Abstract
The Microsporidia Nosema ceranae is an invasive pathogen affecting honey bee health, particularly in warm climates. In this study, N. ceranae was detected for the first time in honey bees (Apis mellifera L.) of the Madeira archipelago, indicating that this pathogen is now spreading across the entire Macaronesia. Nosema apis was not detected, and the high prevalence (67.7%) of N. ceranae indicates its dominance over N. apis.
The archipelago of Madeira, together with that of the Azores and the Canaries, form the biogeographical region of Macaronesia (sensu Beyhl et al. (Citation1995)). These volcanic archipelagos share a mild climate and a highly diverse flora, thereby offering ideal conditions for apiculture. In addition, they share honey bee populations that are genetically close to the Iberian honey bee Apis mellifera iberiensis, as revealed by wing morphometry and mitochondrial DNA (Álvarez et al., Citation1997, Citation2001; Ferreira et al., Citation2020; Miguel et al., Citation2015; Muñoz et al., Citation2013). These findings support historical introductions of A. m. iberiensis into Macaronesia during colonization by the Portuguese and Spanish settlers (Ferreira et al., Citation2020), although natural expansion out of the north African coast has also been proposed (de la Rúa et al., Citation1998, Citation2006).
Over recent decades, beekeeper-mediated introductions of honey bees or hive products brought along Varroa destructor into the three archipelagos and N. ceranae (recently renamed by Tokarev et al. (Citation2020) as Vairimorpha ceranae) into the Canaries and the Azores (Lopes et al., Citation2022; Muñoz et al., Citation2014). However, in the Azores, there are still six islands that are free of V. destructor and two that are free of N. ceranae (Lopes et al., Citation2022). While V. destructor was also introduced on Madeira in 2001 (Silva, Citation2016), the question remains whether this archipelago is free of N. ceranae, such as in the case of the Azorean island of Flores, which, in spite of being V. destructor-colonized, is still naïve to this pathogen.
To gain a fuller picture of the N. ceranae epidemiological status in Macaronesia, in 2014, we conducted a survey on Madeira, the largest island of the Madeira archipelago. On the other inhabited island of the archipelago, Porto Santo, honey bee colonies were re-introduced in 2014 from the Azorean island of Terceira, after the die-off of the managed population due to the arrival of V. destructor in 2003 (Silva, Citation2016). Given that N. ceranae is present on Terceira (Lopes et al., Citation2022), it is possible that the honey bee population of Porto Santo is also infected.
Prior to the N. ceranae jump from Apis cerana to Apis mellifera, Nosema apis was thought to be the single etiological cause of nosemosis in the world (Higes et al., Citation2006; Huang et al., Citation2007). While the role of N. ceranae in colony losses is a contentious matter (reviewed by Martín-Hernández et al. (Citation2018)), there is evidence that it has higher biological potential in warmer climates than N. apis (Martín-Hernández et al., Citation2009). In this context, it has been claimed that N. ceranae is an important stressor in the Mediterranean countries (Hges et al., Citation2010) and likely in Madeira because of the year-round mild climate.
The goal of this study was to survey the honey bee population of Madeira for the presence of N. ceranae and N. apis and evaluate their prevalence and distribution. To achieve this goal, between February and March of 2014, three colonies from 31 apiaries were sampled along the coast where, due to the island’s rough orography, most apiaries are located (). Total DNA was extracted from a pool of 120 workers for each of the 93 samples, following the protocol described by Martín-Hernández et al. (Citation2012). The 93 DNA extracts were simultaneously screened for N. ceranae, N. apis, and an internal control of A. mellifera (COI gene) using the multiplex-PCR assay developed by Martín-Hernández et al. (Citation2012). The assay comprised a negative control of extraction, a non-template control (NTC), a positive control for N. apis, and another for N. ceranae. The QIAxcel Advanced System (Qiagen®) was used to analyse the PCR products. The amplification of the internal A. mellifera control in all 93 samples confirmed the success of the DNA extraction. No amplicons were observed in the extraction controls or NTCs, indicating that there was no cross-contamination during sample processing.
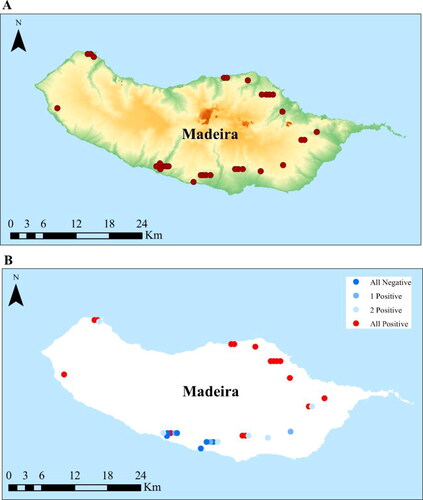
Figure 1. Map of Madeira showing (A) the orography of the island, with the location of the sampled apiaries, and (B) the location of the apiaries with the three sampled colonies N. ceranae-positive (red dots), two colonies N. ceranae-positive (light blue dots), one colony N. ceranae-positive (blue dots), and no N. ceranae-positive colonies (dark blue dots).
Of the 93 colony samples, 30 (32.3%) were negative for both Nosema species (). N. apis was not detected, whereas N. ceranae was found in 63 colonies (67.7%). This finding suggests the dominance of N. ceranae over N. apis and it is unaffected by sampling time because the colonies were sampled in February and March, therefore matching the seasonal peak of N. apis infection (Fries, Citation1993). Interestingly, the prevalence of N. ceranae on Madeira lies between that reported for the Canaries (75%; Muñoz et al. (Citation2014)) and for the Azores (50.7%; Lopes et al. (Citation2022)). This south-north gradient might be explained by N. ceranae arriving at the Canaries first and at the Azores last, which is supported by the observation that there are two Azorean islands still free of N. ceranae (Lopes et al., Citation2022). Alternatively, but not mutually exclusive, this pattern is shaped by the climate in which the warmer and dryer Canaries and Madeira are more favourable to N. ceranae (Martín-Hernández et al., Citation2009).
The analysis by apiary revealed that in 16 of the 31 (51.6%) surveyed apiaries, all the colonies tested positive for N. ceranae, six (19.4%) had two-thirds of positive colonies, three had one-third (9.7%), and six (19.4%) had no positive samples (). This observation suggests that once N. ceranae enters an apiary, it rapidly spreads across all the colonies. Interestingly, most of the N. ceranae-negative colonies are clustered on the southern side of the island, and, except for one single apiary, which only had two positive colonies, all northern apiaries had the three sampled colonies positive for N. ceranae (). This pattern suggests that N. ceranae first entered Madeira from the north, and, at the moment of this study, it was spreading to the south. Alternatively, N. ceranae entered Madeira elsewhere and a more favourable environment for the development of N. ceranae infection in the north explains the higher prevalence. The northern side of Madeira is more humid and colder than the southern side. But more importantly, because it is dominated by the densely-wooded primeval Laurissilva (laurel) forest, the northern side offers poor pollen sources for honey bees, and there is mounting evidence that nutrition has a pivotal role in the development of N. ceranae infection (Castelli et al., Citation2020).
This study constitutes the first report of N. ceranae on Madeira. The lack of detection of N. apis suggests that N. ceranae is possibly the sole species causing nosemosis on Madeira, as reported elsewhere (Cilia et al., Citation2019; Gajger et al., Citation2010; Stevanovic et al., Citation2011). The distributional pattern of the pathogen suggests that it entered Madeira not long before the sampling in 2014 and it is possibly still expanding. Another cross-sectional survey is warranted to assess whether N. ceranae prevalence in the south has reached the levels of the north, which would help understand the dynamics of the dispersion.
Acknowledgements
We are indebted to Enga Berta Correia of the “Divisão de Experimentação e Melhoria Agrícola,” “Direção Regional de Agricultura da Madeira,” for collecting the honey bee samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Álvarez, F. P., da Silva, M. V., Campano, F., Vaquero, E. J., Flores, J., Puerta, F. P., & Bustos, M. (2001). Discriminación entre poblaciones de abejas (Apis mellifera L.) del sur de España, centro de Portugal y Madeira. Archivos de Zootecnia, 50(189), 79–89.
- Álvarez, F. P., Fernández, R. H., López, J. R., Puerta, F. P., Serrano, J. F., & Bustos, M. (1997). Estudio morfológico de las abejas melíferas del Archipiélago Canario (Gran Canaria, Tenerife, La Palma, Gomera). Archivos de Zootecnia, 47, 451–459.
- Beyhl, F. E., Myes, B., & Ohm, P. (1995). Macaronesia: A biogeographical puzzle. Boletim do Museu Municipal Do Funchal (História Natural), 4, 107–113.
- Castelli, L., Branchiccela, B., Garrido, M., Invernizzi, C., Porrini, M., Romero, H., Santos, E., Zunino, P., & Antunez, K. (2020). Impact of nutritional stress on honey bee gut microbiota, immunity, and Nosema ceranae infection. Microbial Ecology, 80(4), 908–919. https://doi.org/10.1007/s00248-020-01538-1
- Cilia, G., Sagona, S., Giusti, M., dos Santos, P. E. J., Nanetti, A., & Felicioli, A. (2019). Nosema ceranae infection in honey bee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi journal of Biological Sciences, 26(7), 1553–1556. https://doi.org/10.1016/j.sjbs.2018.11.017
- de la Rúa, P., Galián, J., Pedersen, B. V., & Serrano, J. (2006). Molecular characterization and population structure of Apis mellifera from Madeira and the Azores. Apidologie, 37(6), 699–708. https://doi.org/10.1051/apido:2006044
- de la Rúa, P., Serrano, J., & Galián, J. (1998). Mitochondrial DNA variability in the Canary Islands honey bees (Apis mellifera L.). Molecular Ecology, 7(11), 1543–1547. https://doi.org/10.1046/j.1365-294x.1998.00468.x
- Ferreira, H., Henriques, D., Neves, C. J., Machado, C. A. S., Azeved, J. C., Francoy, T. M., & Pinto, M. A. (2020). Historical and contemporaneous human-mediated processes left a strong genetic signature on honey bee populations from the Macaronesian archipelago of the Azores. Apidologie, 51(3), 316–328. https://doi.org/10.1007/s13592-019-00720-w
- Fries, I. (1993). Nosema apis - A parasite in the honey bee colony. Bee World, 74(1), 5–19. https://doi.org/10.1080/0005772X.1993.11099149
- Gajger, I. T., Vugrek, O., Grilec, D., & Petrinec, Z. (2010). Prevalence and distribution of Nosema ceranae in Croatian honey bee colonies. Veterinární Medicína, 55(9), 457–462. https://doi.org/10.17221/2983-VETMED
- Hges, M., Martín-Hernández, R., & Meana, A. (2010). Nosema ceranae in Europe: An emergent type C nosemosis. Apidologie, 41(3), 375–392. https://doi.org/10.1051/apido/2010019
- Higes, M., Martín, R., & Meana, A. (2006). Nosema ceranae, a new microsporidian parasite in honey bees in Europe. Journal of Invertebrate Pathology, 92(2), 93–95. https://doi.org/10.1016/j.jip.2006.02.005
- Huang, W. F., Jiang, J. H., Chen, Y. W., & Wang, C. H. (2007). A Nosema ceranae isolate from the honey bee Apis mellifera. Apidologie, 38(1), 30–37. https://doi.org/10.1051/apido:2006054
- Lopes, A. R., Martín-Hernández, R., Higes, M., Segura, S. K., Henriques, D., & Pinto, M. A. (2022). Colonisation patterns of Nosema ceranae in the azores archipelago. Veterinary Sciences, 9(7), 320. https://doi.org/10.3390/vetsci9070320
- Martín-Hernández, R., Bartolomé, C., Chejanovsky, N., Le Conte, Y., Dalmon, A., Dussaubat, C., García-Palencia, P., Meana, A., Pinto, M. A., Soroker, V., & Higes, M. (2018). Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environmental microbiology, 20(4), 1302–1329. https://doi.org/10.1111/1462-2920.14103
- Martín-Hernández, R., Botías, C., Bailón, E. G., Martínez-Salvador, A., Prieto, L., Meana, A., & Higes, M. (2012). Microsporidia infecting Apis mellifera: Coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environmental microbiology, 14(8), 2127–2138. https://doi.org/10.1111/j.1462-2920.2011.02645.x
- Martín-Hernández, R., Meana, A., García-Palencia, P., Marín, P., Botías, C., Garrido-Bailón, E., Barrios, L., & Higes, M. (2009). Effect of temperature on the biotic potential of honey bee microsporidia. Applied and Environmental Microbiology, 75(8), 2554–2557. https://doi.org/10.1128/aem.02908-08
- Miguel, I., Garnery, L., Iriondo, M., Baylac, M., Manzano, C., Steve Sheppard, W., & Estonba, A. (2015). Origin, evolution and conservation of the honey bees from La Palma Island (Canary Islands): Molecular and morphological data. Journal of Apicultural Research, 54(5), 427–440. https://doi.org/10.1080/00218839.2016.1180017
- Muñoz, I., Cepero, A., Pinto, M. A., Martín-Hernández, R., Higes, M., & De la Rúa, P. (2014). Presence of Nosema ceranae associated with honey bee queen introductions. Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 23, 161–168. https://doi.org/10.1016/j.meegid.2014.02.008
- Muñoz, I., Pinto, M. A., & De la Rúa, P. (2013). Temporal changes in mitochondrial diversity highlights contrasting population events in Macaronesian honey bees. Apidologie, 44(3), 295–305. https://doi.org/10.1007/s13592-012-0179-0
- Silva, D. (2016). A realidada apícola na Região Autónoma da Madeira. Comunication presented at the XVII Fórum Nacional de Apicultura e XV Feira do Mel, Terceira-Azores, Portugal.
- Stevanovic, J., Stanimirovic, Z., Genersch, E., Kovacevic, S. R., Ljubenkovic, J., Radakovic, M., & Aleksic, N. (2011). Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie, 42(1), 49–58. https://doi.org/10.1051/apido/2010034
- Tokarev, Y. S., Huang, W. F., Solter, L. F., Malysh, J. M., Becnel, J. J., & Vossbrinck, C. R. (2020). A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. Journal of Invertebrate Pathology, 169, 107279. https://doi.org/10.1016/j.jip.2019.107279
