Abstract
Osperalycus tenerphagus, a new genus and species of Nematalycidae (Acari: Endeostigmata), is described from Ohio, USA, using light microscopy and low-temperature scanning electron microscopy. Specimens were extracted from two different loam soils. This genus can be readily distinguished from the other genera of Nematalycidae by the simple setae that line the opisthosoma. The mouthparts are especially distinct in possessing three-segmented palps, an unusual vessel-shaped structure, and rutella that overlap at the midline. The discovery of rutella in this genus and in two other genera indicates that the Nematalycidae can be more confidently assigned to the Endeostigmata (Sarcoptiformes).
http://www.zoobank.org/urn:lsid:zoobank.org:pub:A64A6612-B66C-4814-80A9-3856DEBE8C46
Introduction
The Nematalycidae is an unusual family of soil mites with a vermiform body that can be extended and contracted via a combination of hydraulic pressure and a continuous muscle layer underlying a thin epidermis (Haupt and Coineau Citation1999). Their unique physiology among mites is almost certainly an adaptation for living in small interstitial spaces in mineral soil and sand (Haupt and Coineau Citation1999). The Nematalycidae also appear to be exclusively thelytokous (Norton et al. Citation1993).
This family is readily distinguished from all other Acari by a combination of a highly elongate idiosoma (adults ≥6 times width when fully extended), a much greater distance between legs II and III relative to legs I and II, and a genital opening much closer to legs IV than to the anal opening. The prodorsum of this family is also distinct from many others in lacking trichobothria and a naso, with fewer than four pairs of setae; the rostral seta is unpaired if present. The Nematalycidae was originally treated as belonging to the Endeostigmata (Sarcoptiformes) (Strenzke Citation1954). It was later assigned to the Tydeoidea (Cunliffe Citation1956; Wainstein Citation1965; Krantz Citation1972). Kethley (Citation1982) placed it outside the Tydeoidea but proposed that derived characters suggested a closer relationship with the Tydeoidea than the Endeostigmata. However, since the discovery of two families – Micropsammidae (Coineau and Theron Citation1983) and Proteonematalycidae (Kethley Citation1989) – that have a transitional morphology between Nematalycidae and other families within the Endeostigmata, the Nematalycidae have been more commonly regarded as belonging to the Endeostigmata (Walter Citation2009; Walter et al. Citation2011).
Four genera of Nematalycidae have been described (Walter et al. Citation2011), all of which are monospecific. Nematalycus was described from groundwater from the Algerian coast (Strenzke Citation1954). Soon afterwards Cunliffea was described from pasture soil from the USA (Cunliffe Citation1956) and has since been collected from sands in Germany (Russell and Alberti Citation2009). Gordialycus was described from fine sands from southern France (Coineau et al. Citation1967). This genus has a worldwide distribution, having been found in southern Africa, Turkmenistan, Venezuela, Cuba, Mauritania, New Caledonia, Australia, Brazil, Hungary and the USA, (Coineau and Theron Citation1983; Silva et al. Citation1989; Thibaud and Coineau Citation1998; Norton and Kinnear Citation1999; Norton et al. Citation2008). Psammolycus was described from sandy soils from Brazil (Schubart Citation1973). This genus may also have been found in the USA (pers. obs.). A published record from Spain (Moraza Citation2008) was in error and is instead Gordialycus (pers. obs.).
A fifth genus and species of Nematalycidae is hereby described. The mites were collected from two different loam soils from relatively disturbed habitats in Ohio – a silty clay loam from a suburban prairie located on a university campus, and a sandy loam from a young chestnut plantation (see Material examined section for more details). Collecting took place throughout 2010 and 2011. As with all other species of Nematalycidae, all adults recovered were female, indicating thelytoky.
Material and methods
For the collection of slide-mounted specimens, soil samples were processed by soil washing in accordance with Kethley (Citation1991). Mites that were recovered using this technique were slide mounted in Hoyer’s medium. Live mites were also collected for the purpose of imaging with a low-temperature scanning electron microscope (LT-SEM) at the US Department of Agriculture, Electron & Confocal Microscopy Unit, Beltsville, MD. This was accomplished by directly removing them from floated material that had not yet been sieved. Searching was undertaken with a dissection microscope.
Live specimens, for LT-SEM, were secured to 15 × 30-cm copper plates, using ultrasmooth round (12-mm diameter), carbon adhesive tabs (Electron Microscopy Sciences, Inc., Hatfield, PA, USA). The specimens were frozen conductively, in a Styrofoam box, by placing the plates on the surface of a pre-cooled (−196°C) brass bar whose lower half was submerged in liquid nitrogen. After 20–30 s, the holders containing the frozen samples were transferred to the Quorum PP2000 cryo-prep chamber (Quorum Technologies, East Sussex, UK) attached to an S-4700 field emission scanning electron microscope (Hitachi High Technologies America, Inc., Pleasanton, CA, USA). The specimens were etched inside the cryotransfer system to remove any surface contamination (condensed water vapour) by raising the temperature of the stage to −90°C for 10–15 min. Following etching, the temperature inside the chamber was lowered below −130°C, and the specimens were coated with a 10-nm layer of platinum using a magnetron sputter head equipped with a platinum target. The specimens were transferred to a pre-cooled (−130°C) cryostage in the SEM for observation. An accelerating voltage of 5 kV was used to view the specimens. Images were captured using a 4pi Analysis System (Durham, NC, USA). Images were sized and placed together to produce a single figure using Adobe® Photoshop CS 5.0.
With the exception of the pre-larva – which, if existing, was not recovered – the different instars were carefully studied for ontogenetic variation. Slide-mounted mites were drawn using a compound microscope (Zeiss Axioscope™) equipped with a camera lucida as well as phase-contrast and differential interference contrast optical systems. Many detailed features of this mite were not readily distinguishable without LT-SEM. For example, the very small size of the mouthparts made it impossible to discern their true shape and structure without LT-SEM. For this reason, LT-SEM was used to make modifications and corrections to drawings where the light microscope provided insufficient detail.
Although the prodorsal, opisthosomal and genital setae tend to project straight out (as revealed by LT-SEM), they are typically pushed into a flat two-dimensional orientation by slide mounting. These setae were drawn as though slide mounted (with vertically oriented setae on the dorsum and ventrum projecting to the side) to reveal their actual lengths. The LT-SEM also revealed minute barbs near the base of many setae. These setae are treated as simple because it is impossible to determine which setae do not have them (being out of view in many LT-SEM images).
Measurements were made using an ocular micrometer and are given to the nearest micrometer. Legs were measured from the base of the trochanter to the tip of the empodium (adjusting for legs in which the empodium was not fully extended). All of the prodorsal and opisthosomal setae were measured (including setae from the genital region). Setae were measured from the centre of the base to the tip. All setal length measurements in the main text are presented as ranges in parentheses with the symbol “µm” omitted.
In the adult female section, reference is occasionally made to images of nymphal instars to illustrate structures in which there is no apparent difference between instars (e.g. integument, ventral furrow and solenidial grooves). Tables pertaining to the ontogeny section give abbreviations for instars: Larva = La; Protonymph = PN; Deutonymph = DN; Tritonymph = TN; Female = F.
Osperalycus tenerphagus Bolton and Klompen gen. nov., sp. nov.
–
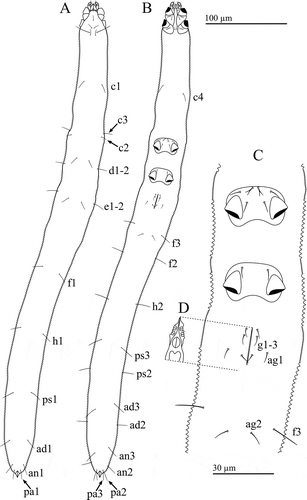
Figure 1. Osperalycus tenerphagus sp. nov. Female: (A) dorsal view; (B) ventral view; (C) metapodosoma and genital region (D) genitalia – internal.
Diagnosis
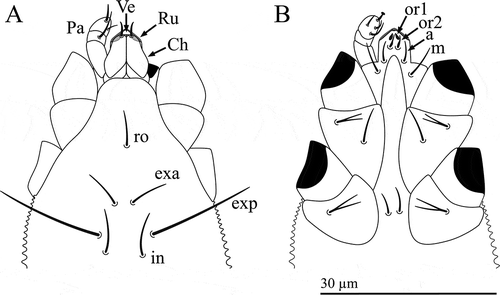
Osperalycus is readily distinguished from other genera of Nematalycidae by the presence of simple setae of similar size along the opisthosoma (A, B); the simple setae next to the anal opening of Gordialycus and Nematalycus are at least three times as long as those along the rest of the opisthosoma, although the opisthosoma of Gordialycus and Nematalycus is often almost completely nude. Cunliffea and Psammolycus have bifurcate or trifurcate setae along the opisthosoma. Osperalycus is also readily distinguished from Gordialycus and Nematalycus by the baculiform solenidion on tarsus II – this solenidion has a distinctly swollen or bulbous tip in Gordialycus and Nematalycus. The most distinct characters of Osperalycus are to be found in the mouthparts (A, B). The vessel (Ve) – modified from the lateral lips – is a very unusual structure that appears to be unique to this genus (A). Markedly short chelicerae (Ch) (<15 µm) are found in both Osperalycus tenerphagus and Psammolycus delamarei, but not Nearctic cf. Psammolycus or any other genus of Nematalycidae. Osperalycus is also distinct from all other genera in having rutella (Ru) that overlap at the midline (A).
Female
General morphology
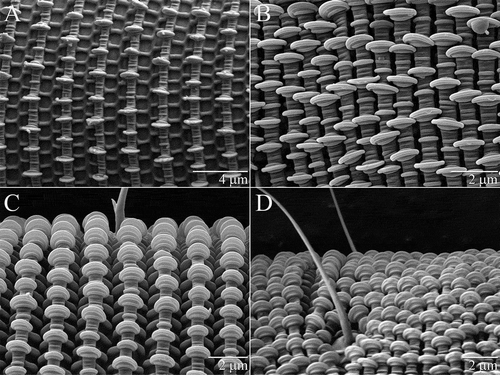
Idiosoma ≈600 µm long (≈12 times longer than wide) when fully extended (A, B). Cuticle with flat round projections, or palettes, sensu Haupt and Coineau (Citation1999), lining the latitudinal annuli (A–D). Trachea and peritremes absent. Lyrifissures absent from idiosoma. Podocephalic canals terminating anteriorly between the bases of the chelicerae; extending back beyond coxae I. Distinct glands leading into podocephalic canals at coxae I. Long narrow oesophagus extends to proximity of metapodosoma.
Prodorsum
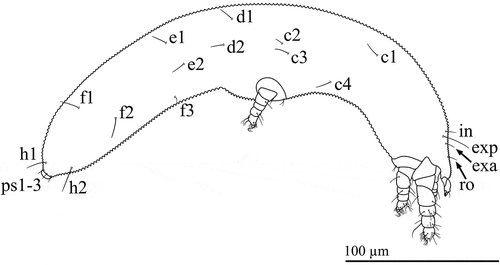
Trichobothria absent; naso absent; eyes absent. Three pairs of setae (exa, in and le) and one unpaired rostral (ro) seta. All setae simple and close to the midline of the prodorsum (A). Setae ro, le and in subequal and short (4–8); exa long (16–22).
Opisthosoma
All setae simple. Setae associated with all nine opisthonotal segments: c1–4, d1–2, e1–2, f1–3, h1–2, ps1–3, ad1–3, an1–3, pa1–3. Frequent and noticeable asymmetry in the longitudinal positions of pairs of setae (A, B). In segmental remnants F-PA, ancestrally dorsolateral setae are displaced to a ventrolateral or ventral position that is usually noticeably more anterior than the dorsomedial setae of the same segment. Segment C very long; position of c2 and c3 much more posterior than c1 and c4.
Setae on segments C–E generally shorter (5–15) than those on segments F-PA (10–19). Setae c2 short (5–8); proximal setae c3 comparatively long (9–13). Longest opisthosomal setae h2 and ps3 (15–19). From segments H to PA, ventral setae (h2, ps3, ad3, an3, pa3) typically 1 to 4 µm longer than lateral and dorsal setae on the same segment; lateral and dorsal setae subequal.
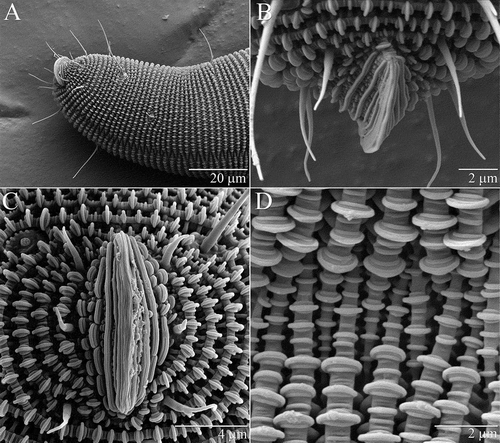
Distinct anal valves projecting from the anus (A–C). Ventral furrow, in which annular ridges terminate (D), extending from proximity of anal valves to genital region (A).
Figure 4. Osperalycus tenerphagus sp. nov. Tritonymph, opisthosoma: (A) anal valves and ventral furrow, ventrolateral view; (B) anal valves, dorsal view; (C) anal valves, posterior view; (D) ventral furrow, close-up.
Three pairs of genital (g) setae and two pairs of aggenital (ag) setae (C). Genital and aggenital setae short (3–7) compared with most other opisthosomal setae. Two pairs of bilobed genital papillae (D).
Podosoma
Large gap separating legs II and III (B). Coxal fields I and II medially separated by intercoxal region (B). Single pair of intercoxal setae (between coxal fields II). Coxal fields III and IV medially fused (C). Each coxal field I with one bifurcate seta and one simple seta; each coxal field II with one bifurcate seta; coxal fields III with one bifurcate seta and two simple setae on each side of the fused coxal plate; coxal fields IV with one simple seta on each side of the fused coxal plate.
Legs
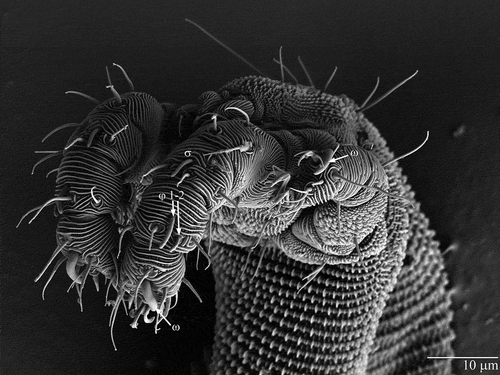
Pretarsi I–IV each with two lateral claws and a ciliated empodium (A–E). Solenidial formulae femora–tarsi for individual legs I–IV: 0-1-2-1; 0-0-0-1; 0-0-0-0; 0-0-0-0. Longitudinal grooves of shortest solenidion phi (φ) (tibia I) restricted to bulbous tip (F). Other solenidia baculiform with longitudinal grooves extending almost all the way to the base. Solenidia omega (ω) on tarsi I and II subequal and noticeably larger than solenidion phi (φ) and sigma (σ) on, respectively, tibia I and genu I ().
Figure 5. Osperalycus tenerphagus sp. nov. Female: (A–E) tarsi I–IV; (A) tarsus I; (B) tarsus II, ε obscured; (C) tarsus II, ε visible; (D) tarsus III; (E) tarsus IV. Deutonymph: (F) tibia I.
Setae absent from trochanters I–IV. Setal formulae femora–tarsi for individual legs I–IV (excluding solenidia and famuli): 4-6-7-16; 2-2-2-9; 1-0-2-6; 1-0-2-5. All setae on femora–tibiae I–IV simple. Tarsi I–IV with various setae (see below). Femur I sometimes lacking the dorsal seta normally added in post-protonymphal instars – some individuals with three setae on one femur I and four on the other.
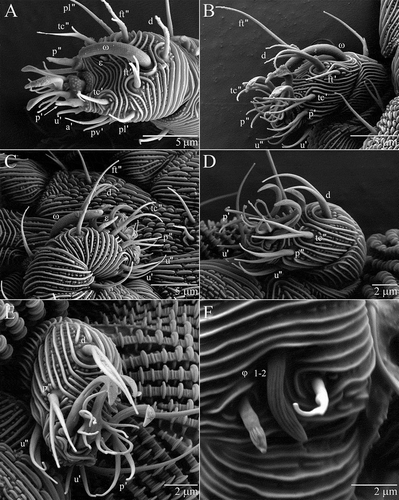
Tarsus I (A). Seven pairs of setae (p, tc, ft, u, a, pv and pl) and two unpaired setae (s and d). Proral (p) setae conical and extending from turbercle. One tectal (tc″) and one fastigial (ft″) seta usually semi-bifurcate, with one branch short and barb-like – sometimes short and basal enough for the seta to qualify as simple (see aforementioned criteria in Material and methods section), or rarely long enough to be bifurcate. Other fastigial (ft') seta usually bifurcate, rarely semi-trifurcate or trifurcate. All other setae simple. Subunguinal (s) seta centred between pair of anterolateral (a) setae. Dorsal (d) seta proximal and posterior to base of solenidion ω. Small stubby famulus (ε) near tc″. Dorsolateral lyrifissure next to posterior margin of tarsus (obscured by overlying integument when viewed under LT-SEM).
Tarsus II (B, C). Four pairs of setae (p, tc, ft and u) and one unpaired seta (d). Setae p, tc and u bifurcate and projecting forward past base of ambulacrum; ft simple; d trifurcate. Long rod-like famulus near tc″. Base of solenidion ω next to posterior margin of tarsus.
Tarsus III (D). Two pairs of setae (p and u) and two unpaired setae (tc″ and d). Setae p, u and tc″ bifurcate and projecting forward past base of ambulacrum; d bifurcate.
Tarsus IV (E). Identical to tarsus III but with tc″ absent.
Gnathosoma
Gnathosoma including a vessel (Ve) – modified from the lateral lips – into which the chelae (opposing digits) slot (A). Chelicerae (Ch) ≈12 µm long and slanting downwards, anteriorly, into the vessel. Single dorsal seta on each chelicera. Rutella (Ru) consisting of a swollen base with a narrow extending digit. The rutella lie against the anterior surface of the vessel and overlap at the midline, appearing as an inverted v – more discernible, under a light microscope, when the chelae are not within the vessel. Palps (Pa) ≈10 µm long and three-segmented, tentatively designated femur, genu and tarsus; solenidia absent; setal formula femur–tarsus for each palp: 0-1-6; distal tarsal seta with cup-shaped tip; all other setae simple. Palp tarsus also with three very small cuticular protuberances (only visible under LT-SEM and omitted from A, B). Subcapitulum with four pairs of setae: m, a, or1–2 (B). Adoral setae (or1–2) typically unevenly tapering (or2 sometimes evenly tapering); anterior pair (or1) vestigial and broad.
Ontogeny
Idiosoma and appendages
From larva to adult, the idiosoma increases greatly in length compared with the appendages (; , A, B). Notably, there is no apparent increase in the length of the palp.
Table 1. Osperalycus tenerphagus sp. nov. Mean length of appendages and idiosoma across instars (µm).
Chaetotaxy
Aside from the legs and coxal fields (see below), setal additions only occur in the genital region and segments AD to PA (). Adults readily distinguished from tritonymphs by the presence of setae ag2 (very posterior and close to f3) and long pa1–3 ().
Table 2. Osperalycus tenerphagus sp. nov. Distinguishing attributes of instars.
New segmental additions of setae to the posterior tip of the opisthosoma always short (3–7), increasing two- to four-fold in length at the next instar (). Several other opisthosomal setae noticeably increase in length from the larval to adult instar (, , 7): seta f3 increases approximately four-fold (from 3–4) to be subequal to f2 and f1 (11–16); setae d2 and e2 increase by about 50% (from 7–10 to 10–15). All other opisthosomal and prodorsal setae increase only slightly (< 30%) or not at all.
Leg setation complete (including coxal fields) by the deutonymphal instar (). Setation of tarsus I is completed by the addition of an unpaired dorsal (d) seta in the protonymph. Chaetotaxy unchanging for tarsi II–IV. Numbers of solenidia, famuli and lyrifissures also unchanging for all leg segments. Gnathosoma without additions of setae or any other structures.
Table 3. Osperalycus tenerphagus sp. nov. Setal addition pattern for legs (including coxal fields) across instars.
Material examined
Holotype female (OSAL 015134), USA, Ohio, Franklin Co., Kinnear Road, 39.9990 N, 83.0468 W, silty clay loam from suburban prairie (including shrubs, grasses and small trees); collector: Samuel Bolton, May 2011, 40 cm deep (SB11-05-I). Same data: 9 F (OSAL 0103239, 0105137, 0105138, 0105147, 0105148, 0105149, 0105153, 0105155, 0105156), 1 TN/F? (OSAL 0105135), 5 TN (OSAL 0105143, 0105146, 0105151, 0105152, 0105157), 1 DN (OSAL 0105145), 1 PN (OSAL 0105150), 3 L (OSAL 0103237, 0105141, 0105142), 1 pharate (TN–F, OSAL 0105154). Same locality and collector, July 2010, 60 cm deep (SB10-0724-I): 4 F (OSAL 0103240, 0105136, 0105139, 0105140), 1 DN, (OSAL 0105144), 2 PN (OSAL 0103241, 0103242),1 L (OSAL 0103245), 2 pharates (L-PN, OSAL 0103243, 0103244). August 2011 (LT-SEM material), 60 cm deep: 9 F, 1 TN/F?, 6 TN, 1 DN/TN?, 1 DN, 1 PN (used for SEM, not recovered). USA, Ohio, Ashtabula Co., West Main Road, 41.9246 N, 80.6138 W, sandy loam in small chestnut plantation, collector: Samuel Bolton, July 2011, 40 cm deep (SB11-07-IV): 1 F (OSAL 0105133), 1 TN (OSAL 0103238), 1 DN (OSAL 0105132).
Type material and depositor
Holotype female (OSAL 0105134) at Ohio State University Acarology Collection (OSAL), Columbus, Ohio, USA. Paratypes: US National Collection (USNM), housed at the Beltsville Agricultural Research Center, USDA, Beltsville, MD, USA: 2 F (OSAL 0105137, 0105147); Natural History Museum (BMNH), London, UK: 2 F (OSAL 0105140, 0105148); all other paratypes at OSAL.
Etymology
Binomial
Osperalycus tenerphagus. Ospera- is a combination of the Latin terms for “mouth” (os) and “purse/bag” (pera) in reference to the soft and unsclerotized vessel of the gnathosoma; -lycus is a Latinized Greek ending given to three of the four other genera of Nematalycidae. The species name, tenerphagus, combines the Latin term for “tender” with the Greek-derived Latin suffix for “feeding”, referring to the delicate mechanism hypothesized to explain how this mite may carefully pick up small micro-organisms and place them into its feeding vessel without rupturing them (Bolton et al. in preparation).
Systematic relationships
Whereas rutella were thought to be absent from the Nematalycidae (Walter Citation2009), they are clearly present in Osperalycus tenerphagus. Rutella also appear to be present in Gordialycus sp. nov. and cf. Psammolycus sp. nov. (pers. obs.). The presence of rutella combined with the absence of a tracheal system more firmly places the Nematalycidae within the Endeostigmata. Other newly observed characters also suggest a closer relationship with the Endeostigmata than the Tydeoidea, e.g. presence of setae for all nine opisthosomal segments and more than two pairs of setae on the C and F segments.
Opisthosomal setae near the anal opening at least three times as long as those along the rest of the opisthosoma, which is often almost completely nude; chelicerae long (>15 µm).2
Setae along the length of the opisthosoma of similar size; chelicerae can be short (<15 µm) or long (>20 µm).3
Legs III and IV noticeably smaller than leg II; body usually extremely elongated (adult length >20× width).Gordialycus
Legs III and IV not noticeably smaller than leg II; body not extremely elongated (adult length <20× width).Nematalycus
Palps with two segments; pretarsi II to IV without claws; tritonymphs and adults with three pairs of simple genital papillae; chelicerae long (>20 µm) but with very short chelae (opposing digits); opisthosomal setae always trifurcate.Cunliffea
Palps with three or four segments; lateral claws present on pretarsi II to IV; tritonymphs and adults with two pairs of bilobed genital papillae; if chelicerae long (>20 µm), chelae distinctly elongated; opisthosomal setae trifurcate, bifurcate or simple.4
Palps with four segments; opisthosomal setae distinctly bifurcate or trifurcate; rutella absent or never overlapping at the midline when present; if chelicerae long (>20 µm), chelae distinctly elongated. Psammolycus
Palps with three segments; opisthosomal setae long and simple (with very small basal barbs that are not visible under a light microscope); rutella overlap at the midline; chelicerae short (<15 µm).Osperalycus
Key to the genera of the Nematalycidae
Acknowledgements
This research was partly funded by the Smithsonian Institution (predoctoral fellowship) for SB. Chris Pooley presented and arranged the LT-SEM image plates. Thanks to the Field Museum, Chicago, for the loan of specimens for comparison. Thanks also to Cal Welbourn for useful suggestions, and to Julie Stehli for permission to collect from her land in Ashtabula County, Ohio, USA. Mention of trade names or commercial products in this publication does not imply recommendation or endorsement by the USDA or The Ohio State University. , , , and in this publication are sourced from: US Department of Agriculture, Agricultural Research Service, Electron and Confocal Microscopy Unit, Beltsville, Maryland, USA. These images are in the public domain.
References
- Coineau Y, Fize A, Delamere Deboutteville C. 1967. Découverte en France des acariens Nematalycidae Strenzke à l‘occasion des traveaux d‘aménagement du Languedoc-Rousillon. C. r. Acad. Sci. Paris 265:685–688.
- Coineau Y, Theron PD. 1983. Les Micropsammidae, n. fam. d‘Acariens Endeostigmata des sables fin. Acarologia. 24:275–280.
- Cunliffe F. 1956. A new species of Nematalycus Strenzke with notes on the family (Acarina, Nematalycidae). Proc Entomol Soc Wash. 58:353–355.
- Haupt J, Coineau Y. 1999. Ultrastructure and functional morphology of a nematalycid mite (Acari: Actinotrichida: Endeostigmata: Nematalycidae): adaptations to mesopsammal life. Acta Zool. 80:97–111.
- Kethley JB. 1982. Acariformes, Prostigmata. In: Parker SB, editor. Synopsis and classification of living organisms. New York (NY): McGraw-Hill, Inc; p. 146–169.
- Kethley JB. 1989. Proteonematalycidae, a new mite family (Acari: Acariformes), from fore-dune sand of the southern shores of Lake Michigan. Int J Acarol. 15:209–217.
- Kethley JB. 1991. A procedure for extraction of microarthropods from bulk samples with emphasis on inactive stages. Agric Ecosyst Environ. 34:193–200.
- Krantz GW. 1972. A manual of acarology. 1st ed. Corvallis (OR): Oregon State University Book Stores, Inc.
- Moraza ML. 2008. First records of Endeostigmata and Sphaerolichina mites (Acari: Sarcoptiformes and Trombidiformes) from the Iberian Peninsula and the Canary Islands. Bol Asoc Esp Entomol. 32:293–304.
- Norton RA, Kethley JB, Johnston DE, OConnor BM. 1993. Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Wrensch DL, Ebbert MA, editors. Evolution and diversity of sex ratio in insects and mites. New York (NY): Chapman & Hall; p. 8–99.
- Norton RA, Kinnear A. 1999. New Australian records of xerophilic acariform mites (Oribatida and Prostigmata). Aust Entomol. 26:53–55.
- Norton RA, Oliveira AR, De Moraes GJ. 2008. First Brazilian records of the acariform mite genera Adelphacarus and Gordialycus (Acari : Adelphacaridae and Nematalycidae). Int J Acarol. 34:91–94.
- Russell DJ, Alberti G. 2009. Actinedid mite community diversity in a succession gradient in continental sand-dune habitats of central Europe. In: Sabelis MW, Bruin J, editors. Trends in Acarology. Proc. of the 12th Intern. Congress of Acarology. 2006. Amsterdam, Dordrecht: Springer-Science & Business Media B. V; p. 135–142.
- Schubart HOR. 1973. The occurence of Nematalycidae (Acari, Prostigmata) in Central Amazonia with a description of a new genus and species. Acta Amazonica. 3:53–57.
- Silva SI, Mackay WP, Whitford WG. 1989. Temporal patterns of microarthropod population densities in fluff grass (Erioneuron pulchellum) litter. Relationship to subterranean termites. Pedobiologia. 33:333–338.
- Strenzke K. 1954. Nematalychus nematoides n. gen. n. sp. (Acarina, Trombidiformes) aus dem Grundwasser der algerischen Küste. Vie Milieu. 4:638–647.
- Thibaud JM, Coineau Y. 1998. New localities for the genus Gordialycus (Acarina: Nematalycidae). Biogeogr. 74:91–94.
- Wainstein BA. 1965. [The system of the aquatic mites and their place in the suborder Trombidiformes, in ecology and biology of aquatic invertebrates]. Tr. Inst. Biol. Vnutr. Vodnany. 8:66–83. Russian.
- Walter DE. 2009. Suborder Endeostigmata. In: Krantz GW, Walter DE, editors. A manual of acarology. Lubbock, TX: Texas Tech University Press; p. 421–429.
- Walter DE, Bolton S, Uusitalo M, Zhang Z-Q. 2011. Suborder Endeostigmata Reuter, 1909. In: Zhang Z-Q, editor. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa; p. 139–140.