Abstract
In this study we compared the effects of transcranial direct current stimulation (tDCS) in the subacute and chronic stages of post-stroke recovery. Anodal/sham tDCS was applied to the primary motor cortex of stroke patients in these stages of recovery in a cross-over design. The Jebsen–Taylor hand function test was employed. The repeated-measure ANOVA showed significant influence of the stimulation type and test performance time (during/after tDCS) with no overall influence of recovery stage. The interaction TYPE*TIME*STAGE was significant. The effect after anodal tDCS in the subacute stage was significantly higher compared to the effects in all relevant conditions including the chronic stage. Therefore, tDCS treatment in the subacute stage of recovery can be superior, at least for some patients, to treatment in the chronic stage.
Introduction
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique, which modulates cortical excitability and activity; it has been explored as a treatment option for various neurological conditions (Brunoni, Nitsche, & Loo, Citation2016; Lefaucheur et al., Citation2017). Influence of tDCS on recovery after stroke, particularly on the motor function, has been examined in numerous studies (Kang, Summers, & Cauraugh, Citation2016; Nowak, Grefkes, Ameli, & Fink, Citation2009; Triccas et al., Citation2016). They report 10% to 30% improvement of forearm motor function in stroke patients. One possible way to increase the effect could be through earlier intervention after disease onset, as neuroplastic changes are more likely to occur at the earlier stages of recovery after stroke (Biernaskie, Chernenko, & Corbett, Citation2004; Hara, Citation2015). However, which stage of recovery is most receptive for tDCS application has not yet been delineated. In this study, we address this question and compare effects of tDCS in subacute and chronic stages of recovery post-stroke.
For rehabilitation after stroke, anodal tDCS of the primary motor cortex (M1) on the lesioned side or cathodal stimulation of M1 on contralateral side is usually employed (Nowak et al., Citation2009). Anodal stimulation leads to the increase in cortical excitability and activity, while cathodal stimulation – decreases it (Utz, Dimova, Oppenlander, & Kerkhoff, Citation2010). Direct current results in subthreshold polarity-specific polarization of neuronal membranes (Bikson et al., Citation2004; Nitsche & Paulus, Citation2000; Purpura & McMurtry, Citation1965). After several minutes of stimulation, excitability changes last after the end of the session (Nitsche & Paulus, Citation2000, Citation2001; Nitsche et al., Citation2003b). These changes are related to glutamatergic plasticity (Nitsche et al., Citation2003a), presumably gated by GABAergic downregulation (Stagg et al., Citation2009). This plasticity is considered to be similar to neurophysiological correlate of learning – long-term potentiation (LTP) and long-term depression (LTD) – since it is associated with N-methyl-D-aspartate (NMDA) receptors, calcium channels and protein synthesis (Fritsch et al., Citation2010; Nitsche et al., Citation2003a). In addition to inducing changes to brain regions under the electrodes, tDCS also influences functional connectivity and activity in the remote areas (Polania, Nitsche, & Paulus, Citation2011a; Polania, Paulus, Antal, & Nitsche, Citation2011b).
Most of the tDCS studies are performed in the chronic stage of recovery post-stroke when functions are stabilized. Although chronic stroke is defined as > 1 month after stroke, in these studies patients with stroke older than 6 months are usually investigated. Yet, neuroplasticity is likely to be greater at the earlier stages of recovery (acute: 1 day – 1 week, subacute: 1 week – 1 month after stroke). As shown in animal models, focal ischemic damage is accompanied by several weeks window of increased plasticity (Biernaskie et al., Citation2004). This corresponds to the fact that almost all recovery from impairment in humans occurs in the first three months. Accordingly, early rehabilitation facilitates motor recovery after stroke more efficiently (Kwakkel et al., Citation2004).
It is also known that re-learning of the lost motor functions after stroke is accompanied by plastic functional reorganization of brain circuits (Pellegrino et al., Citation2012; Várkuti et al., Citation2013). It can therefore be suggested that modulation of the brain excitability with tDCS can be more efficient in the early stages of recovery after stroke.
Most of the studies, investigating tDCS influence in the acute and subacute stage of recovery post-stroke showed beneficial effects (Alber, Moser, Gall, & Sabel, Citation2017; Berretta, Tzeng, & Clarkson, Citation2014; Chang, Kim, & Park, Citation2015; Lee & Chun, Citation2014; Stinear & Byblow, Citation2014; Wang et al., Citation2014). In the study by Chang et al. (Citation2015), improvement in low limb function and higher cortex excitability in the anodal group compared to sham was observed when 24 subacute stroke patients received 10 sessions of anodal/sham tDCS combined with conventional physical therapy. Another investigation revealed significantly greater recovery of visual fields in seven homonymous hemianopia subacute stroke patients treated with anodal tDCS and vision restoration training compared to controls (Alber et al., Citation2017). A plateau in recovery was achieved faster in acute stroke patients after bilateral priming combined with physiotherapy in the study by Stinear and Byblow, (Citation2014).
Yet, other studies have not shown any additional improvement from tDCS (Hesse et al., Citation2011; Kim et al., Citation2014; Leon et al., Citation2017; Mazzoleni, Tran, Iardella, Dario, & Posteraro, Citation2017; Rossi, Sallustio, Di Legge, Stanzione, & Koch, Citation2013; Triccas et al., Citation2015). No group difference was revealed when bilateral movement therapy was combined with anodal, cathodal or sham tDCS in severe impaired subacute stroke patients (Hesse et al., Citation2011). In another study (Rossi et al., Citation2013) no improvement of motor recovery after 5-day anodal tDCS to acute stroke patients was obtained.
In a Cochrane review (Elsner, Kugler, Pohl, & Mehrholz, Citation2013) no effect of different stages of recovery post-stroke (acute, subacute and chronic) was found. Yet, a tendency to greater clinical effects in a sub-group of patients in the subacute stage of recovery after stroke with time from the stroke not more than four weeks was observed when patients were stimulated with cathodal tDCS or cTBS (Nicolo et al., Citation2018).
These studies therefore do not allow concluding which stage of recovery post-stroke is preferential for the stimulation. Absence of the effects of tDCS in the early stages in some studies can depend on other factors than stage of recovery. For example, in the study by Hesse et al. (Citation2011) severity of stroke could be a limiting factor. On the other hand, presence of the effects in the acute/subacute stage does not provide enough information about possible effects of the same treatment in the chronic stage. Also, since tDCS effects in stroke population has a high inter-individual variability (Pavlova et al., Citation2017), we wanted to investigate tDCS effects in the same patients in cross-over design and directly compare motor effects of anodal tDCS of the primary motor cortex in different stages of recovery post-stroke. The study includes subacute (from two to four weeks after stroke) and chronic stages (> six months after stroke). We hypothesized that the effect of the stimulation in the subacute stage exceeds the one obtained in the chronic stage.
Method
Patients. 16 subacute stroke patients (9 women; mean age 67.7 ± 3.4 years, mean time from stroke 24.5 ± 2.9 days) were enrolled in the study. Inclusion criteria were: stroke 2-4 weeks prior to the study, age 18–75 years, moderate/mild paresis of the hand, ability to understand the instruction/to communicate. Exclusion criteria: metallic implants in the head or heart, epilepsy, skin irritation, alcohol and/or drug dependence, other neurologic, psychiatric or severe somatic disorders, pregnancy/lactation. All patients were out-patients; the study was performed at the Moscow Research and Clinical Center for Neuropsychiatry of the Healthcare Department of Moscow.
All patients gave an informed consent. The study was approved by the ethical committee of the Moscow Research and Clinical Center for Neuropsychiatry of the Healthcare Department of Moscow.
Three patients dropped out of the study before participation during the chronic stage – one died and two refused to participate due to fatigability. Thirteen patients were included in the analysis.
Experimental Protocol and Design
This cross-over balanced randomized single-blinded study covered two periods for each patient: subacute (2–4 weeks) and chronic (>6 months after stroke). Each patient participated in a preliminary training session and four stimulation sessions (one anodal and one sham session in each stage of recovery post-stroke). Clinical evaluation of the degree of hand paresis was done by neurologist at each stage of recovery (before first and third tDCS sessions) with help of 0–5 scale (0-no movement, 5-full recovery). The order of sessions was constant for each patient in the two recovery stages and balanced between participants (). One week interval was present between sessions of tDCS to minimize carryover effects.
Figure 1. (A) Time course of the study. (B) Time course of one study session. JTT – Jebsen–Taylor Hand function test.
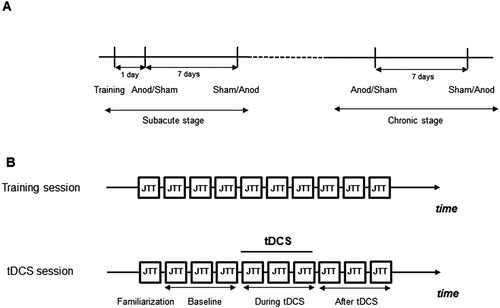
JTT was performed 10 times during the training session to ensure stable results. During stimulation sessions, JTT was performed once for familiarization, three times – before tDCS (baseline), three times – immediately after switching on the stimulator (during tDCS) and three times – immediately after tDCS (; Fregni et al., Citation2005, Hummel et al., Citation2005). Performing JTT three times (18 items altogether) took approximately 10 minutes since the patients needed rest between items, and the next item had to be positioned on the table. At the end of each session, the patients were asked whether they received the real or sham stimulation.
The trial was single-blinded due to the limitations of the employed tDCS equipment which did not allow the performance of double-blinded trials. Randomization and tests sessions were conducted by the non-blinded researcher.
Transcranial Direct Current Stimulation
Bipolar stimulation was delivered by a battery-driven electrical stimulator Reamed-polaris (Vozrojdenie, Russia), developed for the transcranial micropolarization – method of noninvasive brain stimulation with direct current and, when similar parameters of stimulation are applied, is an analog to tDCS. The device is approved for the clinical use in Russia. Iron electrodes, covered with a saline-soaked sponge were placed on the primary motor cortex (size of the sponge electrode was 3.5 × 5 cm). Localization of the position of the primary motor cortex electrode was determined with transcranial magnetic stimulation (TMS) via defining the hot spot of the first dorsal interosseous muscle (FDI) contralateral to the performing hand. The reference electrode was placed above the contralateral orbit (size of the sponge electrode was 5 × 7 cm). For active conditions tDCS intensity was 0.5 mA (resulting in current density of 0.0286 mA/cm2 in active electrode). It is worth noting that the current was reduced from the most commonly used 1 mA to 0.5 mA to compensate for the electrode size and to keep current density constant (0.5 mA/17.5 cm2 = 0.0286 mA/cm2). Stimulation was applied during 10 minutes in active conditions. In the sham conditions, tDCS stimulation was performed for 30 seconds only.
Transcranial Magnetic Stimulation (TMS)
TMS was performed using a standard double (‘figure-of-eight’) coil connected to a biphasic MagPro stimulator (MagVenture GmbH, Willich, Germany). The coil was placed tangentially to the scalp, with the handle pointing posterolaterally at a 45° angle from the midline. Surface electromyography was recorded from both right and left FDI by use of surface electrodes. The site at which TMS of slightly suprathreshold intensity consistently elicited the largest MEP in the FDI muscle was marked as the motor hotspot.
Jebsen–Taylor Hand Function Test
The Jebsen–Taylor Hand function test (JTT) is widely used to evaluate fine hand motor function (Jebsen, Taylor, Trieschmann, Trotter, & Howard, Citation1969). The test assesses a wide range of daily hand functions and included initially seven items. The test has been shown to be valid and reliable (Ferreiro, Santos, & Conforto, Citation2010; Jebsen et al., Citation1969; Stern, Citation1992). As previously suggested, one of the items (writing a sentence) was excluded since some of the patients used their non-dominant hand for writing (Boggio et al., Citation2007; Fregni et al., Citation2005; Hummel et al., Citation2005). Six items were included in the test: turning cards, picking up small objects, picking up beans with a spoon to simulate feeding, stacking checkers, and lifting light and heavy cans. Patients were instructed to perform task as quickly and accurately as possible.
Data Analysis
The results were analyzed with MATLAB R2012b. To evaluate the blindness of patients to tDCS type and possible change of the clinical status of the patients between subacute and chronic stages, sign test was performed. Performance time during the training of the JTT and the baselines of each tDCS experiment were assessed by the repeated-measure ANOVA. The inverted percent change of the total JTT time relative to the baseline was a primary outcome measure of the study and was used as a measure of improvement in the test (positive values represent improved performance). Improvement during and after anodal/sham tDCS was evaluated with the repeated-measure ANOVA with three factors (STAGE, TYPE and TIME). The factor STAGE compared the values between subacute and chronic stages, factor TYPE – different tDCS conditions (anodal vs sham) and factor TIME – performance during or after the stimulation. Post-hoc analysis was performed with the Fisher LSD test. This data were found to be normally distributed (evaluated through the Kolmogorov-Smirnov test). The time taken for the performance of each item and the percent change of the item performance time during/after tDCS relative to baseline was calculated. The improvement (the inverse of the percent change) was assessed with the repeated-measure ANOVA in order to evaluate the impact of tDCS on individual items and, eventually, the types of movements most sensitive to stimulation.
Since two distinct patterns of effects were seen after anodal stimulation in the subacute stage – an improvement of more than 7% or no distinct increase – the patients were distributed into two groups based on the observed patterns. Repeated-measure ANOVA with the additional factor GROUP and relevant post-hoc Fisher LSD tests were performed.
Results
No adverse events/side-effects were observed. Participants could poorly distinguish between real and sham stimulation conditions (according to the results of the questionnaire) (Sign Test, z = 1.77, p = 0.08). The mean time between stroke and the beginning of the study was 24.6 ± 2.6 days. The mean time between stroke and the second part of the study (chronic stage) was 232.3 ± 59.1 days. The degree of hand paresis improved from the subacute to chronic stage in 7 of 13 subjects. No change was seen on the group level (Z = 1.22, p = 0.22). All patients could perform the Jebsen–Taylor test. Performance of the test during preliminary training session for 10 times lead to stabilization of performance already after the fifth time (F(9,135) = 12.07, p = 0.00000; ). Baseline performance during four tDCS sessions did not change (F(3,36) = 0.23, p = 0.87; not shown; for mean baseline values see ). Baseline clinical data are presented in . All patients reported as being right-handed before stroke. All patients had MEPs as revealed with TMS so that the exact position of the M1 could be defined.
Figure 2. The mean total time of Jebsen–Taylor Hand function test during ten sessions of training. - mean, vertical bars denote 0.95 confidence intervals.
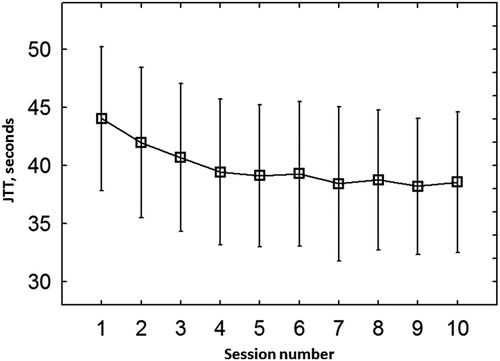
TABLE 1. Clinical characteristics of the patients.
The repeated-measure ANOVA showed significant influence of the stimulation type (anodal or sham) (factor TYPE; F(1,12) = 9.99, p = 0.008), of the Jebsen–Taylor test performance time relative to stimulation (during or directly after tDCS) (factor TIME, F(1,12) = 15.11, p = 0.002), but no influence of the stage of recovery post-stroke (subacute or chronic) (factor STAGE; F(1,12) = 1.49, p = 0.25). The interaction between type of stimulation, stage of recovery and time of the test performance (but not pairwise interactions between factors) was significant (F(1,12) = 7.39, p = 0.02; , ), meaning that outcome of stimulation depended on all three factors, including stage of recovery. Post-hoc comparisons showed a significantly higher effect (p = 0.03) in the subacute stage after the stimulation compared to chronic stage. The comparison between anodal and sham stimulation showed significant difference after tDCS in the subacute (p = 0.0001) but not in the chronic stage (p = 0.39); the effect of anodal tDCS was higher after the stimulation compared to concurrent stimulation in the subacute stage (p = 0.001, ).
Figure 3. Inverted mean percent change of the total JTT time relative to baseline (positive values represent improved performance). Significant interaction between type of stimulation, stage of stroke and time of the test performance was observed (repeated-measure ANOVA, F(1,12) = 7.39, p = 0.02). Relevant post-hoc comparisons (Fisher LSD test) are shown. * p < 0.05. **p < 0.01, ***p < 0.001.
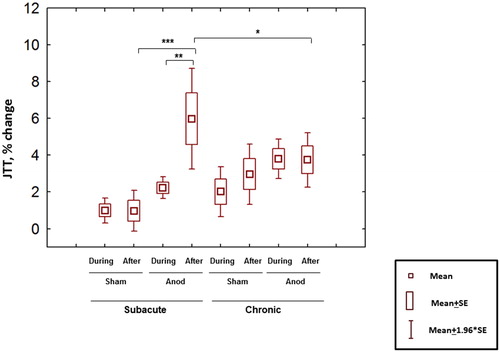
TABLE 2. Results of ANOVA.
The interaction STAGE*TIME*TYPE*SUBTEST was not significant (F(5,60) = 0.78; p = 0.57), meaning that there was no detectable differential effect of tDCS on the individual subtests ().
TABLE 3. Mean time and percent of improvement of the subtests performance in different conditions.
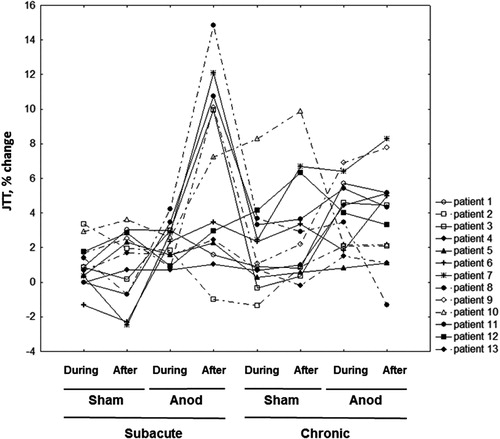
Note, that individual patients’ results showed considerable variability (). One can see some clear responders with improvement of the test performance after the anodal stimulation in the subacute stage (Group 1) and non-responders with no improvement in this condition (Group 2). Six patients were classified as Group 1 (patients 3, 7, 8, 9, 10, 11; ). No difference between Groups 1 and 2 was seen in the degree of paresis in any of recovery stages (one-way ANOVA; F(1,11) = 0.62, p = 0.45 and F(1,11) = 0.28, p = 0.61, respectively), in the type (chi-sqare, 0.75), location of stroke (chi-sqare, 0.31) and age (t = 0.26). Yet, all Group 1 patients were women; six men and one woman belonged to the Group 2 (chi-sqare, 0.002).
Figure 4. Inverted percent change of the total JTT time relative to baseline (positive values represent improved performance) in individual patients (eight measurements with JTT in four sessions).
In the Group 1, post-hoc comparisons obtained from the interaction STAGE*TIME*TYPE*GROUP revealed significant differences between anodal and sham tDCS both during and after stimulation in the subacute stage and during stimulation in the chronic stage. The effect of the anodal stimulation was highest in the subacute stage after stimulation ().
Figure 5. Inverted mean percent change of the total JTT time relative to baseline in Group 1 (A) and Group 2 (B). Repeated-measure ANOVA with factors STAGE, TYPE; TIME and GROUP (F(1,11) = 35.4; p = 0.0001). Relevant post-hoc comparisons (Fisher LSD test) are shown. *p < 0.05. **p < 0.01, ***p < 0.001.
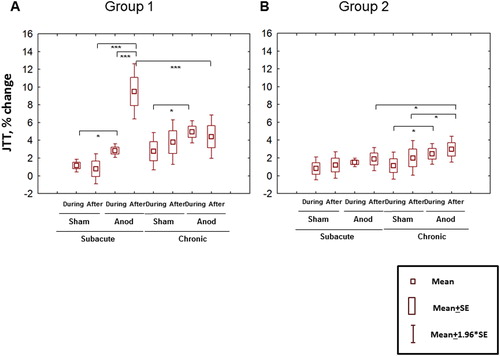
Despite minor effects of tDCS in the Group 2, the differences between anodal and sham tDCS were significant both during and after stimulation but, on contrast to the Group 1, only in the chronic stage; the effect of anodal tDCS after the stimulation was significantly higher in the chronic compared to subacute stage ().
Discussion
The aim of our study was to directly compare the motor effects of single-session anodal tDCS in subacute and chronic stages in a cross-over design. It was revealed that the effect of tDCS depends on several factors, including the stage of the recovery. The highest effect is shown for the anodal tDCS in the subacute stage of recovery when assessed after the end of stimulation.
Post-Stroke Recovery Stages and tDCS
Some positive effects of tDCS on stroke patients have been described from acute to chronic recovery stages (Henrich-Noack, Sergeeva, & Sabel, Citation2017). Yet, the mechanisms of this influence can be different. Upregulation of N-methyl-D-aspartate receptors (Que, Schiene, Witte, & Zilles, Citation1999), cortical disinhibition and dysregulation of GABAergic neurotransmission (Buchkremer-Ratzmann & Witte, Citation1997; Redecker, Wang, Fritschy, & Witte, Citation2002) are observed after stroke. Along with this, neuronal excitability is increased in the early stages of recovery post-stroke. Theoretically, anodal tDCS applied in the acute stage bears the risk of further neural damage, since this stage is characterized by excitotoxicity when neurons can be damaged by excessive excitatory stimulation. Yet, improvement from anodal stimulation is shown in this period, probably, due to the vascular mechanisms (Kurimoto et al., Citation2010).
In the subacute stage repeated tDCS can recruit neuroprotective and anti-inflammatory mechanisms, as well as promote neural growth (Brus-Ramer, Carmel, Chakrabarty, & Martin, Citation2007; Corredor & Goldberg, Citation2009; Henrich-Noack et al., Citation2005). Single-session anodal tDCS could make local reduction of GABA concentration observed after stimulation (Stagg et al., Citation2009) relatively more visible since the baseline GABAergic neurotransmission initially decreased in early stages of recovery (Buchkremer-Ratzmann & Witte, Citation1997; Redecker et al., Citation2002). Among others, this mechanism could potentially increase the effect of tDCS compared to the chronic stage even if only single-session stimulation is applied.
In our study, a significantly greater effect is seen in the subacute stage (compared to the chronic stage) after stimulation with anodal tDCS to the primary motor cortex; however, mean values did not exceed 10% in either condition. The latter fact can be compared with several other studies investigated the effect of single-session tDCS measured with the Jebsen–Taylor test in the chronic stage (Boggio et al., Citation2007; Fregni et al., Citation2005; Hummel et al., Citation2005). 9% improvement was observed in the study by Hummel et al. (Citation2005), when anodal stimulation was applied to M1, 7% and 12% – in the study by Fregni et al. (Citation2005) when anodal tDCS of the affected hemisphere or cathodal tDCS of unaffected hemisphere, respectively, was employed. Repetitive stimulations (five daily sessions of cathodal stimulation) in the study by Boggio et al. (Citation2007) resulted in 17% improvement. Therefore, the mechanisms which define the effects of single-session tDCS do not seem to play a substantial role for the eventual additional benefits of stimulation in the subacute stage of recovery.
In addition to plasticity mechanisms engaged in a single-session tDCS, long-term treatment can support the rescue of the neurons in the immediate vicinity of stroke site (prenumbra), which undergoes apoptosis in subacute stage of recovery post-stroke (Corredor & Goldberg, Citation2009; Guglielmo et al., Citation1998), as well as re-activate functionally inactive neurons in areas remote from the damage (Henrich-Noack et al., Citation2005). Electrical activity also modulates plasticity mechanisms which mediate rewiring of neural connections (Brus-Ramer et al., Citation2007).
Accordingly, some studies investigating tDCS effects after repetitive sessions in the subacute stage show higher effects (Kim et al., Citation2010; You, Kim, Chun, Jung, & Park, Citation2011). In the study by You et al. (Citation2011), effect of 10 sessions of cathodal tDCS of the right Wernicke's area of subacute stroke patients is 25% bigger than in sham tDCS in auditory motor comprehension. In the study by Kim et al. (Citation2010), effect of 10 sessions of cathodal tDCS of M1 in subacute stroke patients exceeds the effect of sham tDCS by 87% and effect of anodal tDCS exceeds the effect of sham by 93% on follow-up when measured by Fugl-Meyer Assessment.
Assessment during and after tDCS
Among other factors, timing of tDCS application is an important factor for the size and direction of stimulation effect (Ziemann & Siebner, Citation2008). In our study, the subacute stage of recovery after stroke is associated with significantly greater effects after tDCS compared to concurrent stimulation. Training during the stimulation has also been shown to lead to improvement in performance (Reis et al., Citation2009; Reis & Fritsch, Citation2011; Stagg et al., Citation2011). On the other hand, deterioration of performance is observed when both anodal and cathodal tDCS are applied prior or after motor task (Stagg et al., Citation2011). The former is explained by gating mechanisms when shift in membrane polarization causes strengthening of synapsis and latter – by homeostatic metaplasticity which stabilizes the system after previous too high activity.
Depolarization which leads to subsequent LTP can be achieved either by disinhibition or by direct activation (Ziemann & Siebner, Citation2008). It was shown that after anodal tDCS local concentration of GABA is decreased and concentration of glutamate did not increase significantly (Stagg et al., Citation2009). Yet, relative contribution of GABA/glutamate in response to anodal tDCS can vary during vs after the stimulation, interact with the baseline neurotransmission and eventually lead to differential motor responses.
In addition to the timing of test, other factors including stimulation protocol and exact learning task can also influence the effect of stimulation. For example, effect of tDCS on the subsequent task learning was shown for explicit but not implicit task (Stagg et al., Citation2011). JTT employed to assess tDCS effects in our study has been used in the number of studies (Boggio et al., Citation2007; Fregni et al., Citation2005; Hummel et al., Citation2005). In the study by Hummel et al. (Citation2005) performed in the chronic stage of recovery post-stroke, the decrease in the JTT time performance was shown both during and after anodal stimulation. The effects were also significant both during and after cathodal stimulation (Boggio et al., Citation2007; Fregni et al., Citation2005). Yet, in the study by Fregni et al. (Citation2005) anodal tDCS was associated with significant effects when the JTT was performed after but not during the stimulation.
The effect was also revealed during tDCS in other studies, using different tests (like the Strength-Dexterity task as in the study by Pavlova, Kuo, Nitsche, and Borg (Citation2014)).
Comparison of Group 1 and Group 2
Group 1 showed a significant difference between anodal and sham tDCS both during and after stimulation in the subacute stage and significant decrease in the chronic stage. This pattern was opposite to the one of the Group 2: Though the effects of tDCS for this group were very small (only 1–1.3% higher improvement in anodal than in sham tDCS in the chronic stage), these differences both during and after tDCS were significant, moreover, significantly higher effects in the chronic compared to subacute stage were observed. Therefore, the possibility that for some patients tDCS treatment in chronic stage can be superior compared to subacute stage, cannot be ruled out. Further studies with optimal parameters of stimulation (for example, stronger current, see Study Limitations) and sufficiently big sample size should be used to address this issue.
Group 1 and 2 patients did not differ in degree of paresis, level or type of stroke. Surprisingly, all Group 1 patients were women whereas six of seven Group 2 patients were men. Although such dramatic difference in tDCS responses between genders was not reported previously, stronger modulatory effects of tDCS in women has been observed in some previous studies (Chaieb, Antal, & Paulus, Citation2008; Kuo, Paulus, & Nitsche, Citation2006). In the study by Chaieb et al. (Citation2008), a higher excitability in women compared to men in response to the excitatory anodal stimulation of visual cortex was observed. On the other hand, stronger/longer inhibitory effects in response to cathodal tDCS of the motor cortex were obtained in women in the study by Kuo et al. (Citation2006). These effects can be attributed to the impact of female gonadal sex hormones which influence cortical plasticity: Estrogens enhance neuronal excitability, in particular, by acting on the NMDA receptors while progesterone diminishes it (Bäckström, Citation1976; Inghilleri et al., Citation2004; Lee et al., Citation2018; Smith, Adams, Schmidt, Rubinow, & Wassermann, Citation2002; Woolley & McEwen, Citation1992). Since NMDA-receptors are upregulated early after stroke (Que et al., Citation1999), gender-related factors can interact with current brain plasticity status and play a role in shaping responses to tDCS in relation to the time-window for stimulation after the stroke.
Study Limitations
The study was single-blinded due to the tDCS device characteristics. This could potentially cause bias in the sessions’ conductance. Another limitation of the present study is a small sample size. Only mechanisms which can be revealed in a single-session tDCS were investigated in the study. In our study, current was decreased to 0.5 mA to compensate for the smaller electrode size and keep the current density 0.029 mA/cm2. However, in some studies current of 2 mA with density 0.057 mA/cm2 were shown to be beneficial for the stroke recovery (Khedr et al., Citation2013). Therefore, the low current may have contributed to the relatively small effect size of this study, especially in its chronic stage part.
Also, since our data were acquired in stroke patients with mild/moderate upper limb paresis and preserved MEP, the obtained information can be applicable only to this patient group. Presence of MEPs on the early stage of recovery post-stroke (<7 days) is a good predictor of the recovery, especially in severe stroke patients (Stinear, Citation2017). Yet, in our study an improvement in the paresis degree from subacute to chronic stage was not seen on the group level (was observed only in seven patients) despite of the presence of the MEPs in the subacute stage in all patients. This could be due to the milder stroke of our patients, insufficient sensitivity of the clinical scale or the timing of MEP assessment – instead of the first seven days as specified by Stinear (Citation2017), in our study the mean time was 24.6 days after the stroke.
Conclusions
Many factors interact to determine the motor effects of a single-session of anodal tDCS to the primary motor cortex, such as: the stage of recovery post-stroke, type of stimulation, timing of the assessment and factors related to gender. Among all investigated conditions, the highest effect of tDCS was seen in the subacute stage when it was assessed after the end of anodal stimulation. Therefore, tDCS treatment in the subacute stage of recovery can be superior over the treatment in the chronic stage. Yet, some patients did not respond to tDCS in the subacute stage (Group 2). Their effect of stimulation increased significantly in the chronic stage though stayed negligibly small. Post-hoc analysis revealed the gender difference between groups. The Group 1 which defined the noticeable response in the subacute stage included only women, whereas Group 2 consists mostly of men. No difference in other clinical parameters was seen.
The study underlies vulnerability of tDCS to the number of factors and importance of methodological issues in tDCS application. Gender should be taken into account when planning tDCS studies, especially involving different stages of recovery post-stroke. Another factor is timing of the assessment; this issue is directly connected to the mechanisms of tDCS action which still needs to be resolved. Also the exact test which is supposed to reveal the effect and previous activity would play a role. The eventual additional benefits of stimulation in the subacute stage of recovery post-stroke (neuroprotection, promotion of neuronal growth, anti-inflammatory properties) should be investigated in the further studies in which repeated sessions of tDCS would be employed.
ACKNOWLEDGMENTS
The authors are grateful to Marina P. Pavlova for the language correction and valuable comments.
References
- Alber, R., Moser, H., Gall, C., & Sabel, B. A. (2017). Combined transcranial direct current stimulation and vision restoration training in subacute stroke rehabilitation: A pilot study. PM&R, 9(8), 787–794. doi:10.1016/j.pmrj.2016.12.003
- Berretta, A., Tzeng, Y. C., & Clarkson, A. N. (2014). Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Review of Neurotherapeutics, 14(11), 1335–1344. doi:10.1586/14737175.2014.969242
- Biernaskie, J., Chernenko, G., & Corbett, D. (2004). Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. Journal of Neuroscience, 24(5), 1245–1254. doi:10.1523/JNEUROSCI.3834-03.2004
- Bikson, M., Inoue, M., Akiyama, H., Deans, J. K., Fox, J. E., Miyakawa, H., & Jefferys, J. G. (2004). Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. The Journal of Physiology, 557 (1), 175–190. doi:10.1113/jphysiol.2003.055772
- Boggio, P. S., Nunes, A., Rigonatti, S. P., Nitsche, M. A., Pascual-Leone, A., & Fregni, F. (2007). Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience, 25(2), 123–129.
- Brunoni, A., Nitsche, M., & Loo, C. (Eds.). (2016). Transcranial direct current stimulation in neuropsychiatric disorders clinical principles and management. Retrieved from https://www.springer.com/us/book/9783319339658
- Brus-Ramer, M., Carmel, J. B., Chakrabarty, S., & Martin, J. H. (2007). Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. Journal of Neuroscience, 27(50), 13793–13801. doi:10.1523/JNEUROSCI.3489-07.2007
- Buchkremer-Ratzmann, I., & Witte, O. W. (1997). Extended brain disinhibition following small photothrombotic lesions in rat frontal cortex. Neuroreport, 8(2), 519–522. doi:10.1097/00001756-199701200-00028
- Bäckström, T. (1976). Epilepsy in women. Oestrogen and progesterone plasma levels. Experientia, 32(2), 248–249. doi:10.1007/bf01937792
- Chaieb, L., Antal, A., & Paulus, W. (2008). Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Visual Neuroscience, 25(1), 77–81. doi:10.1017/S0952523808080097
- Chang, M. C., Kim, D. Y., & Park, D. H. (2015). Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimulation, 8(3), 561–566. doi:10.1016/j.brs.2015.01.411
- Corredor, R. G., & Goldberg, J. L. (2009). Electrical activity enhances neuronal survival and regeneration. Journal of Neural Engineering, 6(5), 055001. doi:10.1088/1741-2560/6/5/055001
- Elsner, B., Kugler, J., Pohl, M., & Mehrholz, J. (2013). Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. The Cochrane Database of Systematic Reviews, 15(11), CD009645.
- Ferreiro, K. N., Santos, R. L., & Conforto, A. B. (2010). Psychometric properties of the Portuguese version of the Jebsen-Taylor test for adults with mild hemiparesis. Revista Brasileira de Fisioterapia (Sao Carlos (Sao Paulo, Brazil)), 14(5), 377–382.
- Fregni, F., Boggio, P. S., Mansur, C. G., Wagner, T., Ferreira, M. J. L., Lima, M. C., … Pascual-Leone, A. (2005). Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport, 16(14), 1551–1555. doi:10.1097/01.wnr.0000177010.44602.5e
- Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., & Lu, B. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron, 66(2), 198–204. doi:10.1016/j.neuron.2010.03.035
- Guglielmo, M., Chan, P., Cortez, S., Stopa, E., McMillan, P., Johanson, C., … Doberstein, C. (1998). The temporal profile and morphologic features of neuronal death in human stroke resemble those observed in experimental forebrain ischemia: The potential role of apoptosis. Neurological Research, 20(4), 283–296. doi:10.1080/01616412.1998.11740520
- Hara, Y. (2015). Brain plasticity and rehabilitation in stroke patients. Journal of Nippon Medical School [Nippon Ika Daigaku Zasshi], 82(1), 4–13. doi:10.1272/jnms.82.4
- Henrich-Noack, P., Gorkin, A. G., Krautwald, K., Pforte, C., Schröder, U. H., & Reymann, K. G. (2005). Tetanus-induced re-activation of evoked spiking in the post-ischemic dentate gyrus. Neuroscience, 133(2), 571–581. doi:10.1016/j.neuroscience.2005.02.044
- Henrich-Noack, P., Sergeeva, E. G., & Sabel, B. A. (2017). Non-invasive electrical brain stimulation: From acute to late-stage treatment of central nervous system damage. Neural Regeneration Research, 12(10), 1590–1594. doi:10.4103/1673-5374.217322
- Hesse, S., Waldner, A., Mehrholz, J., Tomelleri, C., Pohl, M., & Werner, C. (2011). Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: An exploratory, randomized multicenter trial. Neurorehabilitation and Neural Repair, 25(9), 838–846. doi:10.1177/1545968311413906
- Hummel, F., Celnik, P., Giraux, P., Floel, A., Wu, W. H., Gerloff, C., & Cohen, L. G. (2005). Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain, 128(3), 490–499. doi:10.1093/brain/awh369
- Inghilleri, M., Conte, A., Currà, A., Frasca, V., Lorenzano, C., & Berardelli, A. (2004). Ovarian hormones and cortical excitability. An rTMS Study in Humans Clinical Neurophysiology, 115(5), 1063–1068. doi:10.1016/j.clinph.2003.12.003
- Jebsen, R. H., Taylor, N., Trieschmann, R. B., Trotter, M. J., & Howard, L. A. (1969). An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation, 50(6), 311–319.
- Kang, N., Summers, J. J., & Cauraugh, J. H. (2016). Transcranial direct current stimulation facilitates motor learning post-stroke: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry, 87(4), 345–355. doi:10.1136/jnnp-2015-311242
- Khedr, E. M., Shawky, O. A., El-Hammady, D. H., Rothwell, J. C., Darwish, E. S., Mostafa, O. M., & Tohamy, A. M. (2013). Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: A pilot randomized controlled trial. Neurorehabilitation and Neural Repair, 27(7), 592–601. doi:10.1177/1545968313484808
- Kim, D. Y., Lim, J. Y., Kang, E. K., You, D. S., Oh, M. K., Oh, B. M., & Paik, N. J. (2010). Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. American Journal of Physical Medicine & Rehabilitation, 89(11), 879–886. doi:10.1097/PHM.0b013e3181f70aa7
- Kim, Y. J., Ku, J., Cho, S., Kim, H. J., Cho, Y. K., Lim, T., & Kang, Y. J. (2014). Facilitation of corticospinal excitability by virtual reality exercise following anodal transcranial direct current stimulation in healthy volunteers and subacute stroke subjects. Journal of Neuroengineering and Rehabilitation, 18, 11–124. doi:10.1186/1743-0003-11-124
- Kuo, M. F., Paulus, W., & Nitsche, M. A. (2006). Sex differences in cortical neuroplasticity in humans. Neuroreport, 17(16), 1703–1707. doi:10.1097/01.wnr.0000239955.68319.c2
- Kurimoto, T., Oono, S., Oku, H., Tagami, Y., Kashimoto, R., Takata, M., … Mimura, O. (2010). Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clinical Ophthalmology, 4, 1441–1446.
- Kwakkel, G., van Peppen, R., Wagenaar, R. C., Wood Dauphinee, S., Richards, C., Ashburn, A., … Langhorne, P. (2004). Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke, 35(11), 2529–2539. doi:10.1161/01.STR.0000143153.76460.7d
- Lee, S. J., & Chun, M. H. (2014). Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Archives of Physical Medicine and Rehabilitation, 95(3), 431–438. doi:10.1016/j.apmr.2013.10.027
- Lee, S., Chung, S. W., Rogasch, N. C., Thomson, C. J., Worsley, R. N., Kulkarni, J., … Segrave, R. A. (2018). The influence of endogenous estrogen on transcranial direct current stimulation: A preliminary study. European Journal of Neuroscience, 48(4), 2001–2012. doi:10.1111/ejn.14085
- Lefaucheur, J.-P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., … Paulus, W. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128(1), 56–92. doi:10.1016/j.clinph.2016.10.087
- Leon, D., Cortes, M., Elder, J., Kumru, H., Laxe, S., Edwards, D. J., … Pascual-Leone, A. (2017). tDCS does not enhance the effects of robot-assisted gait training in patients with subacute stroke. Restorative Neurology and Neuroscience, 35(4), 377–384. doi:10.3233/RNN-170734
- Mazzoleni, S., Tran, V. D., Iardella, L., Dario, P., & Posteraro, F. (2017). Randomized, sham-controlled trial based on transcranial direct current stimulation and wrist robot-assisted integrated treatment on subacute stroke patients: Intermediate results. IEEE International Conference on Rehabilitation Robotics, 2017, 555–560.
- Nicolo, P., Magnin, C., Pedrazzini, E., Plomp, G., Mottaz, A., Schnider, A., & Guggisberg, A. G. (2018). Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Archives of Physical Medicine and Rehabilitation, 99(5), 862–872. doi:10.1016/j.apmr.2017.10.026
- Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., Lang, N., … Paulus, W. (2003a). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. The Journal of Physiology, 553(1), 293–301. doi:10.1113/jphysiol.2003.049916
- Nitsche, M. A., Nitsche, M. S., Klein, C. C., Tergau, F., Rothwell, J. C., & Paulus, W. (2003b). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114 (4), 600–604. doi:10.1016/S1388-2457(02)00412-1
- Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639. doi:10.1111/j.1469-7793.2000.t01-1-00633.x
- Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901. doi:10.1212/WNL.57.10.1899
- Nowak, D. A., Grefkes, C., Ameli, M., & Fink, G. R. (2009). Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair, 23(7), 641–656. doi:10.1177/1545968309336661
- Pavlova, E., Kuo, M. F., Nitsche, M. A., & Borg, J. (2014). Transcranial direct current stimulation of the premotor cortex: Effects on hand dexterity. Brain Research, 1576, 52–62. doi:10.1016/j.brainres.2014.06.023
- Pavlova, E. L., Lindberg, P., Khan, A., Ruschkowski, S., Nitsche, M. A., & Borg, J. (2017). Transcranial direct current stimulation combined with visuo-motor training as treatment for chronic stroke patients. Restorative Neurology and Neuroscience, 35(3), 307–317.
- Pellegrino, G., Tomasevic, L., Tombini, M., Assenza, G., Bravi, M., Sterzi, S., … Tecchio, F. (2012). Inter-hemispheric coupling changes associate with motor improvements after robotic stroke rehabilitation. Restorative Neurology and Neuroscience, 30(6), 497–510.
- Polanıa, R., Nitsche, M. A., & Paulus, W. (2011a). Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Human Brain Mapping, 32, 1236–1249. doi:10.1002/hbm.21104
- Polanıa, R., Paulus, W., Antal, A., & Nitsche, M. A. (2011b). Introducing graph theory to track for neuroplastic alterations in the resting human brain: A transcranial direct current stimulation study. NeuroImage, 54, 2287–2296. doi:10.1016/j.neuroimage.2010.09.085
- Purpura, D. P., & McMurtry, J. G. (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology, 28(1), 166–185. doi:10.1152/jn.1965.28.1.166
- Que, M., Schiene, K., Witte, O. W., & Zilles, K. (1999). Widespread upregulation of N-methyl-D-aspartate receptors after focal photothrombotic lesion in rat brain. Neuroscience Letters, 273(2), 77–80. doi:10.1016/S0304-3940(99)00598-4
- Redecker, C., Wang, W., Fritschy, J. M., & Witte, O. W. (2002). Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: Mediation by NMDA-dependent processes. Journal of Cerebral Blood Flow & Metabolism, 22(12), 1463–1475. doi:10.1097/01.WCB.0000034149.72481.BD
- Reis, J., & Fritsch, B. (2011). Modulation of motor performance and motor learning by transcranial direct current stimulation. Current Opinion in Neurology, 24(6), 590–596. doi:10.1097/WCO.0b013e32834c3db0
- Reis, J., Schambra, H. M., Cohen, L. G., Buch, E. R., Fritsch, B., Zarahn, E., … Krakauer, J. W. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America, 106(5), 1590–1595. doi:10.1073/pnas.0805413106
- Rossi, C., Sallustio, F., Di Legge, S., Stanzione, P., & Koch, G. (2013). Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. European Journal of Neurology, 20(1), 202–204. doi:10.1111/j.1468-1331.2012.03703.x
- Smith, M. J., Adams, L. F., Schmidt, P. J., Rubinow, D. R., & Wassermann, E. M. (2002). Effects of ovarian hormones on human cortical excitability. Annals of Neurology, 51(5), 599–603. doi:10.1002/ana.10180
- Stagg, C. J., Best, J. G., Stephenson, M. C., O'Shea, J., Wylezinska, M., Kincses, Z. T., … Johansen-Berg, H. (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience, 1347(29), 5202–5206. doi:10.1523/JNEUROSCI.4432-08.2009
- Stagg, C. J., Jayaram, G., Pastor, D., Kincses, Z. T., Matthews, P. M., & Johansen-Berg, H. (2011). Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia, 49(5), 800–804. doi:10.1016/j.neuropsychologia.2011.02.009
- Stern, E. B. (1992). Stability of the Jebsen-Taylor hand function test across three test sessions. The American Journal of Occupational Therapy, 46(7), 647–649. doi:10.5014/ajot.46.7.647
- Stinear, C. M. (2017). Prediction of motor recovery after stroke: Advances in biomarkers. The Lancet. Neurology, 16(10), 826–836. doi:10.1016/S1474-4422(17)30283-1
- Stinear, C. M., & Byblow, W. D. (2014). Predicting and accelerating motor recovery after stroke. Current Opinion in Neurology, 27(6), 624–630. doi:10.1097/WCO.0000000000000153
- Triccas, T. L., Burridge, J. H., Hughes, A. M., Pickering, R. M., Desikan, M., Rothwell, J. C., & Verheyden, G. (2016). Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: A review and meta-analysis. Clinical Neurophysiology, 127(1), 946–955. doi:10.1016/j.clinph.2015.04.067
- Triccas, L. T., Burridge, J. H., Hughes, A. M., Verheyden, G., Desikan, M., & Rothwell, J. (2015). A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation, 37(2), 181–191. doi:10.3233/NRE-151251
- Utz, K. S., Dimova, V., Oppenlander, K., & Kerkhoff, G. (2010). Electrified minds: Transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia, 48(10), 2789–2810. doi:10.1016/j.neuropsychologia.2010.06.002
- Várkuti, B., Guan, C., Pan, Y., Phua, K. S., Ang, K. K., Kuah, C. W. K., … Sitaram, R. (2013). Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabilitation and Neural Repair, 27(1), 53–62. doi:10.1177/1545968312445910
- Wang, Q. M., Cui, H., Han, S. J., Black-Schaffer, R., Volz, M. S., Lee, Y.-T., … Fregni, F. (2014). Combination of transcranial direct current stimulation and methylphenidate in subacute stroke. Neuroscience Letters, 569, 6–11. doi:10.1016/j.neulet.2014.03.011
- Woolley, C. S., & McEwen, B. S. (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience, 12(7), 2549–2554.
- You, D. S., Kim, D. Y., Chun, M. H., Jung, S. E., & Park, S. J. (2011). Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain and Language, 119(1), 1–5. doi:10.1016/j.bandl.2011.05.002
- Ziemann, U., & Siebner, H. R. (2008). Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimulation, 1(1), 60–66. doi:10.1016/j.brs.2007.08.003
