Abstract
Our study aimed to analyze movement control strategies using predefined criteria for amplitude and differences in these strategies between children with and without DCD. Children with (n = 28) and without DCD (n = 15) were included. A video-observation-tool was used to score the moving body parts during a Wii Fit slalom task over multiple time points. Two-step cluster analysis was used to extract distinct movement strategies. Two different movement strategies were identified that were independently validated by a measure of task performance and a subjective mark of quality of the movement. Initial differences between groups and changes over time toward the more successful strategy were found in both groups, albeit in a different percentage. This study shows that the more efficient movement strategy is seen in the majority of the TD children and only in a small number of children with DCD, even after practice.
Background
In a number of sports, like figure skating, freestyle skiing, freestyle snowboarding or gymnastics, a jury evaluating the quality of performance of the athletes uses a set of predefined criteria. Apparently the jury believes that observation will provide reliable information. Likewise, in clinical practice observation is often used to detect deviations from commonly seen coordination patterns. A field where clinical observation of movement patterns is applied on a day-to-day basis is pediatric physical therapy, which deals with developmental motor or coordination problems, such as in developmental coordination disorder (DCD). Children with DCD show problems in fine and/or gross motor function (Geuze, Jongmans, Schoemaker, & Smits-Engelsman, Citation2001; Larkin & Rose, Citation2005), and the majority have problems with static and dynamic balance (Cherng, Hsu, Chen, & Chen, Citation2007; Deconinck et al., Citation2006; Deconinck, Savelsbergh, Clercq, & De Lenoir, Citation2010; Geuze, Citation2003; Grove & Lazarus, Citation2007; Hoare, Citation1994; Jelsma, Geuze, Mombarg, & Smits-Engelsman, Citation2014; Macnab, Miller, & Polatajko, Citation2001; Visser, Kalverboer, & Geuze, Citation1998). Parents and teachers are often the first to notice something is different in movement patterns or motor behavior of children with DCD, not just because they note poor performance, but also because the movements appear unhandy and awkward. Because of this capacity, parents and teachers are considered important sources of information both in screening procedures (through parent and teacher questionnaires) and in support of an indication of motor problems (Schoemaker, Flapper, Verheij, Wilson, Reinders-Messelink, & de Kloet, Citation2006; Schoemaker, Flapper, Reinders-Messelink, & de Kloet, Citation2008; Wilson, Kaplan, Crawford, Campbell, & Dewey, Citation2000). Concerns noticed by nonprofessionals may be followed by formal diagnostic procedures including a standardized motor test to confirm or dispute a motor impairment (American Psychiatric Association, Citation2013; Blank, Smits-Engelsman, Polatajko, & Wilson, Citation2012; Smits-Engelsman et al., Citation2013). However, although parent and teacher observations when compared to formal diagnosis are quite accurate, they also may lead to a considerable proportion of false negatives or positives (respectively Schoemaker et al., Citation2006, Citation2008). Therefore, it is of interest to develop other observational techniques to objectify abnormalities or changes in postures and movements. Preferably techniques that do not rely on high-tech equipment like 3-D motion analysis (Beynon, McGinley, Dobson, & Baker, Citation2010; Mündermann, Corazza, & Andriacchi, Citation2006; Ounpuu, Citation2004), EMG (Logan, Kiemel & Jeka, Citation2017), and accelerometers (Lugade, Fortune, Morrow, & Kaufman, Citation2014), being too costly and complex for usual clinical practice. The current study aims to explore if in a complex dynamic balance task children use different control strategies that can be observed from movement amplitude characteristics, and if the use of different strategies changes over time due to training and differentiates between children with and without DCD in terms of efficiency.
Although clinical observation to detect the difference between normal and deviant movements is used on a daily basis, it has not been studied extensively. Beynon et al. (Citation2010) showed in their study that clinical observation seems valid. Detection of abnormalities in gait by observation of kinematics by clinicians appeared to be similar to the overall laboratory kinematic data of gait deviation (Beynon et al., Citation2010). However, some deviations from normal, like an increase of 3-D knee abduction during squat can hardly be recognized by visual observation (Yamazaki, Muneta, Ju, & Sekiya, Citation2010). Therefore, we explored if a standardized protocol using a set of predefined criteria could provide insight into the kinematic aspects of a dynamic balance task.
VR games are suitable to evaluate distinct balance control strategies. Playing motion steered games may help children to focus on the task and reduce the distraction from the observers or cameras. An optimal strategy will usually unfold after exploring a certain amount of training (Schmidt & Young, Citation1987). An example of a VR study of strategy use is the study of Michalski et al. (Citation2012). A typically developing group of young adults adopted distinct postural control strategies when repeatedly playing the game. The movements became tuned to the task at hand. The game in which a skier has to shift weight in order to pass gates during each run induced a lower extremity strategy (hip and knees) to control the game, while a strategy with more trunk and head movement was used in order to head a soccer ball (Michalski et al., Citation2012). Most of the VR games that involve control of balance use shifting weight while standing on two feet to control the VR-task at hand. Lateral shift of weight is primarily controlled by the hip abductors/adductors (Winter, Prince, Frank, Powell, & Zabjek, Citation1996). This strategy of control is efficient because simple load/unload movements are easier and safer compared to other strategies that use rotation of the trunk or large arm and head movements to induce a change in weight shift.
In the present study, we used video observation to detect effective and ineffective strategies in children with and without DCD. We also investigated if non-optimal weight shifting strategies changed over time (i.e., with experience), to gain insight if participants would change towards the use of more successful strategies. When a task is new, initially several strategies are explored from which one emerges as the dominant one. Normally this will be the optimal strategy, but this change of movement kinematics may be different in children with DCD, given their limited or different ability to adapt their movements (Gentle, Barnett, & Wilmut, Citation2016; Diamond, Downs, & Morris, Citation2014; Du, Wilmut, & Barnett, Citation2015). From previous studies, we know that children with DCD are less successful in playing motion steered computer games (Gonsalves, Campbell, Jensen, & Straker, Citation2015; Jelsma et al., Citation2014; Mombarg, Jelsma, & Hartman, Citation2013). This may be related to the use of less efficient movement strategies.
The specific question addressed in the present study is if observers using a video observation protocol can discriminate efficient from less efficient movement control strategies. A protocol was developed to include observable aspects of the movements of body parts based on available knowledge about balance strategies in the literature. As one of the key features in the majority of children with DCD is poor balance control (Cherng et al., Citation2007; Deconinck et al., Citation2006; Deconinck et al., Citation2010; Geuze, Citation2003; Grove & Lazarus, Citation2007; Hoare, Citation1994; Jelsma et al., Citation2014; Macnab et al., Citation2001; Visser et al., Citation1998), to study these movement control strategies we selected a dynamic balance task in a virtual environment. More specifically we used a virtual reality (VR) game that children like to play – ski slalom – which requires whole body movement to control the game.
It is known that the kinetics of control in a ski slalom task of children with DCD differs from that of TD children at the level of displacement of the center of pressure (COP) during exergaming (Jelsma et al., Citation2016). However, differences in COP displacements may originate in opposite movements of upper or lower body or displacements of the feet, which cannot be detected in the COP only. Structured observation seems to offer a more holistic approach that may enable to detect functional differences of the displacement of body parts to control the VR task. Therefore, we developed an observation tool that quantifies the movements of the different body parts during the ski slalom game of the Nintendo Wii Fit using predefined criteria. First, based on several studies (Gog van et al., Citation2009; Michalski et al., Citation2012) and the theory of kinematics of posture (Gage, Winter, Frank, & Adkin, Citation2004; Horak & Nashner, Citation1986; Winter, Patla, Prince, Ishac, & Gielo-Perczak, Citation1998) it was anticipated that at least two distinct strategies could be used. The shift of weight to the right or the left is primarily controlled by the hip abductors/adductors (Winter et al., Citation1996). This lower limb strategy is efficient because simple load/unload movements are easier and safer compared to strategies that use large arm, head and trunk movements to induce a shift of weight.
The first aim of the study was to identify and validate dynamic balance control strategies. We collected data on the maximum lateral displacement of head, shoulder and hip, involvement of arms and displacement of feet. We used cluster analyses to extract strategies, and validated the efficiency of the clusters by comparing the average performance (the mean number of successfully passed gates) and the average mark for quality of coordination, as independent measures of efficiency. Our second aim was to detect whether TD children and children with DCD differ in the use of movement strategies. We predict that children with DCD will show larger upper body movement and larger movements of the arms. Subsequently we explored whether children change the use of strategies over time or due to intervention, both at group and individual level. We hypothesized that children without DCD would use more efficient strategies than children with DCD and that after intervention children with DCD would use more efficient and consistent strategies.
Methods
Participants
Data were collected from 43 children between 6 and 11 years old, of which 28 children with DCD were recruited from two primary schools for special education and 15 typically developing children from a mainstream primary school in the same region in Groningen, the Netherlands. The children with DCD were selected from referrals to pediatric physical therapy for motor coordination problems as noticed by parents and teachers. All children were tested using the Movement Assessment Battery for Children-2 (MABC-2) (Henderson, Sugden & Barnett, Citation2007; Smits-Engelsman, Citation2010). Children were included in the DCD group if their total test score was below a standard score of 7 (<16th percentile). Typically developing children were included in the TD group if their total test score was at or above a standard score of 10 (≥50th percentile). Children diagnosed with a medical or neurological condition, mental disorder or IQ < 70, as confirmed by either the Wechsler Intelligence Scale for Children (WISC) or the Snijders-Oomen Nonverbal intelligence tests (SON-R test) (Tellegen, Citation1993; Wechsler, Citation2003), were excluded. To make sure, we included children with and without balance problems, we checked the Balance component score of the MABC2 and we set the criterion at or below a standard score of 7 (≤16th percentile) for the children with DCD and at or above a standard score of 10 (≥50th percentile) for the TD children. For this reason, one child with DCD was excluded from the data, since the MABC-2 component balance score was above a standard score of 7. Demographic characteristics and mean MABC-2 scores of both groups are presented in .
TABLE 1. Demographic characteristics and mean MABC-2 scores of the TD and DCD group, tested for differences with the independent sample t-test.
The study was approved by the Ethics Committee of the Department of Psychology, of the University of Groningen and permission to conduct the study was granted by the designated educational authorities. All parents and children gave their informed consent or assent.
Instruments
Movement Assessment Battery for Children, second edition (MABC-2)
The Dutch version of the MABC-2 is a standardized motor test to determine the level of motor proficiency of children aged 3–16 years, compared to peers (Henderson et al., Citation2007) and has norms for the Dutch population (Smits-Engelsman, Citation2010). The total standard score is the summed score of the components manual dexterity, aiming and catching and balance. A score of >7th standard score represents normal motor performance, of 6&7 represents at risk for motor problems and ≤5th standard score a definite motor problem, according to the European Guideline for DCD (Blank et al., Citation2012).
Apparatus
Wii Fit ski slalom test
The Wii Fit ski slalom test consisted of ten repetitions of the ski slalom game. The Nintendo Wii Fit system with balance board was used for the present study (see Appendix 1). To score maximum lateral movement amplitudes, a white paper of 1 m wide and 1.9 m high was attached to the wall. The paper had eleven vertical grid lines 10 cm apart marked from left to right –5; –4; –3; –2; –1; 0; 1; 2; 3; 4; 5, with the zero in the center. The Wii balance board (WBB) was placed 50 cm in front of the zero mark on the paper and the game monitor was placed at a distance of 2 m from the WBB at 75 cm height in line with the balance board and the zero vertical line. A video camera on top of the monitor recorded the child on the balance board frontally and the avatar of the game (Mii) through a mirror. The balance board had two marked spots 25 cm apart for the children to position their feet on these spots.
The Wii Fit ski slalom game was used to study the dynamic control of the body posture, that is, the shifts of weight applied to control the avatar without losing balance. In this game, one can steer the avatar skier (Mii) by shifting weight sideways and control speed by shifting weight forward or backward. Every run had 19 identical gates to be passed at varied lateral and forward distances on the slope. The number of missed gates and time to finish was recorded. The Wii software derives a Wii score from the following equation: Wii Score = T + (# × 7 s), where T is the time taken to reach the end of the slope, # is the number of missed gates and 7 s is the penalty in seconds for each gate missed. Thus, a higher Wii score reflects worse performance. Both the number of passed gates and the Wii score are used for the analysis.
Video observation tool
The video records were scored at slow speed by a group of five trained observers who recorded the lateral displacement of landmarks of head and body, and movement of the extremities. Right before each run the width and position of the shoulders and hips was determined. This ‘baseline’ information was used to center the body landmark data and correct for body width.
The observers selected the two largest excursions that occurred during a run. At these points in time they recorded the lateral amplitude from the background raster on a 5-point scale (range 0–4) of the following observational variables: lateral side of the head at ear height, both sides of the location of the acromion as upper trunk, trochanter major as hip and the outside of both feet. Arm movements were scored according to predefined categorical scores of positions on a 5-point scale (range 0–4), ranging from the arm as relaxed, no large arm movements to swings beyond –5 and 5 of the grid lines. Movement of the feet was scored on a 5-point scale ranging from standing still to a corrective step due to loss of balance (range 0–4). After scoring the observers watched the run at real time speed and a single mark (range 1–10; 1= no; 10= perfect control) was given for each run evaluating the quality of control (smoothness of movements) during the run. For details, see Appendix 1.
Observers
Five students of the Human Movement Science and Psychology departments of the University of Groningen were trained by the first author on scoring the videos according to protocol. After training they scored 7 videos each, the Intraclass Correlation Coefficient (ICCs) between observers was calculated on the agreement of movement scores in the protocol and marks given for the performance of the children in these seven runs. Overall, the reliability between testers was excellent (ICC .98) and split per variable acceptable to high (). The reliability within observers was excellent (all ICCs >.93). All testers were blinded for the type of child and test moment.
TABLE 2. Inter-tester reliability for the video observation protocol (5 testers; 7 videos).
Procedure
Children were first tested with the MABC-2. Children who fulfilled the selection criteria then proceeded to the VR ski slalom test. They started the procedure creating an individual Mii. The WBB was calibrated for the individual height and weight and all children completed the Wii basic balance test in order to get acquainted in a standardized way with steering the Mii. They then performed the Wii Fit ski slalom test. Next, the children of the DCD group were offered a training period of 6 weeks of active gaming on the balance board in half-hour sessions, three times a week. Each practice session comprised of a self-selected choice out of ten Wii balance games (see Appendix 2), except for the ski slalom game, with the restriction that they could only play the same game twice in a session. Specially trained Physical Education and Physiotherapy students instructed and supervised the training. The first author coordinated and supervised the intervention. The children of the TD group were instructed and agreed not to be involved in any practice with the Wii Fit. After 6 weeks, both groups completed the postmeasurement Wii Fit ski slalom test.
Analysis
Forty-three children (DCD group 28 children and TD group 15 children) × 10 runs × 2 (pre- and posttest) yield 860 data points. Missing scores led to 827 complete data entries with five observation variables (head, shoulder, hip, feet and arm), mark, Wii score and passed gates. Differences between groups of the distribution of the scale scores were tested using Chi-square analysis. The mean of the movement scores was calculated per group per run.
Extraction of strategies by cluster analysis
Two step cluster analysis was used because it is a reliable method to identify in an exploratory manner the optimal number of clusters. It selects the number of clusters automatically and aims to explain the greatest amount of variance. Two-step cluster analysis using SPSS Version 23 (SPSS Inc., Citation2001, pp. 361–391) was performed using the five observation variables. The smallest Schwarz’s Bayesian Criterion (BIC) indicating the “best” cluster solution determined the clustering criterion.
Per variable appropriate parametric or non-parametric tests were used to test whether observation variables were significantly different between clusters. ANOVA was used to test whether clusters differed in performance measures (number of passed gates, Wii score: the lower, the better) and subjective mark for quality of movement control (the higher, the better). The size of the between cluster effects was determined by Cohen’s d effect size and classified as: >.20 small, >.50 moderate and >.80 large effect size (Cohen, Citation1988).
Cluster allocation: Consistency of cluster used was determined per test moment per child based on the 10 runs. If 7–10 runs were assigned to the same cluster for the series of 10 runs of that child was recoded as consistent to that cluster. If the child shifted between clusters, that is, if only 4 to 6 runs were in the same cluster, these ten runs were recoded as inconsistent. Frequencies of the cluster allocation at pre- and postmeasurement and between TD children and children with DCD were compared using repeated measures GLM with time (2) as within and group (2) as between factor.
Results
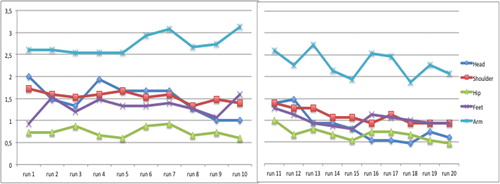
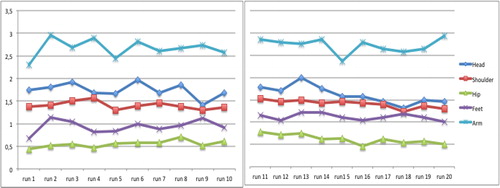
The mean scores of the observed variables per group and per run are displayed in . Distribution of movement scores over sessions was different between groups for head (χ2=65.7, p<.001), hip (χ2=10.0, p=.007), feet (χ2=16.0, p=.003) and arm (χ2=16.0, p=.003), but not for shoulder/upper trunk (χ2=8.0, p=.093), showing large amplitude movements and/or feet displacements being present more frequently within the DCD group. Over time, movement amplitudes decreased in the TD group, especially for head, shoulder/upper trunk and arm movements, while such a decrease was not present in the DCD group. The five observed variable scores of all runs (20 per child) have been used for cluster analysis.
FIGURE 1. (A) Mean scores of the observed variables per run at pretest and 6 weeks later for the TD group; range reflecting minimal displacement (scale score 0) to largest movements (scale score 4).
Clusters
Two-step cluster analysis revealed separation into two clusters, with highest predictor importance for shoulder/upper trunk and head movements. The silhouette measure of cohesion and separation of 0.2 suggested” fair” cluster separation. Ratio of cluster sizes was 1.54, which is considered good, with 501 runs in cluster 1 and 326 in cluster 2. The predominant characteristics of observation variables in the two identified clusters are outlined in . Overall in cluster 1 scores represent minimal movements and in cluster 2 amplitudes are larger. The number of passed gates, Wii score and mark were all significantly different between the clusters (respectively, F(1,826)=10.3, p=.001, Cohen’s d=.23; F(1,826)=11, p=.001, Cohen’s d=.24; F(1,826)=68.4, p<.001, Cohen’s d=.61), validating the difference in efficiency of the clusters, and indicating that cluster 1 was the more successful cluster. Mean number of passed gates, Wii scores and mark of the runs per cluster are displayed in .
FIGURE 2. The observational variables per cluster with the range of score from small (0) towards large movements (4) in percentages. Note that higher columns for values of cluster 1 (blue) on the left display less excursion of the body, while cluster 2 shows more values on the right side of the figures.
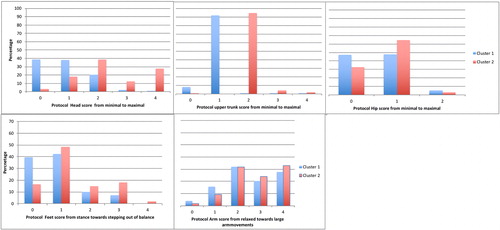
TABLE 3. The mean passed gates, Wii score (the lower, the better) and mark (≥ 5.5 is considered to be a pass) per cluster over all children and test moments.
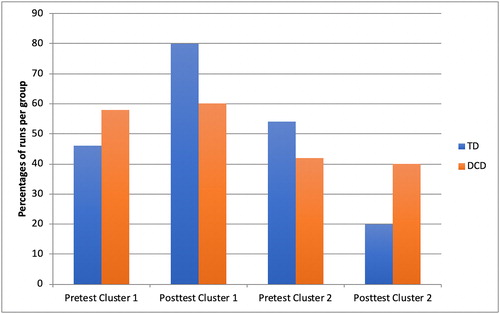
Differences between groups per test moment
Overall, both TD children and children with DCD used more often cluster 1. Over pre- and posttest together runs of the TD children were classified as cluster 1 in 63% and as cluster 2 in 37%. Those of children with DCD were classified as cluster 1 in 59.2% and as cluster 2 in 40.8% of all runs. In , the percentage of runs and the difference between pre- and posttest is depicted. For the TD group, 46% was classified as cluster 1 and 54% as cluster 2 at pretest and at posttest 80% as cluster 1 and 20% as cluster 2. For the DCD group, this change was smaller with 58.2% of the runs classified as cluster 1 and 41.8% of the runs as cluster 2 at pretest; and 60.1% as cluster 1 and 39.9% as cluster 2 at posttest.
Within the clusters the Wii performance was different in the rate of success between the two groups. T-tests revealed significant differences between the TD children and the children with DCD for passed gates, Wii score and mark within cluster 1 and 2 (all p≤.015). Overall, in cluster 1 a significant difference was found for passed gates, Wii score and mark (respectively, t = 16.2, p<.001; t=–16.8, p<.001; t = 9.2, p<.001), which remained significantly different when tested per moment (all p<.001). Within cluster 2 the runs of both groups differed at pretest for passed gates and Wii score (p<.001), but not for mark (p=.223) and at posttest all variables were significantly different (p≤.016) ().
TABLE 4. Mean passed gates (SD) and mean mark (SD) per cluster per group and test moment.
Consistent use of clusters between pre- and posttest
At pretest children within the TD group used cluster 1 more often than cluster 2. Over time more TD children moved towards the characteristics of cluster 1 and became more consistent in cluster use. The DCD group showed a similar pattern of change after intervention (). Repeated measures revealed an effect of time on consistency (F(1,32)=15.3, p<.001, ηp2=.32), but not between groups (F(1,32)=1.4, p=.240, ηp2=.04), nor an interaction between time and group (F(1,32)=9.4, p=.166, ηp2=.06).
TABLE 5. Consistency of cluster allocation over 10 runs per child between groups and test moments in percentages.
Discussion
This study explored the validity of using video observation in detecting efficient versus less efficient movement control strategies in children with and without DCD. Using a structured video observation tool, performance data and cluster analyses to extract control strategies we succeeded to distinguish between two strategies, one being superior to the other in performance and general mark for quality of control. The less efficient strategy was characterized by larger amplitudes of head and shoulder/upper trunk movements. The larger movement amplitudes were used more often during the first ten trials as compared to the second series 6 weeks later, which suggests that an implicit adaptation of movement behavior took place with practice.
Criterion validity of the clusters identified was evident: Cluster analysis yielded two clusters that could be interpreted as strategies and that were different on independent measures of performance and quality of coordination; also, the cluster allocation and the level of success differentiated between groups indicating ecological validity. The two clusters differentiated in passed gates, Wii score and mark. The cluster that resulted in the highest number of passed gates supported the expectation that the best movement control strategy would be small lateral shifts of weight of the different body parts within the base of support with the feet remaining a constant base of support. The lesser successful strategy was characterized by shoulder/upper trunk and head movements often shifting further than the position of the feet as base of support and more frequent lifting of a foot. Although differences in performance of clusters were significant, they were small, indicating that not only the spatial aspects of the task as scored in the VOT are important, but also other factors not captured by rating the lateral shift. Timing the right amount of lateral shift (temporal constraint), is probably another important determining factor for successfully passing gates.
It is known that better postural control, due to preparatory muscle activity that stabilizes the trunk and legs, results in diminished and less frequent oscillations of postural sway in the proximal body segments (Hayes, Citation1982; Odenrick & Sandstedt, Citation1984). Based on the literature it was expected that the children would keep the head, arm and trunk more or less stabilized, while the hip joint would be doing most of the work in shifting the weight in lateral direction. This would be similar to the strategy used by the participants standing on one leg on a narrow ridge (Otten, Citation1999) or on a slackline (Serrien, Hohenauer, Clijsen, Taube, Baeyens & Küng, Citation2017). Stabilizing the head, known as quiet eye phenomenon, will provide visual focus and time to organize visuomotor planning and control of the action (Vickers, Citation2011). It is known that at the age of 6 years or older a transition of postural behavior occurs, with the head becoming the dominant frame of reference (Assaiante & Amblard, Citation1993; Riach & Starkes, Citation1989). During the Wii Fit ski slalom game the eyes should stay fixated on the avatar on the screen. This might explain the minimal head and shoulder/upper trunk movements as a characteristic of this more successful cluster, giving the best opportunity to use visual information for utilizing feed forward control and feedback (Fransson, Johansson, Hafström, & Magnusson, Citation2000; Jelsma et al., Citation2014). In this framework, it fits that the more controlled movement strategy for the ski slalom task (cluster 1) combines minimal displacement, maximization of visual information and task efficiency.
The less successful cluster was characterized by an upper-body strategy, with larger movement amplitudes and a frequent use of heel lift or corrective step. Lifting a heel can be considered a strategy to shift some weight to the other foot, or to hold balance when there is danger of loss of balance to the front (Hayes, Citation1982). A corrective step will be made when there is risk that the center of gravity will move outside the base of support. The more frequent large arm movements may indicate a corrective movement to avoid loss of balance. The larger movements might also reflect a compensation for movements of other parts of the body in the wrong direction or correction needed for inaccuracies caused by the preceding movement – a sign of unproductive task-related movement control. Moving the head to one side has a relatively large effect on balance due to its distance from the axis of the ankle joint, requiring more correction by stabilizing torques in the linking joints of the different segments. Additionally, large head movements will result in less visual control and would require a countermovement in the hips to stay in balance, as shown by one of the children presented in Appendix 3. It is known that children with DCD are limited in moving their center of gravity towards the boundaries of their base of support (Jelsma et al., Citation2016). It may be the case that the larger movements combined with the countermovement in the hips/arms/feet would result in a net lack of weight shift or a less controlled weight shift. For these reasons, the larger movement strategy is the least efficient strategy.
Differences between TD and DCD group
The second research question aimed at differences of movement strategies between the TD and DCD group. At pretest, half of the TD children used consistently and significantly more successful the smaller movement amplitude strategy and passed a mean of 15.2 gates. However, the other half of the children used larger movements in which still a mean of 13 of the 19 gates were passed. These findings suggest that not only the amplitude of the weight shift needed for spatial control of the game is an important indicator for success. Importantly, the children with DCD who used the same small movement strategy at pretest were less successful in passing the gates (9.9 gates) and more often got a mark indicating poor control. Within strategies the TD and DCD groups differed on the independent markers for movement control: performance and mark for quality, which may be explained by the overall less smooth movements of the children with DCD.
The children with DCD were less consistent in their movement strategy at pretest. Apparently, initially both children with and without DCD have the tendency to vary the amplitudes of their movements in order to find the best strategy for success in the game. When looking at the consistent use of strategy at pretest, it appeared that the children with DCD showed more variability in their initial performance. This phenomenon has also been seen in obstacle crossing in the study of Deconinck et al. (Citation2010) in which the children with DCD demonstrated greater mediolateral motion of the Center of Mass during the crossing stride of the obstacle, in comparison to their peers. Inconsistency in repeated task execution or requiring a longer exploration phase may well be a limiting factor in learning and automatizing movement skills.
Change of movement behavior at posttest
At posttest, the majority of the TD children used consistently the more efficient smaller movement amplitude strategy even more successfully. After 6 weeks of training with other VR games, the children with DCD presented less an inconsistent strategy and showed a shift towards the use of the smaller movement amplitude strategy, with better results of both the task and the mark. Apparently, children with DCD do learn to improve their scores after a variable training. However, it appears that this is probably more the effect of improved anticipation and reaction (Bonney, Jelsma, Ferguson, & Smits-Engelsman, Citation2017; Jelsma et al., Citation2014), and to a lesser extent the effect of an adaption of movements to the more efficient strategy.
An unexpected finding was that the TD and the DCD children who used the larger amplitude movement strategy still showed an improvement at posttest. This signifies that even when using a non-optimal control strategy, performance may improve; however, this improvement will likely be less in rate and final level compared to using an optimal control strategy. Former studies showed that children with DCD fail to adapt to a new or more efficient strategy but rather persisted in their less efficient initial strategy (Biotteau, Chaix, & Albaret, Citation2016; Goodgold-Edwards & Cermak, Citation1990). In our study, this was only the case for part of the group, since 20% of the children with DCD shifted towards more consistency and to the more successful smaller movement amplitude strategy. This study partly confirms that children with DCD were inconsistent in the use of strategies, but also showed that part of the children with DCD are able to adapt and explore another movement strategy.
Usefulness for clinical practice
Our video observation tool proved to be a tool that non-clinical testers could use after training in a reliable way. Using video recordings can be an additional tool to detect inefficient movement strategies and changes over time. In dynamic balance tasks, it can be advised to monitor the upper trunk and head movements compared to the hip sway and foot stance in order to evaluate the use of the most efficient smaller movement amplitude strategy. However, improvement of the task can also be found when children are still using a less efficient strategy. This can both be found in children with and without DCD. Besides the spatial control of the task, the temporal control may be equally important to determine control of dynamic balance tasks.
The clinical implication of this study is that Wii Fit balance training can offer children with DCD the opportunity to implicitly explore movement strategies that can help them to adapt to a more efficient strategy with a decrease of movement amplitudes and improve performance. Whether longer training, or combining training with instructions may lead to larger improvement of movement strategies deserves to be investigated.
Strength and limitations
Strengths: this is the first study that explores data driven movement strategies through cluster analysis. This approach proved valid to detect movement control strategies and detect changes over time. Finding a limited number of clusters facilitates functional interpretation. With a large data set and using the Schwarz Bayesian Criterion the cluster analysis resulted in a reliable 2 cluster solution that was validated by independent performance and quality measures and interpreted as efficient control versus less efficient control strategies.
Limitations: our video observation tool focused on the two largest excursions during a run, rating the amplitude of different body parts. This implies that timing, as an important control variable, was not taken into account. This may explain why the best strategy does not have a success rate of 100%. On the other hand one can use inefficient strategies and still be on the right spot on the right time to pass a gate. In order to disentangle actual movements connected to the exact position of the avatar, research with a video recording system that reproduces both the position of the avatar on the screen and simultaneously the position of the child through body markers during the Wii Fit game is recommended.
In this study, only the lateral displacement was studied and not the displacement in forward and backward direction. Since in the Wii game only the lateral displacement is used to position the avatar and the forward and backward displacement is used to control the speed of the avatar, the lateral displacement is the most important factor that determines the success or failure of passing the gates.
Conclusion
Cluster analysis of quantitative observational scores yielded two movement strategies that were different in game performance and qualitative scores as judged by the observers. The clusters display control strategies that differ in movement amplitudes, control of balance and task efficiency. The best strategy presented smaller lateral shifts of the body and feet within the base of support, while the lesser successful strategy was characterized by larger lateral displacement of shoulder/upper trunk, head and feet movements. Most TD children adapted their movement strategy during the second session to consistent use of the more efficient, smaller movement amplitudes strategy, while only a small part of the children with DCD showed the more efficient movement strategy after intervention resulting in better game scores. Implicit learning during the repetition of a VR task is associated with adaptation to a more efficient movement control strategy.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed on the publisher's website.
Supplemental Material
Download MS Word (7 MB)Supplemental Material
Download MS Word (15 KB)Supplemental Material
Download MS Word (466.4 KB)Acknowledgments
We would like to thank all children, parents and the staff of participating schools for their time and cooperation. The work done by the pediatric physical therapists and students of the University of Applied Sciences; Hanze, Groningen and Avans+, Breda and the University of Groningen in helping to collect the data for this study, is highly appreciated. Many thanks to Hans Gankema for recovering lost video fragments after a hard disk crash and therefore saving the project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington DC: Author.
- Assaiante, C., & Amblard, B. (1993). Ontogenesis of head stabilisation in space during locomotion in children: Influence of visual cues. Experimental Brain Research, 93, 488–515.
- Beynon, S., McGinley, J. L., Dobson, F., & Baker, R. (2010). Correlations of the Gait Profile Score and the Movement Analysis Profile relative to clinical judgments. Gait & Posture, 32, 129–132.
- Biotteau, M., Chaix, Y., & Albaret, J.-M. (2016). What do we really know about motor learning in children with Developmental Coordination Disorder? Current Developmental Disorders Reports, 3(2), 152–160.
- Blank, R., Smits-Engelsman, B., Polatajko, H., & Wilson, P. (2012). European Academy for Childhood Disability. Developmental Medicine and Child Neurology, 54(1), 54–93.
- Bonney, E., Jelsma, D., Ferguson, G., & Smits-Engelsman, B. (2017). Variable training does not lead to better motor learning compared to repetitive training in children with and without DCD when exposed to active video games. Research in Developmental Disabilities, 62, 124–136.
- Cherng, R. J., Hsu, Y. W., Chen, Y. J., & Chen, J. Y. (2007). Standing balance of children with developmental coordination disorder under altered sensory conditions. Human Movement Science, 26(6), 913–926.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates.
- Deconinck, F. J., De Clercq, D., Savelsbergh, G. J., Van Coster, R., Oostra, A., Dewitte, G., & Lenoir, M. (2006). Visual contribution to walking in children with Developmental Coordination Disorder. Child: Care, Health and Development, 32(6), 711–722. doi:10.1111/j.1365-2214.2006.00685.x
- Deconinck, F. J. A., Savelsbergh, G. J. P., Clercq, D., & De Lenoir, M. (2010). Balance problems during obstacle crossing in children with Developmental Coordination Disorder. Gait & Posture, 32, 327–331.
- Diamond, N., Downs, J., & Morris, S. (2014). “The problem with running”-Comparing the propulsion strategy of children with Developmental Coordination Disorder and typically developing children. Gait & Posture, 39, 547–552.
- Du, W., Wilmut, K., & Barnett, A. L. (2015). Level walking in adults with and without Developmental Coordination Disorder: An analysis of movement variability. Human Movement Science, 43, 9–14.
- Fransson, P.-A., Johansson, R., Hafström, A., & Magnusson, M. (2000). Methods for evaluation of postural control adaptation. Gait and Posture, 12(1), 14–24.
- Gage, W. H., Winter, D. A., Frank, J. S., & Adkin, A. L. (2004). Kinematic and kinetic validity of the inverted pendulum model in quiet standing. Gait and Posture, 19(2), 124–132.
- Gentle, J., Barnett, A. L., & Wilmut, K. (2016). Adaptations to walking on an uneven terrain for individuals with and without Developmental Coordination Disorder. Human Movement Science, 49, 346–353.
- Geuze, R. H., Jongmans, M. J., Schoemaker, M. M., & Smits-Engelsman, B. C. M. (2001). Clinical and research diagnostic criteria for developmental coordination disorder: A review and discussion. Human Movement Science, 20, 7–47.
- Geuze, R. H. (2003). Static balance and developmental coordination disorder. Human Movement Science, 22(4-5), 527–548.
- Gog van, T., Paas, F., Marcus, N., Ayres, P., & Sweller, J. (2009). The mirror neuron system and observational learning: Implications for the effectiveness of dynamic visualizations. Educational Psychology Review, 21, 21–30.
- Gonsalves, L., Campbell, A., Jensen, L., & Straker, L. (2015). Children with developmental coordination disorder play active virtual reality games differently than children with typical development. Physical Therapy, 95(3), 360–368.
- Goodgold-Edwards, S. A., & Cermak, S. A. (1990). Integrating motor control and motor learning with neuropsychological perspectives on apraxia and developmental dyspraxia. American Journal of Occupational Therapy, 44(5), 431–439.
- Grove, C. R., & Lazarus, J.-A. C. (2007). Impaired re-weighting of sensory feedback for maintenance of postural control in children with developmental coordination disorder. Human Movement Science, 26(3), 457–476.
- Hayes, K. C. (1982). Biomechanics of postural control. Exercise and Sport Sciences Reviews, 10, 363–391.
- Henderson, S. E., Sugden, D. A., & Barnett, A. L. (2007). Movement assessment battery for children - second edition (movement ABC-2) examiner’s manual. London: Harcourt Assessment.
- Hoare, D. (1994). Subtypes of developmental coordination disorder. Adapted Physical Activities Quarterly, 11(2), 158–169.
- Horak, F. B., & Nashner, L. M. (1986). Central programming of postural movements: Adaptation to altered support-surface configurations. Journal of Neurophysiology, 55(6), 1369–1381.
- Jelsma, D., Geuze, R. H., Mombarg, R., & Smits-Engelsman, B. C. M. (2014). The impact of Wii Fit intervention on dynamic balance control in children with probable Developmental Coordination Disorder and balance problems. Human Movement Science, 33, 404–418. doi:10.1016/j.humov.2013.12.007
- Jelsma, L. D., Smits-Engelsman, B. C. M., Krijnen, W. P., & Geuze, R. H. (2016). Changes in dynamic balance control over time in children with and without Developmental Coordination Disorder. Human Movement Science, 49, 149–159.
- Larkin, D., & Rose, E. (2005). Assessment of developmental coordination disorder. In D. Sugden & M. Chambers (Eds.), Children with developmental coordination disorder (pp. 135–154). London:Whurr.
- Logan, D., Kiemel, T., & Jeka, J. J. (2017). Using a system identification approach to investigate subtask control during human locomotion. Frontiers in Computational Neuroscience, 10, 146.
- Lugade, V., Fortune, E., Morrow, M., & Kaufman, K. (2014). Validity of using tri-axial accelerometers to measure human movement – Part I: Posture and movement detection. Medical Engineering & Physics, 36, 169–176.
- Macnab, J. J., Miller, L. T., & Polatajko, H. J. (2001). The search for subtypes of DCD: Is cluster analysis the answer? Human Movement Science, 20(1-2), 49–72.
- Michalski, A., Glazebrook, C. M., Martin, A. J., Wong, W. W. N., Kim, A. J. W., Moody, K. D., … Zabjek, K. F. (2012). Assessment of the postural control strategies used to play two Wii Fit™ videogames. Gait & Posture, 36(3), 449–453. doi:10.1016/j.gaitpost.2012.04.005
- Mombarg, R., Jelsma, D., & Hartman, E. (2013). Effect of Wii-intervention on balance of children with poor motor performance. Research in Developmental Disabilities, 34(9), 2996–3003.
- Mündermann, L., Corazza, S., & Andriacchi, T. P. (2006). The evolution of methods for the capture of human movement leading to markerless motion capture for biomechanical applications. Journal of NeuroEngineering and Rehabilitation, 5, 3–6.
- Odenrick, P., & Sandstedt, P. (1984). Development of postural sway in the normal child. Human Neurobiology, 3, 241–244.
- Otten, E. (1999). Balancing on a narrow ridge: Biomechanics and control. Philosophical Transactions of the Royal Society of London. Series B Biological Sciences, 29 354, 869–875.
- Ounpuu, S. (2004). Patterns of gait pathology. In: J. Gage (Ed.). Treatment of gait problems in cerebral palsy. London: MacKeith.
- Riach, C. L., & Starkes, J. L. (1989). Visual fixation and postural sway in children. Journal of Motor Behavior, 21(3), 265–276.
- Schmidt, R. A., & Young, D. E. (1987). Transfer of movement control in motor skill learning. In S. Cormier & J. Hagman (Eds.), Transfer of learning (pp. 47–79). Orlando FL: Academic Press.
- Schoemaker, M. M., Flapper, B., Verheij, N. P., Wilson, B. N., Reinders-Messelink, H. A., & de Kloet, A. (2006). Evaluation of the developmental coordination disorder questionnaire as a screening instrument. Developmental Medicine & Child Neurology, 48, 668–673.
- Schoemaker, M. M., Flapper, B. C. T., Reinders-Messelink, H. A., & de Kloet, A. (2008). Validity of the motor observation questionnaire for teachers as a screening instrument for children at risk for developmental coordination disorder. Human Movement Science, 27(2), 190–199.
- Serrien, B., Hohenauer, E., Clijsen, R., Taube, W., Baeyens, J.-P., & Küng, U. (2017). Changes in balance coordination and transfer to an unlearned balance task after slackline training: A self-organizing map analysis. Experimental Brain Research, 235(11), 3427–3436. doi:10.1007/s00221-017-5072-7
- Smits-Engelsman, B. C. M. (2010). Movement ABC-2-NL Dutch manual. Amsterdam: Pearson.
- Smits-Engelsman, B. C., Blank, R., van der Kaay, A. C., Mosterd-van der Meijs, R., Vlugt-van den Brand, E., Polatajko, H. J., & Wilson, P. H. (2013). Efficacy of interventions to improve motor performance in children with developmental coordination disorder: A combined systematic review and meta-analysis. Developmental Medicine & Child Neurology, 55(3), 229–237. doi:10.1111/dmcn.12008
- SPSS Inc. (2001). The SPSS TwoStep cluster component: A scalable component enabling more efficient customer segmentation. Technical Report, Chapter 16, pp. 361–391.
- Vickers, J. N. (2011). Mind over muscle: The role of gaze control, spatial cognition and the quiet eye in motor expertise. Cognitive Processing, 12(3), 219–222. doi:10.1007/s10339-011-0411-2
- Tellegen, P. J. (1993). A nonverbal alternative to the Wechsler scales: The Snijders-Oomen nonverbal intelligence tests. In First annual South Padre Island international interdisciplinary conference on cognitive assessment for children and youth in school and clinical settings: .compendium of proceedings. For Worth, TX: Cyberspace Publishing Corporation.
- Visser, J., Kalverboer, A. F., & Geuze, R. H. (1998). The relationship between physical growth, the level of activity and the development of motor skills in adolescence: Differences between children with DCD and controls. Human Movement Science, 17(4-5), 573–608.
- Wechsler, D. (2003). The Wechsler intelligence scale for children (4th ed.). London: Pearson.
- Wilson, B. N., Kaplan, B. J., Crawford, S. G., Campbell, A., & Dewey, D. (2000). Reliability and validity of a parent questionnaire on childhood motor skills. American Journal of Occupational Therapy, 54(5), 484–493.
- Winter, D. A., Prince, F., Frank, J. S., Powell, C., & Zabjek, K. F. (1996). Unified theory regarding A/P and M/L balance in quiet stance. Journal of Neurophysiology, 75(6), 2334–2343.
- Winter, D. A., Patla, A. E., Prince, F., Ishac, M., & Gielo-Perczak, K. (1998). Stiffness control of balance in quiet standing. Journal of Neurophysiology, 80(3), 1211–1221.
- Yamazaki, J., Muneta, T., Ju, Y. J., & Sekiya, I. (2010). Differences in kinematics of single leg squatting between anterior cruciate ligament-injured patients and healthy controls. Knee Surgery, Sports Traumatology, Arthroscopy, 18(1), 56–63. doi:10.1007/s00167-009-0892-z