temperate climate and its effect on queen performance. Journal of Apicultural Research, 42(4), 61–64. https://doi.org/10.1080/00218839.2003.11101094
- Giovenazzo, P., & Dubreuil, P. (2011). Evaluation of spring organic treatments against Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies in eastern Canada. Experimental & Applied Acarology, 55(1), 65–76. doi:10.1007/s10493-011-9447-3
- Harp, E. R. (1967). Storage of queen bees. American Bee Journal, 107, 250–251.
- Hatjina, F., Bieńkowska, M., Charistos, L., Chlebo, R., Costa, C., Dražić, M. M., Filipi, J., Gregorc, A., Ivanova, E. N., Kezić, N., Kopernicky, J., Kryger, P., Lodesani, M., Lokar, V., Mladenovic, M., Panasiuk, B., Petrov, P. P., Rašic, S., Skerl, M. I. S., Vejsnaes, F., & Wilde, J. (2014). A review of methods used in some European countries for assessing the quality of honey bee queens through their physical characters and the performance of their colonies. Journal of Apicultural Research, 53(3), 337–363. https://doi.org/10.3896/IBRA.1.53.3.02
- Hristov, P., Shumkova, R., Palova, N., & Neov, B. (2020). Factors associated with Honey bee colony losses: A mini-review. Veterinary Sciences, 7(4), 166. https://doi.org/10.3390/vetsci7040166
- Hung, K. L. J., Kingston, J. M., Albrecht, M., Holway, D. A., Kohn, J. R. (2018). The worldwide importance of honey bees as pollinators in natural habitats. Proceedings of the Royal Society B-Biological Sciences, 285, 2017–2140. https://doi.org/10.1098/rspb.2017.2140
- Kono, Y., & Kohn, J. R. (2015). Range and frequency of africanized honey bees in California (USA). PLoS One, 10(9), e0137407. doi:10.1371/journal.pone.0137407
- Laidlaw, H. H., Jr., & Eckert, J. E. (1962). Queen rearing. University of California Press.
- Levesque, M. (2022). Effets de l‘hivernage en banques sur la survie et la qualité reproductive des reines d‘abeilles mellifères (Apis mellifera L.). Mémoire de maitrise. Université Laval. http://hdl.handle.net/20.500.11794/73071
- Levinsohn, M., & Lensky, Y. (1981). Long-term storage of queen honeybees in reservoir colonies. Journal of Apicultural Research, 20(4), 226–233. https://doi.org/10.1080/00218839.1981.11100501
- Manitoba Beekeepers’ Association (MBA). (2015). Importation of packages honey bees from California, United States to Manitoba, Canada. http://manitobabee.org/hive/wp-content/uploads/2015/01/WhitePaper_CaliforniaPackagedHoneybeeImportation_v7.1.pdf
- Mattila, H., Harris, J., & Otis, G. (2001). Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux, 48(2), 88–93. https://doi.org/10.1007/PL00001764
- Melathopoulos, A. (2007). The biology and management of colonies in winter. CAPA.
- Merz, R., Gerig, L., Wille, H., & Leuthold, R. (1979). The problem of short- and long-lived bees in the pre- and post-wintering phase of the honeybee colony (Apis mellifera L.): A study of behaviour. Revue Suisse De Zoologie, 86, 663–671. https://doi.org/10.5962/bhl.part.82329
- Moritz, R. F. A. (1984). The effect of different diluents on insemination success in the honeybee using mixed semen. Journal of Apicultural Research, 23(3), 164–167. https://doi.org/10.1080/00218839.1984.11100626
- Mutinelli, F. (2011). The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Revue Scientifique Et Technique (International Office of Epizootics), 30(1), 257–271. doi:10.20506/rst.30.1.2033
- Nelson, D. L., & Gary, N. E. (1983). Honey productivity of honeybee colonies in relation to body weight, attractiveness and fecundity of the queen. Journal of Apicultural Research, 22(4), 209–213. https://doi.org/10.1080/00218839.1983.11100589
- Neumann, P., Moritz, R. F. A., & van Praagh, J. (1999). Queen mating frequency in different types of honey bee mating apiaries. Journal of Apicultural Research, 38(1-2), 11–18. https://doi.org/10.1080/00218839.1999.11100990
- Pettis, J. S., Westcott, L. C., & Winston, M. L. (1998). Balling behaviour in the honey bee in response to exogenous queen mandibular gland pheromone. Journal of Apicultural Research, 37(2), 125–131. https://doi.org/10.1080/00218839.1998.11100964
- Poole, H. K., Edwards, J. F., Taber, S., & Mills, J. P. (1973). Storage of honeybee queens in the laboratory: An appraisal. The American Bee Journal, 113, 376–378.
- Prešern, J., & Smodiš Škerl, M. I. (2019). Parameters influencing queen body mass and their importance as determined by machine learning in honey bees (Apis mellifera carnica). Apidologie, 50(5), 745–757. https://doi.org/10.1007/s13592-019-00683-y
- R Core Team. (2021). R: A language and environment for statistical computing.
- Rangel, J., Keller, J. J., & Tarpy, D. R. (2013). The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insectes Sociaux, 60(1), 65–73. https://doi.org/10.1007/s00040-012-0267-1
- Reid, M. (1975). Storage of queen bees. Bee World, 56(1), 21–31. https://doi.org/10.1007/s00040-012-0267-1
- Robinson, G. E. (1984). Worker and queen honey bee behavior during the balling of foreign queens. Insectes Sociaux, 31(3), 254–263. https://doi.org/10.1007/BF02223610
- Rousseau, A., & Giovenazzo, P. (2021). Succesful indoor mass storage of honeybee queens (Apis mellifera) during winter. Agriculture, 11(5), 402. https://doi.org/10.3390/agriculture11050402
- Rousseau, A., Fournier, V., & Giovenazzo, P. (2015). Apis mellifera (Hymenoptera: Apidae) drone sperm quality in relation to age, genetic line, and time of breeding. The Canadian Entomologist, 47, 1–10. https://doi.org/10.4039/tce.2015.12
- Seeley, T. D., & Visscher, P. K. (1985). Survival of honeybees in cold climates: The critical timing of colony growth and reproduction. Ecological Entomology, 10(1), 81–88. https://doi.org/10.1111/j.1365-2311.1985.tb00537.x
- Sharaf, H. (2016). Honeybee queens’ performance in relation to their long period storage in queen-right colonies. Cairo University https://doi.org/10.13140/RG.2.2.16521.85602
- Shehata, S. M., Townsend, G. F., & Shuel, R. W. (1981). Seasonal physiological changes in queen and worker honeybees. Journal of Apicultural Research, 20(2), 69–78. https://doi.org/10.1080/00218839.1981.11100475
- Shimanuki, H., & Knox, D. A. (1997). Bee health and international trade in Contamination of animal products: Prevention and risks for animal health. International Office of Epizootics, 16, 172–176. https://doi.org/10.20506/rst.16.1.1008
- Stabentheiner, A., Pressl, H., Papst, T., Hrassnigg, N., & Crailsheim, K. (2003). Endothermic heat production in honeybee winter clusters. The Journal of Experimental Biology, 206(Pt 2), 353–358. doi:10.1242/jeb.00082
- Sumpter, D. J. T., & Broomhead, D. S. (2000). Shape and dynamics of thermoregulating honey bee clusters. Journal of Theoretical Biology, 204(1), 1–14. doi:10.1006/jtbi.1999.1063
- Szabo, T. I. (1975). Overwintering of honeybee queens. 1. Maintenance of honeybee queens in solitary confinement. Journal of Apicultural Research, 14(2), 69–74. https://doi.org/10.1080/00218839.1975.11099805
- Szabo, T. I., & Lefkovitch, L. P. (1991). Development of overwintering honey bee colonies with one- and two-year-old queens. Bee Science, 1, 144–150.
- Woyke, J. (1971). Correlations between the age at which honeybee brood was grafted, characteristics of the resultant queens, and results of insemination. Journal of Apicultural Research, 10(1), 45–55. https://doi.org/10.1080/00218839.1971.11099669
- Woyke, J. (1988). Problems with queen banks. American Bee Journal, 128, 276–278.
- Woyke, J., & Ruttner, F. (1958). An anatomical study of the mating process in the honeybee. Bee World, 39(1), 3–18. https://doi.org/10.1080/0005772X.1958.11095028
- Wyborn, M. (1991). Mass storage of honey bee queens during the winter. Simon Fraser University.
- Wyborn, M. H., Winston, M. L., & Laflamme, P. H. (1993). Mass storage of honey bee (Hymenoptera: Apidae) queens during the winter. The Canadian Entomologist, 125(1), 113–128. https://doi.org/10.4039/Ent125113-1
- Yadava, R. P., & Smith, M. V. (1971). Aggressive behavior of Apis mellifera L. workers towards introduced queens. 3. Relationship between the attractiveness of the queen and worker aggression. Canadian Journal of Zoology, 49(10), 1359–1362. doi:10.1139/z71-203
