 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The authors characterized how motor planning influences temporal order judgment (TOJ) tasks. They examined this by applying vibrotactile stimulation during the planning stages of a bimanual arm movement that would bring the arms into a crossed configuration. The authors have previously shown that planning to cross the arms induces a subjective reversal of spatially defined temporal order judgments that evolves over the course of the planning period. It was unclear, however, whether this effect is modulated by the extent to which the arms would be crossed after movement. The authors examined this issue by having participants plan to move to 4 different targets that would leave the arms in crossed configurations of varying extents. The results demonstrate that even though cutaneous stimuli were applied before the movements, if participants were planning to move into a more crossed configuration, performance on the TOJ task worsened depending on where they were in the planning process. This data suggest the brain uses planning signals to predict sensations from impending movements in a context-dependent manner.
Present theories of motor control suggest that coordinated movement is largely influenced by the predictive capabilities of the brain systems that plan and execute limb movements (Desmurget & Grafton, 2000; Diedrichsen et al., 2010; Miall, Christensen, Cain, & Stanley, Citation2007; Miall & Wolpert, 1996). When performing limb movements, these motor planning signals can be used to make anticipatory adjustments (Makoshi, Kroliczak, & van Donkelaar, Citation2011) or even attenuate sensory signals (Buckingham, Carey, Colino, deGrosbois, & Binsted, Citation2010) to allow for differentiation between self- and externally imposed cutaneous sensations.
Our understanding of these predictive processes has come mainly from studies examining sensorimotor adaptation, which are limited to making indirect inferences based on the changes in motor output during and after the adaptation protocol (for a review, see Shadmehr, Smith, & Krakauer, Citation2010). These techniques typically use a force-field to acclimate the participant to reaching against or perpendicular to a load. When the load is suddenly removed, participants must make corrective movements to reach the desired target. Although the method of using adaptation to investigate online corrections can provide insight into planning processes in general; it is limited by observing corrective behavior during the first few trials following adaptation.
Given that predictive motor planning clearly occurs during movements performed under normal (unadapted) circumstances, it is important to develop experimental protocols that allow insight into these processes. We suggest that the use of a temporal order judgment (TOJ) task used in conjunction with an upper-limb movement can do so. In particular, by examining the systematic changes in TOJ errors during the period leading up to movement onset, we can more directly probe the predictive characteristics of the planning process. This becomes especially apparent under the special circumstances associated with bimanual arm crossing movements. Previous work by Yamamoto and Kitazawa (Citation2001) and others (Schicke & Röder, Citation2006; Shore, Spry, & Spence, Citation2002) has demonstrated that under static conditions, TOJ errors are larger when the arms are crossed compared to when they are uncrossed. One potential explanation for the impaired performance on TOJ tasks while the arms are crossed is that the normally congruent coding of stimulus location via egocentric and allocentric frames of reference is disrupted by the incongruency of the crossed configuration of the arms (Shore et al., Citation2002). Furthermore, it is apparent that errors in tactile localization also occur when the limb is moving or even during the planning process prior to movement onset. In particular, Dassonville (Citation1995) has shown that when a tactile stimulus is applied to the limb, participants mislocalize the stimulus in the direction of impending or ongoing movement, suggesting that tactile localization is influenced by the predictive planning signals associated with the upcoming position of the limb.
In the current experiment, we investigated the predictive planning process by using a protocol, which examines the effects of movement planning on a concurrent TOJ task (Hermosillo, Ritterband-Rosenbaum, & van Donkelaar, Citation2011; Ritterband-Rosenbaum, Hermosillo, Kroliczak, & van Donkelaar, Citation2014). Recent work from our lab (Hermosillo et al., Citation2011) has shown that simply planning to cross the arms systematically influences TOJ error rates in a manner that reflects the final, as opposed to current, configuration of the arms. In the present experiment, we extend this work to investigate how TOJ error rates are modulated by planning to move the limbs into progressively more crossed postures. We hypothesized that altering the planned movement endpoints would systematically influence predictive planning, indicating that the details of the movement rather than movement per se drive the changes in TOJ error rate that are observed.
Methods
Participants
Ten participants (M age = 23.4 ± 3.28 years; three women; all right-handed) completed the experiment. All participants gave their informed consent prior to participating in the experiment, which was approved by the Behavioral Research Ethics Board at the University of British Columbia. Exclusion criteria included no previous history of sensorimotor deficits, and no range of motion limitations.
Experimental Setup
Each participant sat in a chair ∼15 cm from a table with their eyes open and hands resting palms down 15 cm apart on the table with the index fingers pointing at two home targets. Custom-built piezoelectric vibrotactile stimulators were attached to the underside of the distal segment of the extended index finger pad of each hand with Velcro straps. Vibrotactile stimulation was achieved by applying a train of three rectangular voltage pulse (5 V, 2 ms/pulse) to the piezoelectric device producing a small displacement (1 mm) of the contact point (2 mm2) so as to contact the surface of the skin. Participants were provided with 2–3 practice trials, so that they would know what the stimuli would feel like.
Experimental Task
For the TOJ task, the vibrotactile stimuli were delivered to each index finger separated by an interstimulus interval of 100 ms (). This interstimulus interval results in TOJ error rates above baseline but below chance levels (Hermosillo et al., Citation2011; Shore et al., Citation2002; Yamamoto & Kitazawa, 2001). On half the trials the right index finger was stimulated first, whereas on the other half the left index finger was stimulated first. The first vibrotactile stimulus was delivered either coincident with or 250 ms after an auditory cue to move. These two stimulus onset asynchronies (SOAs) were chosen because TOJ decisions based on vibrotactile stimuli delivered coincident with the cue to move reflect the present posture of the hands, whereas those based on vibrotactile stimuli delivered well after the cue (250 ms) to move reflect the future posture (Hermosillo et al., Citation2011).
FIGURE 1. (A) Temporal order judgment (TOJ) task. We instructed participants to move as soon as they were given an auditory cue to one of four different sets of targets. An auditory cue to move was given 250 ms prior to, or coincident with, the first stimulus prior to the first hand stimulated. Tactile stimuli were applied 100 ms apart. Participants were instructed to move their hands to the targets as soon as they heard the tone, and then indicate verbally which hand was stimulated first. (B) Schema of arm configurations while performing TOJ. Participants were instructed to move to a specific target that would place their arms 45°, 90°, 135°, and 180° rotated from the starting position. Target location was held constant within each block.
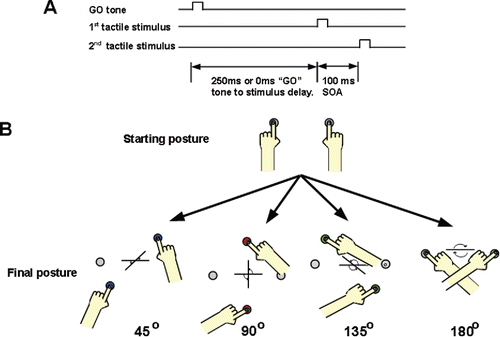
Participants performed a bimanual arm-crossing movement combined with a TOJ task (Hermosillo et al., Citation2011). For the arm-crossing task, participants were instructed to move as quickly and accurately as possible after the auditory cue in the transverse plane from the start location to one of four different sets of targets. The targets were located 45°, 90°, 135°, and 180° away from the starting position, such that resulting movement amplitude and degree of arm crossing varied systematically. A schema of the starting and final hand configurations is shown in . Infrared-emitting diodes were placed on each index finger and movements were recorded with an Optotrak Certus motion capture system (Northern Digital Inc., Waterloo, Ontario, Canada) at 500 Hz.
After the stimulation to both fingers and the arm-crossing movement, participants were required to make an unspeeded, forced-choice verbal TOJ decision indicating which finger (right or left) was stimulated first. Responses were made verbally and were recorded by the experimenter. Four separate blocks of 40 trials were completed in a random order for each of the four movement amplitudes. Each block comprised 10 trials of each combination of the two SOAs (0 or 250 ms) and hand stimulated first (left or right). Although the movement amplitudes were blocked and this experimental approach thereby minimized components of the planning process, the resulting variation in movement initiation times across trials (see Results) was nevertheless consistent with substantial planning occurring during the period leading up to movement onset. The order in which participants performed each movement amplitude block was randomized.
Data Analysis
The main dependent variables were the TOJ error rate, reaction time (time from go cue to movement initiation), movement time, peak acceleration, peak velocity, peak deceleration, and endpoint accuracy. The data were analyzed with a 2 SOA (0 vs. 250 ms) × 4 Movement Amplitude (45°, 90°, 135°, and 180°) repeated measures analysis of variance (ANOVA) with movement variables analyzed for each hand separately. Post hoc pairwise t tests (Bonferroni corrected) were completed to characterize any significant interactions.
Results
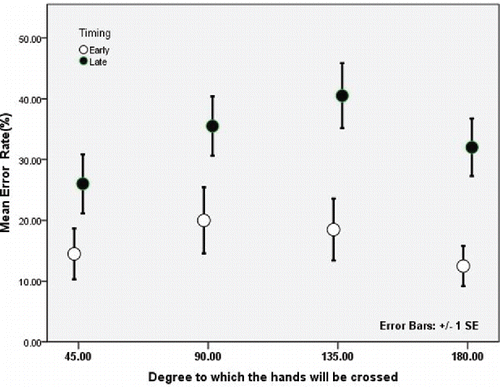
Consistent with our previous study (Hermosillo et al., Citation2011), we found a significant effect of SOA, such that tactile stimulation that was given early (i.e., coincident with the cue to move) resulted in lower TOJ errors than that given late (i.e., just before the participants started to move), F(1, 9) = 20.598, p < .001, partial η2 = 0.696 (). We also observed a significant effect of movement amplitude, F(3, 27) = 3.833, p = .021, partial η2 = .299, with crossing movements to targets at 45° leading to significantly lower TOJ error rates compared to the larger crossing configurations. Finally, there was a significant interaction between SOA and movement amplitude as well, F(3, 27) = 3.758, p < .022, partial η2 = 0.295. This interaction was driven by differences in TOJ error in early versus late trials for the 90°, 135°, and 180° targets, 90°: t(9) = −3.3109, p = .0091; 135°: t(9) = −3.2554, p = .0099; 180°: t(9) = −5.6488, p = .0003, that were not present for the 45° target, t(9) = −2.644, p = .0267, not significant after Bonferroni correction. This demonstrates that the larger amplitude movements that resulted in the arms being more crossed led to greater differences between the early and late presentations of the tactile stimulation. To further examine this issue, we completed a follow-up 2 × 2 repeated measures ANOVA that combined the error rates in the smaller amplitude (45° and 90°) versus larger amplitude (135° and 180°) movements to give a better sense of how the degree to which the hands were crossed at the end of the movement affected the error rates in the TOJ decisions and how this was influenced by the timing of the tactile stimulation. The results showed a significant interaction between the amplitude and SOA, F(1, 9) = 12.125, p = .007, partial η2 = .574, confirming that the TOJ error was larger when the hands ended up in a more crossed posture at the end of the movement.
FIGURE 2. Temporal order judgment (TOJ) error rates expressed as a function of cue time and the degree to which the hands were crossed. Error rates for TOJs in the early condition (−250 ms) are shown in white circles and those in the late condition (0 ms) are shown in black circles.
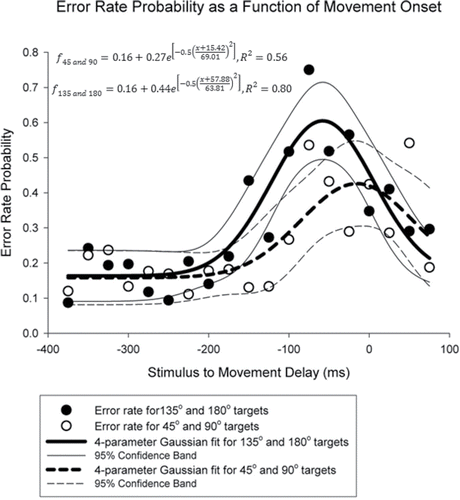
Because TOJ error rate may be a function of where a particular participant is in the planning process (Hermosillo et al., Citation2011), we reanalyzed the data by expressing it as a function of when the first vibrotactile stimulus was delivered relative to movement initiation (defined as < 20 mm/s movement velocity for each hand) for the smaller (45° and 90° targets) and the larger (135° and 180° targets) movement amplitudes separately. We realigned TOJ decisions into 25 ms time bins based on when the first vibrotactile stimulus given beginning at 500 ms prior to until 175 ms after movement initiation. This range reflects individual variation in movement initiation time. Next, we plotted the resulting error scores as a function of when the first vibrotactile stimulus occurred relative to movement onset. Time bins that contained fewer than five trials were not used for analysis, resulting in time bins ranging from −375 ms to +100 ms relative to movement onset. We fitted the two distributions mathematically (Sigmaplot, Systat Software Inc., San Jose, CA) using a four-parameter Gaussian fit across all participants to characterize the evolution of error rate probability across the planning period leading up to the beginning of the movement using equation Equation1(1)
(1) .
(1)
(1)
where y0 corresponds with the baseline error rate, α is the gain of the Gaussian, β describes the width of the distribution, and x0 corresponds with the position of the peak of the distribution.
The results of this analysis showed that both distributions were significantly fit by the Gaussian model, 45° and 90° conditions: F(3, 18) = 6.3348, p = .0055; 135° and 180° conditions: F(3, 18) = 20.257, p < .0001 (). Moreover, the TOJ errors in both distributions increased as movement onset was approached with the overall magnitude of the distribution being larger in the 135°/180° conditions (gain [α] value: 0.44 in 135°/180° conditions vs. .27 in 45°/90° conditions) and the peak occurring sooner in the 135°/180° conditions (distribution peak [x0] value: 57.879 ms prior to movement onset in 135°/180° conditions vs. 15.416 ms prior to movement onset in 45°/90° conditions). By contrast, the remaining parameters of the Gaussian fit were similar across the two sets of conditions. Unfortunately, because data were pooled from participants to generate mean error rates a statistical treatment beyond reporting the parameter values qualitatively was not possible.
FIGURE 3. Temporal order judgment (TOJ) error rate binned across different stimulation-reaction time onset asynchronies. Filled circles indicate TOJ error rates when participants moved to the more crossed configurations (135° and 180°) and open circles indicate TOJ error rates when participants moved to the less crossed configurations (45° and 90°). Lines represent a four-parameter Gaussian fit for these two groups. Negative values indicate that the first stimulus was applied to the hand prior to movement onset; positive values indicate that the first stimulus was applied to the hand after movement onset.
In addition to the TOJ errors, we also monitored the movements themselves to see if there were any differences induced by the TOJ task. One potential explanation of the changes observed in TOJ errors could be that the different movement amplitudes produce different reaction times, causing some TOJs to be worse simply because the reaction times were different. However, we did not observe any differences in reaction time among the degree of crossing, right hand: F(3, 27) = 0.564, p = .664; left hand: F(3, 27) = 0.869, p = .469, or interaction between the degree of crossing and SOA, right hand: F(3, 27) = 0.688, p = .567; left hand: F(3, 27) = 1.23, p = .671.
We observed a faster reaction time when tactile stimuli were applied at the same time as the cue to move, compared to when stimuli were given just before movement onset, for the both the left and right hand: left hand (304 ms vs. 372 ms), F(1, 9) = 14.452, p = .004; right hand (292 ms vs. 356 ms), F(1, 9) = 13.028, p = .006. We also observed a main effect of SOA on the time to peak acceleration for both the left and right hand, left hand (87 ms vs. 94 ms), F(1, 9) = 10.967, p = .009; right hand (92 ms vs. 102 ms), F(1, 9) = 8.357, p = .018, and on the time to peak velocity for the left hand only, left hand (187 ms vs.198 ms), F(1, 9) = 14.038, p = .005); right hand (220 ms vs. 229 ms), F(1, 9) = 4.701, p = .058. Movement amplitude affected multiple kinematic variables as expected (see ). In addition, there was a significant interaction between when the stimuli were given and the amplitude of the limb movement on peak velocity for the left hand, such that peak velocity increased more for larger amplitude movements when stimuli were applied early compared to late, F(3, 27) = 6.165, p = .002. Additionally, when stimuli were applied early (0 ms SOA) compared late (250 ms SOA), peak deceleration for the left hand increased most for the 90°, 135°, and 180° movements, F(3, 27) = 4.983, p = .007.
TABLE 1. Kinematic variables
Discussion
The ability to produce smooth, coordinated upper-limb movement is the result of a complex sensorimotor transformation involving multiple cortical and subcortical brain regions. It is clear that the planning and execution of these movements must be flexible to allow for online adjustments that become necessary when the trajectory is less accurate or more variable than originally intended. While sensory feedback may provide relevant information for such adjustments, it is thought to be too sluggish to be effective during the movement. Rather, it is clear that predictive signals related to the expected sensory consequences of the planned movement are likely at the root of any online adjustments that occur. While such signals are clearly required from a theoretical perspective, the ability to directly infer their influence has been a challenge. The TOJ-bimanual arm-crossing task that we have developed (Hermosillo et al., Citation2011) allows direct insight not only into the contribution of such predictive signals to ongoing movement, but also how this contribution evolves over the course of the planning period.
In the current study, we sought to gain further insight into how these signals are modulated by the characteristics of the movement itself. In particular, we asked how the predictive signals were influenced by the magnitude of the arm crossing movements. This was accomplished by varying the amplitude of the targets to which the movements were made such that the arms ended up in a more or less crossed configuration at the end of the movement. We found that planning to cross the arms caused an increase in TOJ error rates that slowly evolved as the onset of movement was approached, despite the fact that participants started in the same position. The pattern of results suggests that as the planning period progresses, TOJ errors initially reflect the uncrossed limb configuration, but ∼200 ms prior to movement onset errors begin to resemble that of the final crossed posture. We propose that this finding is consistent with sensory prediction signals being used during the planning period which, in turn, bias the TOJ decision and that these signals are directly influenced by the extent to which the hands will cross. Previous work has demonstrated TOJ performance becomes progressively worse when participants limbs are placed in increasingly more crossed postures in a stationary manner (Yamamoto & Kitazawa, Citation2001). Here, we demonstrate that the limbs need not be physically crossed to see an increase in TOJ error rate, rather the participant simply needs to plan to be have his or her limbs crossed in the future.
In our previous study, the movements made with each hand were equal in amplitude, opposite in direction, and ended at the starting location of the other hand (Hermosillo et al., Citation2011). Under such circumstances, the predictive planning may be relatively simple. If this is true, then movements in which these details are varied should result in systematic changes in predictive planning as revealed by the pattern of TOJ responses and characteristics of the movements themselves. Indeed, we have shown that when a small and large amplitude movement are combined in our task, the movements take the same amount of time (Kelso, Southard, & Goodman, Citation1979) even though the larger movements lead to greater TOJ errors when the hand making the larger movement is stimulated first (Hermosillo et al., Citation2011). This suggests that the predictive planning induced by movement may be amplitude dependent and led us to speculate that manipulating characteristics such as movement amplitude would result in changes in the predictive planning process. The present results are consistent with this speculation.
Multiple neural structures are thought to be involved with the predictive process, including the cerebellum (Miall et al., Citation2007), the posterior parietal cortex (Mulliken, Musallam, & Andersen, Citation2008), and premotor cortex (Churchland, Yu, Ryu, Santhanam, & Shenoy, Citation2006). A recent study from our group demonstrated that a single pulse TMS 200 ms prior to hand stimulation over the posterior parietal cortex was sufficient to increase TOJ error rates when the contralateral hand was stimulated first (Ritterband-Rosenbaum et al. Citation2014). Interestingly, when the arms were crossed, TMS to the same region caused an increase in TOJ error rates for the ipsilateral hand, suggesting that the posterior parietal cortex is responsible for keeping track of the time of cutaneous stimuli, possibly for the purposes of predictive planning. Additionally, this suggests that spatial information related to limb postures is encoded in a global reference frame within the posterior parietal cortex, in a manner suitable for reaching. It is important to note that when we fitted Gaussian distributions to the data, we observed a marked increase in the gain of the error rates when participants were planning to physically cross their limbs compared to simply moving their hands to an uncrossed posture, further reinforcing the hypothesis that motor planning processes influence future spatial limb localization.
Extrapolation of these findings suggests that disruption of the cortical mechanisms responsible for movement prediction during the planning process should modulate TOJ errors due to interference of the predicting final limb posture. Because the precise timing of motor planning signals coupled with sensory information needs to be coordinated on a millisecond time scale, investigation of this process through functional magnetic resonance imaging is not yet possible. Additional experiments targeting specific anatomical structures associated with motor prediction with TMS would help elucidate how they contribute to forward modeling.
ACKNOWLEDGMENTS
Mr. Hermosillo and Dr. van Donkelaar designed the experiment. Mr. Hermosillo, Ms. Carmody, and Mr. Ugoalah carried out the experiments under the supervision of Dr. van Donkelaar. Drs. Binsted and Lee provided help in data analysis/interpretation and manuscript editing. Mr. Hermosillo and Dr. van Donkelaar analyzed the data and wrote the manuscript.
Funding
This research was supported by National Science and Engineering Research Council of Canada (NSERC) Discovery Grants and Canadian Foundation of Innovation Grants awarded to Drs. van Donkelaar and Binsted. Mr. Hermosillo was supported by the Graduate School at the University of British Columbia.
REFERENCES
- Buckingham, G., Carey, D. P., Colino, F. L., deGrosbois, J., & Binsted, G. (2010). Gating of vibrotactile detection during visually guided bimanual reaches. Experimental Brain Research, 201, 411–419.
- Churchland, M. M., Yu, B. M., Ryu, S. I., Santhanam, G., & Shenoy, K. V. (2006). Neural variability in premotor cortex provides a signature of motor preparation. Journal of Neuroscience, 26, 3697–3712.
- Dassonville, P. (1995). Haptic localization and the internal representation of the hand in space. Experimental Brain Research, 106, 434–448.
- Desmurget, M. & Grafton, S. (2000). Forward modeling allows for feedback control for fast reaching movements. Trends in Cognitive Sciences, 4, 432–431.
- Diedrichsen, J. Shadmehr, R., & Ivry. R. B. (2010). The coordination of movement: optimal feedback control and beyond. Trend in Cognitive Sciences, 14, 31–39.
- Hermosillo, R., Ritterband-Rosenbaum, A., & van Donkelaar, P. (2011). Predicting future sensorimotor states influences current temporal decision making. The Journal of Neuroscience, 31, 10019–10022.
- Kelso, J. A., Southard, D. L., & Goodman, D. (1979). On the nature of human interlimb coordination. Science, 203, 1029–1031.
- Makoshi, Z., Kroliczak, G., & van Donkelaar, P. (2011). Human supplementary motor area contribution to predictive motor planning. Journal of Motor Behavior, 43, 303–309.
- Miall, R. C., Christensen, L. O. D., Cain, O., & Stanley, J. (2007). Disruption of state estimation in the Human Lateral Cerebellum. PLOS Biology, 5, e316.
- Miall, R. C., & Wolpert, D. M. (1996). Forward models for physiological motor control. Neural Networks, 9, 1265–1279.
- Mulliken, G. H., Musallam, S., & Andersen, R. A. (2008). Forward estimation of movement state in posterior parietal cortex. Proceedings of the National Academy of Sciences, 105, 8170–8177.
- Ritterband-Rosenbaum, A., Hermosillo, R., Kroliczak, G., & van Donkelaar, P. (2014). Hand position dependent modulation of errors in vibrotactile temporal order judgments: The effects of transcranial magnetic stimulation to the human posterior parietal cortex. Experimental Brain Research, 232, 1689–1698.
- Schicke, T., & Röder, B. (2006). Spatial remapping of touch: Confusion of perceived stimulus order across hand and foot. Proceedings of the National Academy of Sciences, 103, 11808–11813.
- Shadmehr, R., Smith, M. A., & Krakauer, J. W. (2010). Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience, 33, 89–108.
- Shore, D. I., Spry, E., & Spence, C. (2002). Confusing the mind by crossing the hands. Cognitive Brain Research, 14, 153–163.
- Yamamoto, S., & Kitazawa, S. (2001). Reversal of subjective temporal order due to arm crossing. Nature Neuroscience, 4, 759–765.
