Abstract
Two new Ecuadorian wasp species attacking pyralid larvae are described: Meteorus albisericus and Meteorus pyralivorus, as well as a new Ecuadorian distribution record for Meteorus desmiae Zitani, another pyralid parasitoid, previously known from Costa Rica and Colombia. The hosts of M. albisericus were found feeding on Clibadium glabrescens S. F. Blake (Asteraceae), Diplazium costale var. robustum (Sodiro) Stolze (Dryopteridaceae), Brunellia tomentosa Bonpl. (Brunelliaceae) and Cavendishia sp. Lindl. (Ericaceae). Chusquea scandens Kunth (Poaceae) is the associated plant for M. pyralivorus hosts. Meteorus. albisericus is the first Neotropical Meteorus species with dorsopes whose biology is known. We hypothesize that the parasitism of pyraloid caterpillars has originated at least twice in Neotropical Meteorus.
http://zoobank.org/urn:lsid:zoobank.org:pub:B603F36B-4645-490D-AD4D-469088EEF36F
Introduction
Meteorus Haliday, 1835, is a cosmopolitan genus of koinobiont endoparasitoids of Coleoptera and Lepidoptera composed of 326 named species to date (Jones and Shaw Citation2012; Yu Citation2012). The genus tends to be especially species-rich in high mountains of the Neotropical region (Aguirre et al. Citation2011; Jones and Shaw Citation2012). The Ecuadorian fauna has received special attention thanks to the project ‘Caterpillars and Parasitoids of the Eastern Andes of Ecuador’ (Dyer et al. Citation2012), and an extensive body of data is growing concerning their ecological interactions; recently 11 new species from Napo province have been described and details have been provided about their biology (Shaw and Jones Citation2009; Jones and Shaw Citation2012).
Twenty-nine species of Meteorus are known as parasitoids of 72 pyralid species around the world and only three of them (Meteorus camilocamargoi Zitani Citation1998, Meteorus desmiae Zitani Citation1998 and Meteorus sterictae Zitani Citation1998) were known to occur in the Neotropics (Yu Citation2012). Pyralid moths are widespread and range from alpine to middle and low elevation in tropics. Most larvae are concealed feeders, living inside fruits, seeds or stems, or constructing nest-like silk shelters among leaves (Powell and Opler Citation2009). Since the focus of Neotropical inventories of caterpillars and their parasitoids are often on exposed hosts, the parasitoids of pyralids are not commonly reared.
This article describes two new species of Meteorus from Ecuador parasitizing Pyralidae caterpillars, and the first record of Meteorus desmiae for Ecuador, which expands its distribution southward as it was previously known for Costa Rica (Zitani et al. Citation1998) and Colombia (Aguirre et al. Citation2011).
Material and methods
Taxonomy
Morphological terminology follows Sharkey and Wharton (Citation1997). Explanatory illustrations can be consulted in Aguirre et al. (Citation2011). Terminology for sclerite surface sculpturing follows Harris (Citation1979). Body colour is qualitatively described. Metasomal tergites are referred as T1, T2, T3 and so on. The specimens were measured using a Leica M80 stereomicroscope with micrometer on a 10 × ocular. Images were captured with a Leica M205C stereomicroscope with digital Leica DFC295 camera kit and processed with Leica Application Suite Version 3.8.0 auto-montage software. Holotypes and paratypes are deposited at the University of Wyoming Insect Museum (UWIM).
Biology
The caterpillars were collected during the ‘Caterpillars and Parasitoids of the Eastern Andes of Ecuador’ project (Dyer et al. Citation2012) based at the Yanayacu Biological Station and Center for Creative Studies (00°35.9' S, 77°53.4' W, elevation 2163 m). The forest is categorized as tropical montane moist forest based on Holdridge (Citation1967). Eighty per cent of the land around the station comprises primary forest; the remaining land is abandoned cattle pasture (Greeney Citation2012). Details about the rearing techniques are provided in Shaw and Jones (Citation2009) and Jones and Shaw (Citation2012).
Results
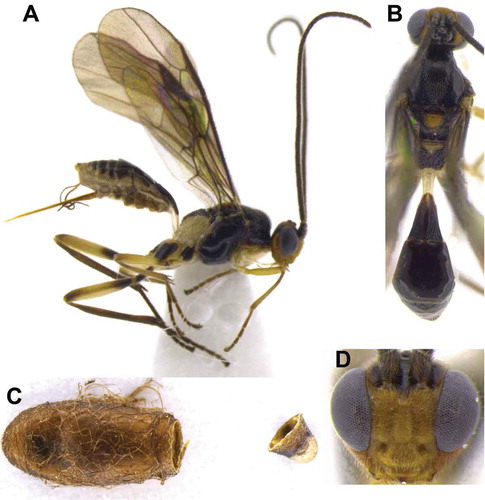
Meteorus albisericus Aguirre and Shaw, sp. nov. (, )
Figure 1. Meteorus albisericus sp. nov. (A) Habitus in lateral view; (B) habitus in dorsal view; (C) head in frontal view.
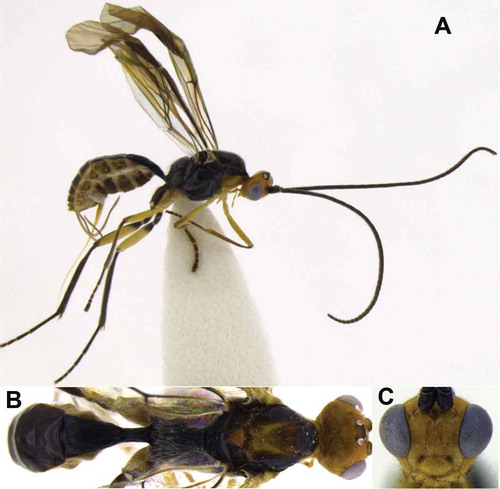
Figure 2. Meteorus albisericus sp. nov. (A) Mesonotum with distinctive notauli; (B) habitus male in lateral view; (C) tergite one (T1) – the upper white arrow indicates the dorsope, the lower white arrow indicates the laterope; (D) cocoon – the white arrow indicates the presence of a pad on anterior end seen from inside.
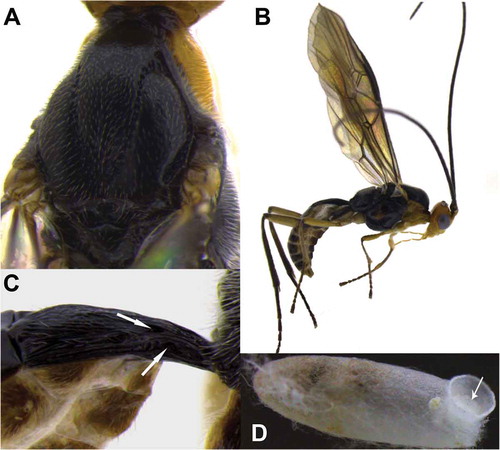
Diagnosis
Mandible moderately twisted, ocelli ocular distance 1.6–1.7 × ocellar diameter, head height 1.6–1.8 × eye height, occipital carina incomplete, notauli distinct, wings hyaline, hind coxa punctate and polished, tarsal claw with a large lobe, dorsope present, laterope present, ventral borders of first tergite widely separated, ovipositor bent or sinuous, 2.7–2.8 × longer than first tergite.
Body colour
Body black except antennae dark brown; head yellow with area between ocelli dark brown; mesosoma with pronotum ventrally and propleuron yellow; prothoracic and mesothoracic leg yellow; metathoracic leg with dorsal area of coxa, trochanter, trochantellus, base and apex of femur, tibia and tarsus dark brown, the remaining yellow; metasoma with dorsal area of T2 and T3 dark brown, dorsal area of T4–T8 brown lighter than previous segments, lateral area of tergites yellow-white with brown punctures, sternum 1 dark brown, sterna 2–6 light brown, hypopygium white; wings hyaline.
Body length
4.3 mm.
Description
Head
Antenna with 34 flagellomeres; flagellar length/width ratios as follows: F1 = 2.8; F2 = 4; F3 = 3.2; F29 = 1.7; F30 = 1.6; F31 = 1.1; F32 = 1.2; F33 = 1.2; F34 = 1.5; head 1.2 × wider than high; occipital carina incomplete; ocelli ocular distance 1.6 × ocellar diameter; head height 1.6 × eye height; temples length 0.7 × eyes length in dorsal view; frons smooth and polished; maximum face width 1.2 × minimum face width; face punctate; minimum face width 1.3 × clypeus width; clypeus smooth and polished; malar space length equal to mandible width basally; mandible moderately twisted.
Mesosoma
Pronotum in lateral view carinate-foveate; propleuron slightly puncticulate; notauli distinct and foveolate; mesonotal lobes well defined; central lobe slightly puncticulate; scutellar furrow with one distinctive carina; mesopleuron puncticulate; sternaulus long, narrow and foveolate; metapleuron puncticulate; propodeum carinate rugose and densely pubescent; median longitudinal carina on propodeum slightly present; median depression on propodeum absent.
Legs
Hind coxa punctate polished; tarsal claw with an internal large lobe.
Wings
Forewing length 4.8 mm; vein r 0.7 × length of 3Rsa; vein 3RSa 0.9 × length of rm; vein m-cu antefurcal; vein 1M 1.6 × length of cu-a; vein 1M 0.9 × length of 1r-m.
Metasoma
Dorsope present; laterope present; ventral borders of first tergite widely separated; first tergite rugulose costate with costae convergent; ovipositor 2.7 × longer than first tergite and sinuous.
Cocoon
Elongate-oval, length 5.9 mm, width 2 mm, completely white-translucent, remnants of emergence could be seen from outside, tightly enveloped by dense silk threads; rounded at the anterior end, slightly tapered at the posterior end, the edge of emergence hole is rough due to the presence of imbricate fibres around it, the cap is loosely attached at the anterior end by some threads and the silk of the apex is composed from slender fibres.
Female variation
Body length 4.3–4.4 mm; pedicel and scape yellow with brown patches; pronotum completely yellow; posterior area of mesopleuron yellow; median and posterior area of mesonotum, and scutellum yellow-orange; antenna with 35 flagellomeres; head 1.2 × wider than high; ocelli ocular distance 1.7 × ocellar diameter; head height 1.8 × eye height; malar space length 1.1 × mandible width basally; propodeum rugose or carinate-rugose with distinct longitudinal and slightly transverse carinae; wing length 4.3–4.8 mm; vein 3RSa 0.9 × length of rm; vein 1M 1.2 × length of cu-a; vein 1M 1.2 × length of 1r-m; ovipositor bent, 2.8 × longer than first tergite.
Male variation
Body length 4.3–4.6 mm; eyes smaller than females, head height 1.8 × eye height; temples length 0.6 × eyes length in dorsal view; minimum face width 1.3 × clypeus width; malar space length equal to mandible width basally; sometimes mesonotal pubescence denser.
Comments
Based on the revisionary works and keys of Zitani et al. (Citation1998) and Aguirre et al. (Citation2011), M. albisericus is similar to Meteorus micrommatus Zitani and Meteorus guineverae Aguirre and Shaw. These three species share the following characteristics: the mandible moderately twisted, notauli distinct, and hind coxa punctate and polished. Meteorus albisericus can be distinguished from M. guineverae by the incomplete occipital carina and the head being mostly orange, and from M. micrommatus by having wings hyaline (slightly infuscated in M. micrommatus), propodeum carinate-rugose with median longitudinal carina present (aerolate-rugose in M. micrommatus), tarsal claw with large lobe (simple in M. micrommatus), dorsopes and lateropes present (absent in M. micrommatus), ventral borders of first tergite separated (touching for a short distance in M. micrommatus) and ovipositor more than twice the length of first tergite (less than twice in M. micrommatus).
Holotype
Female (point mounted), ECUADOR, Napo province, Yanayacu Biological Station, Isla las Palmas, 00°35.9' S, 77°53.4' W, 2163 m, collected as parasitoid cocoon on Clibadium glabrescens (Asteraceae), 29 May 2009, parasitoid emerged 11 June 2009, UWIM.
Paratypes
One female, same data as holotype except collected 11 February 2010, as parasitoid on Pyralidae third instar larva associated with Diplazium costale var robustum (Dryopteridaceae), pupated 2 March 2010, parasitoid emerged 13 March 2010; 1 male, same data as before except collected 18 March 2006 at Vinillos Guacamayos, 2135 m, as parasitoid of Pyralidae second instar larva associated with Cavendishia sp. (Ericaceae), parasitoid pupated 31 May 2006, adult wasp emerged 23 June 2006; 1 male, same data as before except collected March 2011, as Pyralidae parasitoid on D. costelae; 1 male, same data as before except collected at Mirador de Yanayacu, as Pyralidae second larval instar parasitoid feeding on Brunellia tomentosa (Brunelliaceae), adult parasitoid emerged 7 June 2011.
Distribution
ECUADOR, Napo province, Yanayacu Biological Station, High Andean Cloud Forest, 2135–2163 m,
Biology
This species is a parasitoid of second and third instar larva of Pyralidae ().
Etymology
Named for the white cocoon silk, which is unusual among Meteorus.
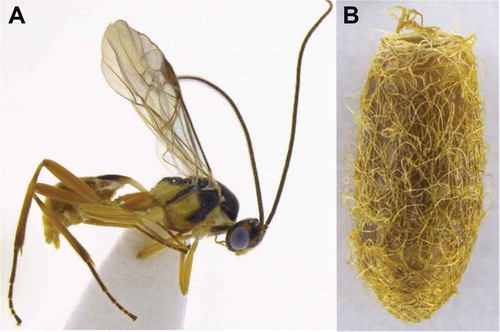
Meteorus pyralivorus Aguirre and Shaw, sp. nov. ()
Diagnosis
Mandible strongly twisted; occipital carina complete; wings slightly infuscated; propodeum rugose; hind coxa strigate to strigate-punctate; tarsal claw with large lobe; dorsopes absent; ventral borders of first tergite joined completely along basal half of segment; ovipositor 1.9–2.1 × longer than first tergite; thorax colour alternating between white, black and small areas yellow ().
Description
Body colour
Antennae dark brown; head orange except frons, area between and around the ocelli, vertex behind the lateral ocellus, temples and occiput black; propleuron yellow; pronotum dorsally black, ventrally dark brown; mesonotum black with scutellum yellow; mesopleuron black; metapleuron white. Propodeum dorsally black; posteriorly, laterally and a small area adjacent to insertion of petiole white; a small yellow patch on the anterior area touching the metanotum (). Prothoracic legs yellow except tibia and tarsus light brown; mesothoracic legs yellow except coxa dorsally black, apex of trochanter, trochantellus and femur basally dark brown, tibia and tarsus light brown; metathoracic legs yellow except coxa dorsally, trochanter, trochantellus and femur basally black, apex of femur, tibia and tarsus brown; metasoma with T1 basally white, medially and apically black; T2–T7, T9 dark brown dorsally, T8 yellow, T2–T9 yellow laterally with small and scattered brown punctures; sterna light brown. Wings slightly infuscated.
Body length
3.8 mm.
Head
Antenna with 28 flagellomeres; flagellar length/width ratios as follows: F1 = 2.6; F2 = 2.8; F3 = 2.6; F26 = 2.1; F27 = 1.7; F28 = 1.1; head 1.7 × wider than high; occipital carina complete; ocelli ocular distance 1.2 × ocellar diameter; head height 1.5 × eye height; temples length 0.6 × eyes length in dorsal view; frons smooth and polished; maximum face width 1.1 × minimum face width; face strigulate; minimum face width equal to clypeus width; clypeus with fine rugulose wrinkles; malar space length 0.8 × mandible width basally; mandible twisted.
Mesosoma
Pronotum in lateral view carinate to carinate-rugulose; propleuron puncticulate and polished; notauli not distinct and rugulose, with a broad and rugose posterior area; mesonotal lobes not well defined; central lobe of mesonotum slightly rugulose-puncticulate; scutellar furrow with one carina; mesopleuron puncticulate and polished to smooth and dull above the sternaulus; sternaulus long, narrow and foveate; metapleuron slightly rugulose-puncticulate; propodeum rugose; median longitudinal carina on propodeum absent; median depression on propodeum absent.
Legs
Hind coxa strigate to strigate-punctate; tarsal with a large lobe.
Wings
Forewing length 3.8 mm; vein r 0.6 × length of 3Rsa; vein 3RSa 0.8 × length of rm; vein m-cu postfurcal; vein 1M 1.4 × length of cu-a; vein 1M 0.9 × length of 1r-m.
Metasoma
Dorsope absent; laterope absent; ventral borders of first tergite joined completely along basal half of segment; first tergite with costae convergent posteriorly; ovipositor 1.9 × longer than first tergite and thickened at the base.
Cocoon
Elongate-oval, length 4.9 mm, width 1.7 mm; honey-brown translucent, loosely enveloped by thread, the edge of emergence hole and cap smooth and neat, posterior end nipple-shaped.
Female variation
Body length 4.1 mm; head 1.2 × wider than high; ocelli ocular distance 1.4 × ocellar diameter; forewing length 4.1 mm; vein 3RSa 0.9 × length of rm; vein 1M 1.5 × length of cu-a; vein 1M 1.2 × length of 1r-m; ovipositor bent, 2.1 × longer than first tergite.
Male variation
Males unknown.
Comments
Meteorus pyralivorus shares with Meteorus rugonasus Shaw and Jones, Meteorus oviedoi Shaw and Nishida, Meteorus restionis Shaw and Jones and Meteorus zitaniae Jones the following combination of characters: mandible twisted; carina occipital complete, notauli not distinct; tarsal claw with large lobe; ventral borders of first tergite joined completely along basal half of segment. However, M. pyralivorus can be separated from these species by it having vertex behind the lateral ocellus, temples and occiput, as well as the pronotum, black; the same areas are mostly or thoroughly pale yellow in the other three species. Additionally, M. pyralivorus lacks the coarsely rugose sculpturing present on the clypeus of M. rugonasus. Based on the mandibles being twisted, presence of a distinctive basal lobe in the tarsal claw and ventral margins of T1 completely joined along basal half of segment this species can be assigned to colon-IIA clade (Maeto Citation1990; Stigenberg and Ronquist Citation2011. See discussion below).
Holotype
Female (point mounted), ECUADOR, Napo Province, Yanayacu Biological Station, Sendero de Macuculoma, 00°35.9' S, 77°53.4' W, 2163 m, 17 November 2007, host plant: Chusquea scandens (Poaceae), host fourth instar caterpillar Pyralidae, solitary parasitoid.
Paratypes
One female, ECUADOR, Napo Province, Yanayacu Biological Station, 00°35.9' S 77°53.4' W, 2163 m, 19 September 2007, host third instar caterpillar Pyralidae, host plant: Chusquea scandens (Poaceae), solitary parasitoid.
Distribution
ECUADOR, Napo province, Yanayacu Biological Station, High Andean Cloud Forest, 2163 m.
Biology
This species is a parasitoid of third and four instar larva of Pyralidae.
Etymology
The name of this species is derived from the Greek pyrali- relating to the host family Pyralidae, and meaning fire, and the Latin -vorare meaning ‘devour’.
First record of Meteorus desmiae Zitani Citation1998 for Ecuador (Figures 5)
Male variation in respect of previous descriptions
Antennae with 32 flagellomeres.
Comments
Meteorus desmiae shares with Meteorus arizonensis, Meteorus laphygmae and Meteorus dos the following combination of characters: mandible strongly twisted, occipital carina complete, notauli not distinct, dorsopes absent, and ventral borders of first tergite joined completely along basal half of segment. Meteorus desmiae can be separated from these species by its long ovipositor, which is 2.3–2.6 × longer than first tergite and the contrasting yellow-orange-black body colour pattern. Compared with other Ecuadorian species, M. desmiae resembles Meteorus bustamanteorum Jones but can be separated from it by having 30–32 antennal flagellomeres (28–29 in M. bustamanteorum), occiput, temples, propleuron and mesonotal lobes black (yellow, honey or light brown in M. bustamanteorum), tarsal claw with large lobe, and ventral borders of first tergite joined completely along basal half of segment (ventral borders of first tergite touching for short distance in M. bustamanteorum). Based on the mandibles twisted, presence of a distinctive basal lobe in the tarsal claw and ventral margins of T1 completely joined along basal half of segment this species can be assigned to colon-IIA clade (Maeto Citation1990; Stigenberg and Ronquist Citation2011. See Discussion below).
Material examined
ECUADOR, Napo Province, 1 male, Yanayacu Biological Station, Cascadas San Rafael, 00°35.9' S, 77°53.4' W, 2163 m, host caterpillar Pyralidae, collected 3 June 2005, parasitoid pupated 28 June 2005.
Distribution
Meteorus desmiae Zitani was described from Costa Rica as a parasitoid of Desmia tages (Cram.) (Pyralidae) feeding on Hamelia patens Jacq. (Rubiaceae) (Zitani et al. Citation1998). Hamelia patens is a shrub that grows as a tree in the tropical lowlands of Costa Rica (Howard Citation1989; Paciorek et al. Citation1995). Their native range extends from southern Florida to Argentina (Little et al. Citation1974). Desmia tages is distributed from Mexico to Brazil including the Antilles, Cuba, Jamaica and Santo Domingo (Druce Citation1891–1900). It is not known if the distribution of M. desmiae coincides with that of its host because the Meteorus fauna from Central America, except Costa Rica, has been poorly studied. Southwards the distribution of M. desmiae was reported from lowland to montane forest in Colombia (Aguirre et al. Citation2011) and the new report from Ecuador could indicate that its range might extend to Brazilian wet forests and Bolivian montane forests following the southward distribution of its possible hosts.
Biology
In Costa Rica M. desmiae is known to parasitize larvae of the pyralid leaf roller Desmia tages (Cramer) (Lepidoptera: Pyraloidea: Crambidae) which feeds on Hamelia patens Jacq. (Rubiaceae) (Zitani et al. Citation1998). The specimen from Ecuador lacks a specific identification for its host caterpillar but it belongs to the superfamily Pyraloidea.
Discussion
The intensive caterpillar-rearing programmes conducted in Costa Rica by Daniel Janzen and Winnie Hallwachs (Janzen and Hallwachs Citation2012) and in Ecuador by Lee Dyer and co-workers (Dyer et al. Citation2012) have allowed us to know the biology for several species of Neotropical Meteorus. Interestingly, all of them, so far, belong to species-groups lacking dorsopes (large pits near the base of the first metasomal tergum) (Zitani et al. Citation1997, Citation1998; Shaw and Jones Citation2009; Jones and Shaw Citation2012). The presence or absence of dorsopes has proven useful in the taxonomy of the genus (Muesebeck Citation1923; Huddleston Citation1980; Zitani et al. Citation1998) and it seems to be correlated with its biology; all the Meteorus parasitoids of Coleoptera and most of the parasitoids of concealed-feeding Lepidoptera larvae are species with dorsopes (Muesebeck Citation1923; Huddleston Citation1980; Maeto Citation1990). Meteorus albisericus is the first Neotropical species with dorsopes for which the biology is known and, although the specific identity of its host is not known, the fact that it belongs to the Pyralidae is interesting because this agrees with the predicted biology for the Meteorus species displaying this character.
The phylogenetic analysis of meteorines by Maeto (Citation1990) shows that the Meteorus species having dorsopes are the stem paraphyletic group of the species lacking it, and this arrangement is related to the transition from the habit of attacking concealed-feeding larvae through the habit of attacking exposed-feeding caterpillars. However, in the phylogenetic schemes presented by Zitani and Shaw (Citation2002) and Stigenberg and Ronquist (Citation2011) the species having dorsopes appear derived. Based on the last two phylogenetic hypotheses, which include a broader set of information than that of Maeto, the use of concealed hosts, such as in the case of M. albisericus, seems to be derived with respect to the use of exposed-feeding caterpillars.
Most of the larvae of Meteorus species parasitizing exposed caterpillars spin their cocoons far out from body of the dying caterpillar employing a long suspending thread (3 m reported for M. congregatus Muesebeck, Zitani and Shaw Citation2002) attached to the substrate, which keeps the parasitoid cocoon hanging from the host plant. It is thought to be an adaptation to avoid predators crawling on the surfaces of leaves or branches, which might attack the Meteorus pupa (Shaw and Huddleston Citation1991; Shirai and Maeto Citation2009). However, a few other species display very short suspending threads or lack them. Zitani (Citation2003) explained the loss of long suspending threads with two scenarios: in some cases, parasitoids attacking exposed caterpillars might profit from the chemicals defences of their host to deter predators (Meteorus papiliovorus Zitani Citation1997), so long threads are not needed; whereas in other cases, parasitoids attacking concealed-hosts in sheltered locations may be protected by the cryptic concealment of the host; the last condition is present in of M. albisericus and others species having dorsopes.
Meteorus species, for which cocoon information is available, commonly consist of yellowish-brown silk, and a white silk is an unusual condition just found in some species attacking concealed-hosts such as in Meteorus obfuscatus (Nees 1811), Meteorus ictericus (Nees 1811), Meteorus trachynotus Viereck 1912 (Zitani Citation2003) and M. albisericus. In the case of M. albisericus, the white cocoon might be an adaptation to avoid hyperparasitoids because it would be cryptic and more difficult to see if formed on the white silk webbing of its pyralid host.
The presence of slender and twisted mandibles, ocelli ocular distance less than 2.0 times the ocellar diameter, tarsal claw with a large lobe and ventral borders of first tergite joined completely along basal half of segment in M. camilocamargoi, M. desmiae, M. pyralivorus and M. sterictae provide evidence for assigning these species into the clade IIA of Stigenberg and Ronquist’s phylogenetic analysis (Stigenberg and Ronquist Citation2011) and agree with the Maeto’s colon subgroup of the pulchricornis group (Maeto Citation1990). In both phylogenetic analyses, the colon-clade IIA subgroup is a derived clade with respect to both those having dorsopes and those sharing the following traits: lacking dorsopes, lacking large lobe on the tarsal claw, and having stout and untwisted mandibles. The fact that these species have pyraloid caterpillars as hosts demonstrates that convergent evolution toward the use of concealed or semi-concealed caterpillars has occurred at least twice among Neotropical Meteorus: once with M. albisericus (species with dorsopes) and once in the group composed by M. camilocamargoi, M. desmiae, M. pyralivorus and M. sterictae. Whether or not the last five species comprise a single lineage of pyralid parasitism, or whether they represent independent evolutions of that behaviour, is yet to be determined.
TNAH_909061_Geolocation_coordinates.xlsx
Download MS Excel (9.9 KB)Acknowledgements
Harold Greeney and Andrew Townsend are thanked for providing local arrangements and hospitality while at the Yanayacu Biological Station. Wilmer Simbaña, Luis Salagaje and volunteer students assisted with caterpillar rearing. Lee Dyer provided photographs of the Pyralidae hosts. This research was supported by National Science Foundation grants BSI-03-46729, BSI-07-17458, DEB-07-17034, DEB-10-20751 (Caterpillars and parasitoids in the Eastern Andes of Ecuador, CAPEA), and NSF Research Experience for Undergraduates (REU) supplemental grants DEB-08-23094, DEB-09-13110, and DEB-10-26103. Any opinions, findings and conclusions expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation. Travel to Ecuador was also supported, in part, by grants from U.W. International Programs, U.W. Environment and Natural Resources Program, Wyoming NASA Space Consortium, and U.W. Global Perspectives Program.
References
- Aguirre H, Sarmiento CE, Shaw SR. 2011. Taxonomic revision and morphometric analyses of Meteorus Haliday, 1835 (Hymenoptera: Braconidae: Meteorinae) from Colombia. Zootaxa. 2938:1–68.
- Druce H. 1891–1900. Biologia Centrali-Americana. Insecta. Lepidoptera-Heterocera. Vol. II. [Internet]. London: published for the editors by R.H. Porter; [cited 2012 Dec 30]. Available from: http://www.sil.si.edu/DigitalCollections/bca/navigation/bca_15_02_00/bca_15_02_00select.cfm
- Dyer LA, Miller JS, Rab Green SB, Gentry GL, Greeney HF, Walla TW. 2012. Caterpillars and parasitoids of the Eastern Andes in Ecuador. [Internet]. [cited 2012 Dec 10]. Available from: http://www.caterpillars.org
- Greeney HF. 2012. Yanayacu biological station and center for creative studies. [Internet]. [cited 2012 Nov 9]. Available from: www.yanyacu.org
- Harris AH. 1979. A glossary of surface sculpturing. Occas Pap Entomol. 28:1–31.
- Holdridge LR. 1967. Life zone ecology. San Jose, Costa Rica: Tropical Science Center.
- Howard RA. 1989. Flora of the Lesser Antilles, Leeward and Windward Islands. Vol. 6. Jamaica Plain, MA: Arnold Arboretum, Harvard University.
- Huddleston T. 1980. A revision of the western Palaeartic species of the genus Meteorus (Hymenoptera: Braconidae). Bull Br Museum (Nat Hist); Entomol. 41:1–58.
- Janzen D, Hallwachs W. 2012. Area de Conservación Guanacaste (ACG), northwestern Costa Rica. Caterpillars, pupae, butterflies and moths. [Internet]. [cited 2012 Dec 10]. Available from: http://janzen.sas.upenn.edu/caterpillars/database.lasso
- Jones GZ, Shaw SR. 2012. Ten new species of Meteorus (Braconidae: Hymenoptera) from Ecuador reared at the Yanayacu Biological Center for Creative Studies. Zootaxa. 3547:1–23.
- Little Jr EL, Woodbury RO, Wadsworth FH. 1974. Trees of puerto rico and the virgin islands. Vol. 2. Agriculture Handbook 449. Washington, DC: U.S. Department of Agriculture.
- Maeto K. 1990. Phylogenetic relationships and host associations of the subfamily Meteorinae Cresson (Hymenoptera: Braconidae). Jpn J Entomol. 58:383–396.
- Muesebeck CFW. 1923. A revision of the North American species of ichneumon-flies belonging to the genus Meteorus Haliday. Proc U S Natl Mus. 63:1–44.
- Paciorek CJ, Moyer BR, Levin RA, Halpern SL. 1995. Pollen consumption by the hummingbird flower mite, Proctolaelaps kirmsei, and possible fitness effects on Hamelia patens. Biotropica. 27:258–262.
- Powell JA, Opler PA. 2009. Moths of western North America. Los Angeles, CA: University of California Press.
- Sharkey MJ, Wharton RA. 1997. Morphology and terminology. In: Wharton RA, Marsh PM, Sharkey MJ, editor. Manual of the New World genera of the family Braconidae (Hymenoptera). Washington, DC: International Society of Hymenopterists; p. 19–37.
- Shaw MR, Huddleston T. 1991. Classification and biology of braconid wasps (Hymenoptera: Braconidae). In: Dolling WR, Askew RR, editors. Handbooks for the identification of British insects. Vol. 7, part 11. Cromwell Road, London: Royal Entomological Society of London.
- Shaw SR, Jones GZ. 2009. A new species of solitary Meteorus (Hymenoptera: Braconidae) reared from caterpillars of toxic butterflies (Lepidoptera: Nymphalidae) in Ecuador. J Insect Sci. 9:1–8. Available from: insectscience.org/9.34.
- Shirai S, Maeto K. 2009. Suspending cocoons to evade ant predation in Meteorus pulchricornis, a braconid parasitoid of exposed-living lepidopteran larvae. Entomol Sci. 12:107–109.
- Stigenberg J, Ronquist F. 2011. Revision of the Western Palaeartic Meteorini (Hymenoptera: Braconidae), with a molecular characterization of hidden fennoscandian species diversity. Zootaxa. 3084:1–95.
- Yu DS. 2012. Home of Ichneumonoidea. [Internet]. [cited 2012 Dec 10]. Available from: www.taxapad.com.
- Zitani NM. 2003. The evolution and adaptive significance of silk in the Meteorinae [dissertation]. Laramie (WY): University of Wyoming.
- Zitani NM, Shaw SR. 2002. From meteors to death stars: variations on a silk thread (Hymenoptera: Braconidae: Meteorinae). Amer Entomol. 48:228–235.
- Zitani NM, Shaw SR, Janzen DH. 1997. Description and biology of new species of Meteorus Haliday (Hymenoptera: Braconidae, Meteorinae) from Costa Rica, parasitizing larvae of Papilio and Parides (Lepidoptera: Papilionidae). J Hymenopt Res. 6:178–185.
- Zitani NM, Shaw SR, Janzen DH. 1998. Systematics of Costa Rican Meteorus (Hymenoptera: Braconidae: Meteorinae) species lacking a dorsope. J Hymenopt Res. 7:182–208.