Abstract
A survey conducted as part of the National Honey Bee Survey from 2013 to 2020 quantified the managed honey bee hives on Guam, sampled accessible wild-living colonies, and described associated diseases and pests. During this period the number of managed bee colonies increased from 20 to 121 which was associated with the formation of the Guam Beekeepers Association. Managed Apis mellifera colonies on Guam had significantly fewer parasites and diseases than reported in the USA. The Varroa mite, Varroa destructor, was detected in 2014 in a wild-living colony in southern Guam. The greater banded hornet, Vespa tropica, was first observed on Guam in 2016, with honey bee depredations observed in 2017. Nosema ceranae was initially detected in wild-living and managed colonies in 2013 and has been consistently observed since that time with little apparent effect on colony health as there have been no recorded colony losses in apiaries. No small hive beetle, Aethina tumida, Tropilaelaps mite, Tropilaelaps spp, American foulbrood, Paenibacillus larvae, European foulbrood, Melissococcus plutonius, chalkbrood, Ascosphaera sp. and 11 additional viral diseases of the 22 known to infest honey bees, including deformed wing virus, were observed.
Introduction
The western honey bee, Apis mellifera, with a worldwide distribution (Moritz et al., Citation2005), is the most studied insect in the world (Villalobos, Citation2016). The widespread distribution and isolation of A. mellifera have allowed for the evolution of at least 30 subspecies (Ruttner, Citation1988). A. mellifera subspecies have been dispersed globally by humans while other honey bees, such as the Asian honey bee, Apis cerana, are not as widely distributed. The global distribution of A. mellifera was largely driven by human desire for honey and beeswax and by the pollination services provided for many crop plant species.
Apis mellifera was first introduced to Guam in 1907 by bees previously introduced to Hawaii from North America in 1857 (Crane, Citation1999). In 1914, the governor of Guam, Capt. W.J. Maxwell, added a course on beekeeping in local schools in hopes that Guam would become more agriculturally self-sufficient. Italian honey bees, Apis mellifera linguistica, were introduced to Guam from Hawaii as pollinators for imported nectar-producing plants and to increase honey production (Thompson, Citation1914). Local honey and beeswax production were anticipated to satisfy local needs as well as be exported (Hartenbower, Citation1914). Since that time, honey bee pollination and honey and beeswax production in Guam and in the Pacific Basin has been poorly studied (Mortensen et al., Citation2008). Interest in honey bee pollination services has increased because ecological services once provided by Guam’s native birds and fruit bats, were lost following the accidental post-World War II introduction of the brown tree snake, Boiga irregularis, on repatriated military equipment from the South Pacific (Fritts & Rodda, Citation1998).
The spread of honey bee pests and parasites is likely related to the global distribution of A. mellifera (Le Conte et al., Citation2010). The Varroa mite, Varroa destructor (hereafter Varroa), a parasitic mite of A. cerana has become widespread by transport of A. mellifera colonies to other regions outside of Europe and North America (Villalobos, Citation2016). In the mid-twentieth century, Varroa has expanded out of its native host range, to its novel host, A. mellifera. Similarly, these parasites vectors for viruses, such as deformed wing virus (DWV), where they are amplified in honey bee populations invaded by Varroa (Wilfert et al., Citation2016).
The primary objectives of this study were to (1) describe the distribution of managed A. mellifera colonies on Guam, and (2) describe the incidence and distribution of honey bee predators, parasites, and diseases on Guam in managed and wild-living colonies.
Materials and methods
The study was conducted on Guam, the largest and southernmost of the Mariana Islands located at 13.444° N and 144.7937° E in the tropical Western Pacific Ocean. Honey bee colonies were sampled and first mapped on Guam as part of the National Honey Bee Survey which started on Guam in 2014. In the absence of satellite imagery, managed and wild-living honey bee colonies were identified from information reported by the general public and by local beekeepers. Survey methodology followed that developed for the National Honey Bee Pest and Disease Survey Sampling Protocol (USDA-APHIS, Citation2024). Data in this study were collected from 2014 to 2020.
Field samples for diseases and parasites
The latitude and longitude for each wild-living or managed colony were recorded using a Garmin eTrex® handheld GPS unit (Garmin Ltd., Olathe, KS, USA) and uploaded onto a Microsoft Excel spreadsheet (Microsoft, Redmond WA). All locations of managed honey bee colonies and wild-living colonies sampled between 2013 and 2020 were mapped using QGIS (QGIS Development Team, London, UK). Wild-living colonies sampled in this study consisted of accessible open nests in urban and natural areas. In addition, wild-living colonies in cavities of homes were removed and sampled as part of this survey. Wild-living colonies nesting in cavities and along Guam’s cliff lines were not accessible and therefore not included in this study. Wild-living colonies on restricted-access military lands in central and northern Guam were included in this study. However, all managed colonies in this study were sampled. Managed colonies consisted of hives maintained in Langstroth, Top Bar, or Warré hive boxes. An apiary consists of two or more managed colonies maintained at a single location by a beekeeper.
Adult bees collected from managed colonies and from accessible wild-living colonies were examined for brood diseases using procedures developed by USDA-ARS (Shimanuki & Knox, Citation2000). In the examination of a managed colony, a frame of brood was removed and examined for the presence of American foulbrood, European foulbrood, sacbrood, and chalkbrood. Honey comb containing brood was further categorized according to appearance, age, color, odor, and characteristics of dead brood. When examining a wild-living colony, brood comb was completely removed from its nest and examined as was done for a managed hive.
Adult honey bees from the same single frame containing developing brood from a managed hive were also sampled for Varroa and for spores of Nosema sp. Honey bees from the frame were brushed into a 1.9 l metal pan containing 250 ml of 95% ethanol. About ½ cup of bees were placed into ethanol and poured into a 500 ml Nalgene® bottle. Any remaining adult bees on the frame were then brushed into the colony. Both sides of the now bee-less frame were then tapped to dislodge any small hive beetles and Tropilaelaps mites into the metal pan containing a thin film of 95% ethanol. The contents of the metal pan were then filtered onto a fine mesh nylon cloth which was then folded and placed in a 125 ml Nalgene® bottle with the ethanol filtrate. The bottles containing the mesh cloth filters and decanted ethanol were sent to the USDAARS Bee Research Laboratory in Beltsville, MD for examination for the presence of small hive beetles and Tropilaelaps mites. When sampling a wild-living colony, a single comb containing brood was removed from the hive and processed as described for managed hives. Combs that were easily moldable were rubber banded onto a frame and then tapped as described as done for a managed hive.
Sampling for viruses
A cup full of live bees from each colony in each apiary was collected into a live bee shipping box. Collected bees were stored in the box in a freezer at −20 °C. After 24 h 50 bees were removed from the box and frozen in liquid nitrogen at −196 °C. Frozen bees were pulverized using a mortar and pestle. The resulting pulverized bee sample was then placed into a 15 ml conical centrifuge tube with 5 ml of RNAlater® (Qiagen, Germantown, MD). The Bee-RNAlater® suspension was mailed via USPS to the USDA-ARS-Bee Research Laboratory, Beltsville, Maryland, for virus analysis.
Mapping predators, parasites, and diseases
Results of assays for diseases, predators, parasites, and viruses received from the USDA-ARS Bee Research Laboratory were entered into QGIS using a template created in Google Maps (Citation2024). Summarized pest data was provided to local and regional beekeepers upon request.
Results
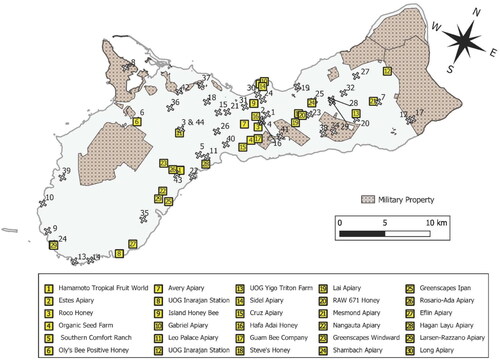
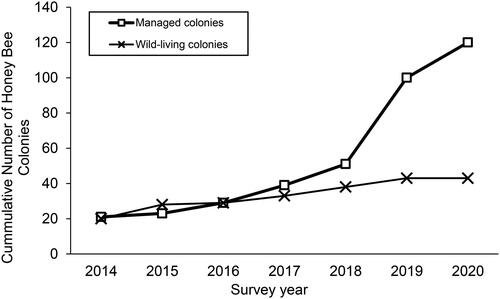
The cumulative number of managed honey bee colonies and sampled wild-living colonies during this period increased from 41 to 164 (). The number of managed colonies increased from 21 to 120 during this period, while the number of sampled wild-living colonies increased from 20 to 44 (). Most apiaries and wild-living colonies sampled were located in central Guam. No apiaries were established nor were wild-living colonies observed in southwest Guam, which consists largely of savannah grassland on ancient volcanic hills (). Wild-living colonies were found on restricted-access military lands near Apra Harbor in central Guam and in northeastern Guam on Andersen Air Force Base. No apiaries were established on military lands.
Figure 1. Cumulative number of managed honey bee colonies and of accessible sampled wild-living colonies on Guam from 2014 to 2020.
Pests and predators
Of the 163 honey bee colonies surveyed from 2014 to 2020, 94 managed colonies in 22 apiaries were observed with wax moths while 26 managed colonies had none. All 43 wild-living colonies sampled were infested with wax moths ().
Table 1. Results of National Honey Bee Survey from managed colonies and wild-living colonies in Guam from 2014 to 2020.
Wild-living colonies
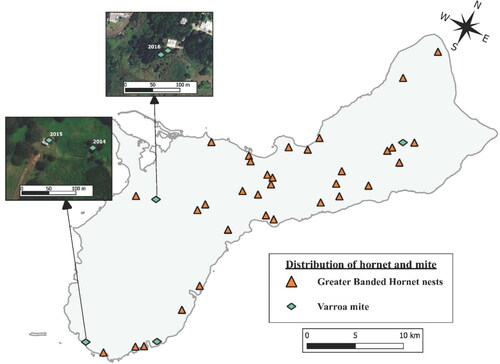
GBH was first observed on Guam in 2016 by Guam beekeepers. In 2017, three managed colonies in two apiaries and seven wild-living colonies were observed being attacked by GBH. Since the introduction of GBH on Guam, 23 managed apiaries have been attacked by GBH (). The number of wild-living colonies attacked by GBH is not known. However, 32 GBH nests have been detected and destroyed ().
Figure 3. Distribution of Vespa tropica nests, and Varroa infestations in managed and wild-living honey bee colonies on Guam from 2014 to 2020.
Since the onset of GBH depredations on Guam, local beekeepers have developed excluders, hardware cloth barriers, and traps to exclude GBH from colonies within apiaries. While substantially reducing hive depredations, Guam beekeepers still kill by hand GBH hovering near hive entrances. Honey bee balling behavior, where bees physically swarm over the invading hornet resulting in the hornet’s death, has occasionally been observed at unprotected hive entrances (Dennis Larson, personal communication, November 21, 2018).
No small hive beetles were found in either managed or wild-living colonies.
Parasitic mites
Varroa was detected in a wild-living located at the Southern Comfort Ranch in southern Guam in 2014. Varroa was then detected in a managed bee colony used as a sentinel within the same property. Varroa was also observed in two wild-living colonies in the village of Santa Rita in central Guam in 2016, in the village of Yigo in northern Guam in 2016, and in a managed colony in the village of Inarajan in southeast Guam in 2017 (, ). All colonies with Varroa were destroyed. No Varroa has ever been found in any managed or wild-living colony on Guam since 2017. No Tropilaelaps mites or tracheal mites were found at any time in Guam from 2014 through 2020 ().
Pathogens
Nosema ceranae
Nosema ceranae was found in 25 of 30 sampled apiaries and was observed in 32 of 44 wild-living colonies sampled.
Brood diseases
American foulbrood, European foulbrood, sacbrood, and chalkbrood were not detected in any apiary or wild-living colony sampled in Guam.
Viruses
No viruses were detected in wild-living colonies or managed apiaries sampled in Guam ().
Discussion
The annual surveys conducted on Guam as part of the National Honey Bee Survey were the first assessments of honey bee health in Guam since the early 1900s when quarantine restrictions on honey bee importation were implemented. Surveys were initiated in 2014 by inviting the public through press releases to report wild-living bee colonies in Guam. Guam beekeepers then allowed their personal honey bee colonies to be included in the survey. The heightened interest in honey bees on Guam resulted in the formal establishment of the Guam Beekeepers Association (GBA) whose membership rapidly increased from 10 in 2015 to 55 by 2020. In addition, the relative ease of keeping honey bees on Guam in the absence of predators and diseases, coupled with the year-round availability of tropical nectar-producing plants, attracted many first-time beekeepers to join the association. Due to low parasite prevalence and favorable foraging conditions in Guam, wild-living colonies are probably thriving and much more numerous than managed colonies. The high volume and exceptional quality of honey, evidenced by a Good Food Award (Good Foods Foundation, Fort Mason Center, San Francisco, CA, USA) in 2020 to a local beekeeper, produced by Guam honey bees has led to small-scale localized commercial sales of honey bee products. Income from local honey bee product sales also supplemented the income of beekeepers whose businesses were severely impacted by the COVID-19 pandemic from 2020 to 2021. Surveys continue on Guam until the present day and have recently expanded to include the islands Saipan, Tinian, and Rota in the Commonwealth of the Northern Marianas Islands.
Despite the high number of managed bee colonies, there are bee swarms reported by the community at least 3–4 times a month (Dennis Larsen, personal communication, September 15, 2019). It is unknown whether swarms are coming from wild-living colonies or managed colonies. Honey bee swarm removal services have also been provided free of charge to government agencies and to private businesses and home owners by GBA. All apiaries established since 2014 were generated from swarms collected by members of GBA, again suggesting the existence of a large wild-living population.
Detection of Varroa and Nosema ceranae
The discovery of Varroa on Guam in 2014 was the first detection of this parasite in the Mariana Islands Archipelago (Rosario et al., Citation2014). Varroa loads in wild-living and managed colonies on Guam sampled between 2014 to 2017 were below the national average of 3.37 mites/100 bees (Fahey et al., Citation2019). The lack of Varroa on Guam since 2017 has stimulated interest by local beekeepers in breeding and rearing honey bee queens, with the long-term objective of developing a queen and nuc export industry.
Nosema ceranae in wild-living and managed colonies appears to have no detrimental effect on honey bees on Guam. Since its first global detection in 2006, there have been Numerous reports suggest that despite its global distribution (Klee et al., Citation2007), N. ceranae appears to have little effect on honey bee health or behavior (Goblirsch et al., Citation2013; Schüler et al., Citation2023). The high prevalence of N. cerenae in Guam is likely attributed to 100% humidity all year round.
Absence of pests, parasites, disease, and viruses
Small hive beetle, Aethina tumida, currently impacts Hawaii’s apiculture and queen bee export industry (Martin, Citation2013), and is a common honey bee pest in the USA, Canada, and Australia (Cuthbertson et al., Citation2013). The absence of this mite and other viruses in Guam has resulted in the strict enforcement of laws prohibiting the importation of honey bees and honey bee products (Lumsden et al., Citation1956).
Beekeeping on Guam
Beekeeping in the humid tropical environment of Guam is different from that of temperate regions. Temperate regions typically experience four distinct seasons where winter is characterized by low temperatures and a lack of floral resources. In temperature regions and under winter conditions honey bee colonies cease to produce brood. Guam seasonality includes a “winter” dry season, a “summer” rainy season, and two transitional seasons. Nectar flow occurs year-round with limited seasonal variation, and brood is produced continually (W. Long, personal communication, June 9, 2017). Honey bee colonies in Guam frequently overpopulate due to the abundant food sources and warm climate. Swarming occurs frequently, allowing captured swarms to become the primary source of newly managed hives. Commonly occurring tropical storms and typhoons have caused Guam and the Marianas Archipelago to be placed on a constant 72-h alert for severe weather events. However, while such severe weather may damage managed colonies in apiaries, it appears to have little effect on wild-living colonies in cliff line cavities or in hollow trees. Guam’s high annual rainfall, averaging over 258 cm/year (Lander & Guard, Citation2003), results in the waterlogging of wooden hives which must be replaced every five to six years (P. Packbier, personal communication, September 2, 2016). Termites on Guam destroy the untreated wood in imported wooden bee boxes. As a result, local beekeepers have resorted to importing hives made of Styrofoam or making their own hives of concrete.
Acknowledgements
We thank members of the Guam Beekeepers Association for allowing access to their apiaries and continuing interest in promoting beekeeping on Guam and in the Mariana Islands.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Crane, E. (1999). The world history of beekeeping and honey hunting (1st ed.). Routledge. https://doi.org/10.4324/9780203819937
- Cuthbertson, A. G. S., Wakefield, M. E., Powell, M. E., Marris, G., Anderson, H., Budge, G. E., Mathers, J. J., Blackburn, L. F., & Brown, M. A. (2013). The small hive beetle, Aethina tumida: A review of its biology and control measures. Current Zoology, 59(5), 644–653. https://doi.org/10.1093/czoolo/59.5.644
- Fahey, R., Rennich, K., Nessa, A., Swan, N., Steinhauer, N., Eversole, H., Reynolds, D., Ryan, J., Rose, R., Evans, J., van Engelsdorp, D. (2019). 2017–2018 APHIS National Honey Bee Disease Survey summary report. Retrieved November 29, 2021, from https://www.aphis.usda.gov/2017-2018-honey-bee-disease-summary-report
- Fritts, T. H., & Rodda, G. H. (1998). The role of introduced species in the degradation of island ecosystems: A case history of Guam1. Annual Review of Ecology and Systematics, 29(1), 113–140. https://doi.org/10.1146/annurev.ecolsys.29.1.113
- Goblirsch, M., Huang, Z. Y., & Spivak, M. (2013). Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLOS One, 8(3), e58165. https://doi.org/10.1371/journal.pone.0058165
- Google Maps (2024). Guam, USA. Retrieved January 14, 2024, from https://www.google.com/maps/@13.4023543,144.8312165,11.74z?authuser=0&entry=ttu
- Hartenbower, A. C. (1914). Report of the special agent in charge (Annual Report of the Guam Agricultural Experimental Station. for 1914, 16). Guam Agricultural Experiment Station.
- Klee, J., Besana, A. M., Genersch, E., Gisder, S., Nanetti, A., Tam, D. Q., Chinh, T. X., Puerta, F., Ruz, J. M., Kryger, P., Message, D., Hatjina, F., Korpela, S., Fries, I., & Paxton, R. J. (2007). Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. Journal of Invertebrate Pathology, 96(1), 1–10. https://doi.org/10.1016/j.jip.2007.02.014
- Lander, M. A., & Guard, C. P. (2003). Creation of a 50-year database, annual rainfall climatology, and annual rainfall distribution map for Guam (WERI Technical Report No. 102, p. 32).
- Le Conte, Y., Ellis, M., & Ritter, W. (2010). Varroa mites and honey bee health: An Varroa explain part of the colony losses? Apidologie, 41(3), 353–363. https://doi.org/10.1051/apido/2010017
- Lumsden, D. V., Jans, F. C., Koenig, N., McGourin, T. F., & Wakefield, W. C. (1956). The agricultural needs of Guam (Report on the Agricultural Experiment Stations, p. 63). US Department of Agriculture.
- Martin, S. J. (2013). Double trouble in paradise: Small hive beetle joins Varroa in Hawaii. American Beekeeping Journal, 153, 529–532.
- Moritz, R. F. A., Härtel, S., & Neumann, P. (2005). Global invasions of the western honey bee (Apis mellifera) and the consequences for biodiversity. Ecoscience, 12, 289–301.
- Mortensen, H. S., Dupont, Y. L., & Olesen, J. M. (2008). A snake in paradise: Disturbance of plant reproduction following extirpation of bird flower-visitors on Guam. Biological Conservation, 141(8), 2146–2154. https://doi.org/10.1016/j.biocon.2008.06.014
- Rosario, C., Moore, A., & Miller, R. (2014). Varroa mite, Varroa destructor (Acari: Varroidae). Guam New Invasive Species Alert No. 2014-04 (2014). Retrieved from https://guaminsects.net/anr/sites/default/files/varroa%20mite_0.pdf
- Ruttner, F. (1988). Biogeography and taxonomy of honeybees. Springer-Verlag.
- Schüler, V., Liu, Y. C., Gisder, S., Horchler, L., Groth, D., & Genersch, E. (2023). Significant, but not biologically relevant: Nosema ceranae infections and winter losses of honey bee colonies. Communications Biology, 6(1), 229. https://doi.org/10.1038/s42003-023-04587-7
- Shimanuki, H., & Knox, D. A. (2000). Diagnosis of honey bee diseases. In Agriculture Handbook No. AH690. United States Department of Agriculture.
- Thompson, J. B. (1914). Report of the special agent in charge (Annual Report of the Guam Agricultural Experimental Station for 1913, 21). Guam Agricultural Experiment Station.
- USDA-APHIS (2024). National honey bee pests and diseases survey sampling protocol. Retrieved June 1, 2024, from https://www.aphis.usda.gov/sites/default/files/honey-bee-sampling-protocol.pdf
- Villalobos, E. (2016). The mite that jumped, the bee that traveled, the disease that followed. Science, 351(6273), 554–556. https://doi.org/10.1126/science.aaf0938
- Wilfert, L., Long, G., Leggett, H. C., Schmid-Hempel, P., Butlin, R., Martin, S. J. M., & Boots, M. (2016). Deformed wing virus is a recent global epidemic in honey bees driven by Varroa mites. Science, 351(6273), 594–597. https://doi.org/10.1126/science.aac9976