ABSTRACT
The genus Papawera includes two species of haminoeid snails found only in temperate waters of New Zealand and southeastern Australia. In this work, we redescribe the Papawera species based on characters of their external morphology, shells, and anatomical features such as radulae, jaws, gizzard plates, and male reproductive systems, using for the first time, scanning electron microscopy. A multi-locus phylogenetic hypothesis and the species delimitation method Automatic Barcode Gap Discovery based on DNA sequences of the cytochrome c oxidase subunit I gene were used to corroborate species status. The type species of the genus, P. zelandiae, is restricted to New Zealand and P. maugeansis is only known from South Australia, Victoria, and Tasmania. These species are easily distinguished externally by the shape of the shell, colouration of the living animal, and morphology of the cephalic shield. Anatomically they have differences in the number of marginal teeth, distribution of rods in the gizzard plates, and anatomy of the fundus in the male reproductive system.
Introduction
The genus Papawera Oskars and Malaquias, Citation2019 was erected to accommodate two haminoid species of temperate affinity distributed across southern Australia and northern New Zealand (Oskars and Malaquias Citation2019), namely Papawera maugeansis (Burn, Citation1966a; originally Haminoea maugeansis) and P. zelandiae (Gray Citation1843; originally Bulla zelandiae). The species P. maugeansis is restricted to southeastern Australia, where it occurs intertidally in tide pools on rocky platforms and subtidally on muddy seagrass beds (Burn Citation1966a, Citation1969; Hales Citation2010; Burn and Wilson Citation2011). Papawera zelandiae is endemic to New Zealand, where it is relatively common on intertidal and subtidal sheltered sandy-mud flats and on rocky shores with algae turfs (Rudman Citation1971a, Citation1971b; Powell Citation1979; Morley and Hayward Citation2015).
Despite the early description of P. zelandiae by Gray (Citation1843) and its junior synonym Haminea obesa (Sowerby, Citation1868), the anatomy and ecology of this species was only studied much later. Rudman (Citation1971a, Citation1971b) first described several anatomical features and its diet. Willan (Citation1979), Hayward (Citation1979) and Morley and Hayward (Citation2015) also provided data on diet as well as ecological aspects such as habitat and distribution.
Burn (Citation1966a) described the species Haminoea maugeansis from Victoria, South Australia, and Tasmania, based on the shell, external morphology, and several anatomical features of the digestive system (jaws, radula and gizzard plates) but only included a drawing of the shell and one row of the radula (Burn Citation1966a, p. 330, , ). Before the description of H. maugeansis, the name Haminoea tenera (Adams, Citation1850) was often employed to refer to haminoeid snails from the southern states of Australia, mostly because the influential Australian naturalist George French Angas (Citation1871) used this name for shells from New South Wales. Consequently, the binomen was largely adopted by many authors to refer to Haminoea-like shells in these Australian regions (e.g. Pritchard and Gatliff Citation1903; MacPherson and Gabriel Citation1962; Burn Citation1966a, Citation1966b; Jansen Citation1995).
Figure 1. Bayesian phylogeny depicting relationships and species diversity of the genus Papawera. Hypothesis based on the combined analysis of the mitochondrial COI, 16S rRNA and 12S rRNA and nuclear 28S rRNA and Histone H3 genes. The tree is a composite based on Figures 1 and from Oskars & Malaquias (Citation2019). Values above branches are BI posterior probabilities and below branches are bootstrap values derived by ML (Oskars & Malaquias, Citation2019). Images of Lamprohaminoea cymbalum, Mozambique (TH64), courtesy of Y. Tibiriçá; Bakawan rotundata, Singapore (496), courtesy of K. Jensen; Smaragdinella cf. sieboldi, Mozambique (TH55), courtesy of Y. Tibiriçá; Haminoea alfredensis, South Africa, NHMUK 20070315 (174), courtesy of G. Branch and C. Griffiths
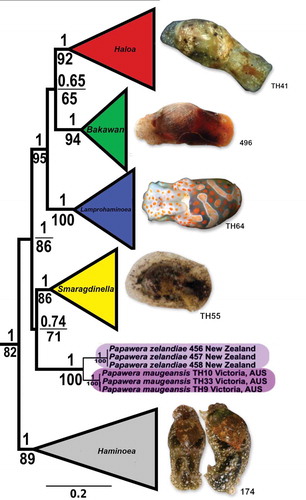
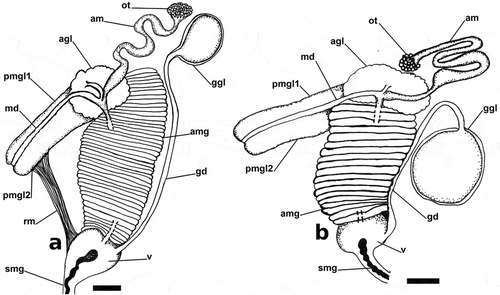
Figure 2. Female reproductive system. (a) Papawera zelandiae, Hokianga, Northland, New Zealand, (AM 79176, H = 11 mm). (b) Papawera maugeansis, Curlewis Reef, Clifton Springs, Victoria, Australia (ZMBN 125459 ex NVM F194630, H = 5.7 mm). Abbreviations: agl, albumen gland. am, ampulla. amg, anterior mucous gland. gd, gameloytic duct. ggl, gametolytic gland. md, medial duct. ot, ovotestis. pmgl1, posterior mucous gland lobe 1, pmgl2, posterior mucous gland lobe 2. rm, retractor muscle. smg, external seminal groove. v, vestibule. Scale bars: a, b = 0.5 mm
Burn (Citation1966a) maintained the species in the genus Haminoea Turton and Kingston Citation1830 but discussed possible different generic assignments. For example, he considered Haloa Pilsbry, Citation1921 because features of the shell matched Pilsbry’s (Citation1921) original description. These included the thick, reflected columellar lip separated by a furrow. Burn (Citation1966a) also considered Lamprohaminoea Habe, Citation1952 (as described by Habe Citation1952; Kuroda and Habe Citation1952) because of the colouration of the animal – orange blotches combined with a smooth and polished shell.
Later Burn (Citation1969, p. 68, fig. 1) illustrated a complete animal and the Hancock’s organ and stated that the ‘ribbed’ Hancock’s organ with its dorsal branches prevented the species from being included in Lamprohaminoea, which Er. Marcus and Burch (Citation1965) showed to have a ridge-like Hancock’s organ. Interestingly, Oskars and Malaquias (Citation2019) found this organ to have relevant differences at the genus level. Notably, the presence of a Hancock’s organ with a central ridge and prominent dorsal and ventral branches was considered by the authors an apomorphy of the genus Papawera (Oskars and Malaquias, Citation2019).
The anatomy of P. zelandiae and P. maugeansis is relatively well known thanks to the works by Rudman (Citation1971a, Citation1971b) and Burn (Citation1966a, Citation1969, Citation1974). However, modern techniques of electron microscopy were never employed to look at certain details of the radula and gizzard plates and, the male reproductive system of P. maugeansis has never been studied. In this work, we use the molecular phylogenetic framework by Oskars and Malaquias (Citation2019) to discuss the systematics of the genus and we redescribe its two species based on fine anatomical work, scanning electron microscopy, and DNA barcoding.
Materials and methods
Taxa sampling and morpho-anatomical work
Specimens were obtained by loan from the collections of The Australian Museum, Sydney, Australia (AMS), Museum Victoria, Melbourne, Victoria, Australia (NMV), The Auckland War Memorial Museum Tāmaki Paenga Hira, Aukland, New Zealand (AM) and The Natural History Museum, London, United Kingdom (NHMUK).
The body of the animal was gently separated from the shell with the aid of forceps. The female reproductive systems, male reproductive systems, gizzards, and buccal bulbs were dissected out of the animals under a stereo-microscope. Shells were measured (total height, H) and imaged with a DSLR camera equipped with macrolens. The reproductive systems were drawn using a stereo-microscope fitted with a drawing tube. The gizzard and buccal bulb were dissolved in a solution containing 180 µl buffer ATL and 20 µl of proteinase K, incubated at 56°C for 4–6 hours to separate and dissolve tissue surrounding the gizzard plates, jaws, and radulae (protocol modified from Holznagel (Citation1998) and Vogler (Citation2013)) [buffer and enzymes were obtained from the Qiagen DNeasy® Blood and Tissue Kit, catalogue no. 69504]. Gizzard plates and jaws were critical-point dried to maintain natural shape and, together with radulae, were mounted on metallic stubs using carbon sticky tabs and coated with gold-palladium for scanning electron microscopy. All samples were scanned and imaged with a Zeiss Supra 55VP scanning electron microscope.
Phylogenetic analyses and molecular species delimitation
In this work, the phylogenetic hypothesis generated by Oskars and Malaquias (Citation2019) for the genus Papawera based on the combination of three mitochondrial genes (cytochrome c oxidase subunit I [COI] + 16S rRNA + 12S rRNA) and two nuclear genes (28S rRNA + Histone H3) was used as our framework for species recognition and relationships (; see Oskars and Malaquias Citation2019 for methodological details). The DNA sequences of the COI gene used to estimate genetic distances and test species delimitation hypotheses were those included in Oskars and Malaquias (Citation2019) (see ).
Table 1. List of specimens used in the ABGD analyses including voucher and GenBank accession numbers
COI uncorrected p-distances were calculated in MEGA 7 (Kumar et al. Citation2016) (). The species delimitation method Automatic Barcode Gap Discovery (ABGD; Puillandre et al. Citation2012) was employed to test species hypotheses by using default settings and three different models (Jukes-Cantor [JC69], Kimura TS/TV = 2.0 [K80], Simple Distance).
Table 2. COI inter- and intra-specific uncorrected p-distances (%) calculated in MEGA 7
All figures were made in Inkscape 0.92 (Inkscape Team Citation2015) and Gimp 2.10 (Mattis et al. Citation1995; Natterer Citation2018).
Results
The phylogeny of Papawera (Oskars and Malaquias, Citation2019) suggests the presence of two species in this genus, which is corroborated by ABDG analysis using all three-distance methods (Figure S1). The minimum COI uncorrected p-distance between both species was 13.5% ().
Taxonomy
Class Gastropoda Cuvier Citation1795
Sub-class Heterobranchia Burmeister Citation1837
Order Cephalaspidea P. Fischer Citation1883
Family Haminoeidae Pilsbry Citation1895
Genus Papawera Oskars and Malaquias Citation2019
Bulla (in part) – Gray Citation1843, p. 243.
Haminea (in part) – Pilsbry Citation1895, p. 365. Kobelt Citation1896, p. 118. E. A. Smith Citation1873, p. 5. Pritchard and Gatliff Citation1903: 217. Thompson Citation1976, p. 34, 45.
Haminoea (in part) – MacPherson and Gabriel Citation1962, p. 243. Burn Citation1966a, p. 330, Citation1966b, p. 266, Citation1969, p. 68. Rudman Citation1971a, p. 545, Citation1971b, p. 649. Burn Citation1974, p. 48. Powell Citation1979, p. 275. Willan Citation1979, p. 269. Jansen Citation1995, p. 86. Rudman Citation1999. Furneaux Citation2003. Rudman Citation2003. Burn Citation2006, p. 8. Rudman Citation2006. Eichler Citation2007. Rudman Citation2007. Hales Citation2010, p. 249. Burn and Wilson Citation2011. Burn Citation2015, p. 65. Morley and Hayward Citation2015, p. 57. Grove Citation2018.
Type species
Bulla zelandiae Gray, Citation1843. Holotype untraceable; iconotype illustrated in Smith (Citation1873, p. 5, Table 1, fig. 10).
Type locality
New Zealand (Gray Citation1843).
Diagnosis
Animal light grey, yellowish-grey or brown to nearly black; orange blotches and opaque white dots may be present. Cephalic shield, broad, shallowly or deeply bilobed; periocular area pigmented or unpigmented; always minute. Hancock organ ridge-like, with prominent dorsal and ventral branches. Shell oval to rounded; smooth with faint growth lines; whitish in colour; periostracum transparent to yellowish; aperture wide, tapering posteriorly, columella thick, separated from last whorl by short furrow; furrow covered by callus posteriorly. Radula formula 27–5.1.1.1.5–27; rachidian tricuspid, elongate, central cusp longer, lateral cusps reduced; outer lateral teeth hook shaped, smooth, decreasing in size outwardly; cusp of inner lateral slightly bulbous, tip pointed. Jaws semi-circular, rods slightly serrated on outer edge. Gizzard plates with 8–20 transverse ridges, top of ridges covered in minute rods, smooth in between ridges. Three gizzard bristled spines, resembling feather-like dusters inserted in raised triangular fleshy base. Reproductive system with oblong annulated prostate; short seminal duct, with semi-enclosed hollow duct; fundus semi-enclosed with glandular epithelium; thick, non-muscular atrium. Female reproductive system with elongate, lamellate anterior mucous gland; thick, blunted and indented bilobed posterior mucous gland, lobes separated by median duct; albumen gland globular with irregular surface, partially surrounding posterior mucous gland and ampulla; gametolytic gland, rounded (Gray Citation1843; Sowerby Citation1868; Pilsbry Citation1895; Burn Citation1966a, Citation1969, Citation1974. Rudman Citation1971a; Rudman Citation1971b, Citation1999, Citation2003, Citation2006, Citation2007; Furneaux Citation2003; Eichler Citation2007; Burn and Wilson Citation2011; Grove Citation2018).
Remarks
The female reproductive system of P. zelandiae was found to be consistent with the description by Rudman (Citation1971a, p. 552, fig. 7a). The system differs from that of taxa within the genera Bakawan, Haloa, and Lamprohaminoea by having a straight and shorter posterior mucous gland and a rounded albumen gland with an irregular surface. The female reproductive system of P. maugeansis was also consistent with P. zelandiae, only differing in the shape of the gametolytic gland, which is nearly twice the size in P. maugeansis (,b). A retractor muscle connecting the posterior mucus gland with the vagina was not observed in the studied specimens of P. maugeansis.
Papawera zelandiae (Gray Citation1843)
(a), , ,
Figure 3. Live animals of Papawera zelandiae (Gray, Citation1843) (a–c), Papawera maugeansis (Burn, Citation1966a) (d–h). (a) Blockhouse Bay, Auckland, New Zealand (D. Crisp, CC BY-NC, iNaturalist.org). (b) Ambury Farm, Mangere Bridge, Auckland, New Zealand, courtesy of J. Geux. (c) Mahurangi West, North Island, New Zealand (D. Wilson, CC BY-NC, iNaturalist.org). (d) Hamers Haven, courtesy of J. Hales. (e) Inverloch, Victoria, Australia, courtesy of J. Hales. (f) Australia, Victoria, Westernport, Shoreham, (AMS c.105854, H = 11 mm), courtesy of G. Millen. (g) Victoria, Australia, courtesy of L. Altoff. (h) Cape Paterson, Inverloch, Victoria, Australia, courtesy of L. Altoff
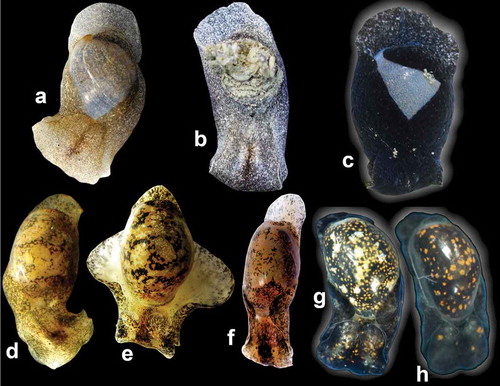
Figure 4. Papawera zelandiae (Gray, Citation1843). (a) apertural view of shell (AM 79,176, H = 11 mm; New Zealand). (b) apertural view of shell (NHMUK 20170322, Haminea obesa, holotype; H = 17 mm; New Zealand). (c) apertural view of shell (AMS C.159944, H = 22 mm; New Zealand). (d) apertural view of shell, (AMS c.457260, H = 27 mm; New Zealand). (e) SEM, whole radula (AM 119920, H = 11 mm; New Zealand). (f) SEM, detail of rachidian and first laterals in central part of radula (AM 119920, H = 11 mm; New Zealand). (g) Right lateral view of whole gizzard plate (AM 119920, H = 11 mm; New Zealand). (h) SEM, dorsal surface of whole gizzard plate (AM 119920, H = 11 mm; New Zealand). Scale bars: e, g, h = 100 µm. f = 0.15 µm
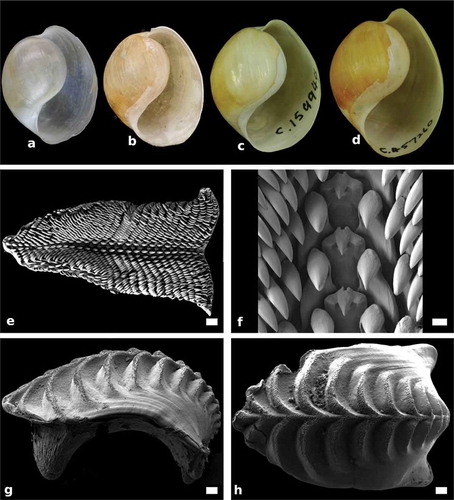
Figure 5. Papawera zelandiae (Gray, Citation1843). (a) SEM, detail of ridges of gizzard plate (AM 119920, H = 11 mm; New Zealand). (b) SEM, Detail of jaws (AM 119920, H = 11 mm; New Zealand). (c) detail of external male reproductive system (AM 119920, H = 11 mm; New Zealand). (d) detail of interior of ventrally opened atrium and fundus (AM 119920, H = 11 mm; New Zealand). Abbreviations: as, atrium sheet. at, atrium. bc, body cavity. fu, fundus. pr, prostate. sd, seminal duct. smg, seminal groove. rm, retractor muscles. Scale bars: a = 20 µm. F = 4 µm. c, d = 0.25 mm
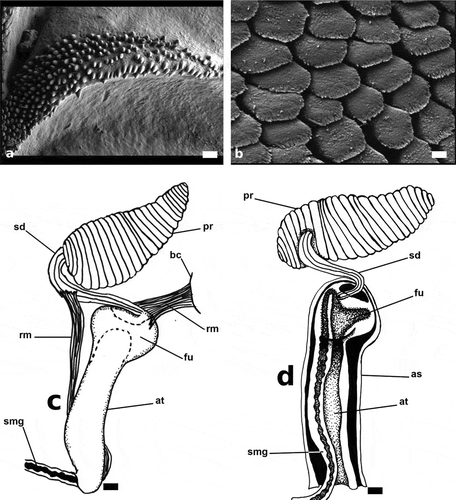
Bulla zelandiae Gray, Citation1843, p. 243.
Haminea zelandiae – E. A. Smith Citation1873, p. 5. Kobelt Citation1896, p. 118. Suter Citation1913, p. 538.
Haminea zelandica (misspelled) – Pilsbry Citation1895, p. 373 (as synonym of Haminea pemphix).
Haminea pemphix (misspelled) – Pilsbry Citation1895, p. 373.
Haminoea zelandiae – Rudman Citation1971a, p. 545–559., Citation1971b, p. 647–675. Hayward Citation1979, p. 175. Powell Citation1979, p. 275. Willan Citation1979, p. 269. Rudman Citation1999. Furneaux Citation2003. Rudman Citation2003. Morley and Hayward Citation2015, p. 57.
Haminea obesa Sowerby II in Reeve Citation1868: no page numbers, pl. 2, fig.13.
Papawera zelandiae – Oskars and Malaquias Citation2019, p. 6.
Diagnosis
Animal light grey or brown to completely black. Shell rounded, bulbous; smooth with faint growth lines; whitish-translucent in colour; periostracum transparent to yellowish; aperture wide, tapering posteriorly, columella thick, separated from last whorl by short furrow, furrow covered by callus posteriorly. Cephalic shield, broad, shallowly bilobed; large parapodial and pallial lobes covering nearly whole shell; periocular area pigmented or small, unpigmented. Hancock’s organ thick ridge, with prominent dorsal and ventral branches. Radula formula 16–25 x 27–22.1.1.1.22–27; rachidian tricuspid, central cusp triangular, narrow, pointed; lateral cusps, reduced, barely raised form base. Gizzard plates with 8–16 transverse ridges; minute rods present along upper inner part of posterior side of ridges and rachis only; rachis present. Male reproductive system with oblong, tapering, annulated prostate, fundus semi-enclosed with glandular epithelium. Distribution restricted to New Zealand (Gray Citation1843; Sowerby Citation1868; Pilsbry Citation1895b; Rudman Citation1971a; Citation1971b, Citation1999, Citation2003; Rudman Citation2006; Furneaux Citation2003).
Type locality
New Zealand (Gray Citation1843). Holotype untraceable; iconotype illustrated in Smith (Citation1873, p. 5, Table 1, fig. 10).
Material examined
New Zealand, 1 shell, Haminea obesa Sowerby Citation1868, holotype, NHMUK 20170322; H = 17 mm. New Zealand, Auckland, Waitemata Harbour, 3 specimens (1 dissected, 3 sequenced), AM 119920, H = 11 mm. New Zealand, Waitemata Harbour, Herne Bay, AMS c.159944, H = 22 mm. New Zealand, Auckland, Manu Kau Harbour, 7 shells, NHMUK unnumbered, Col. Cornwallis in 1946, Stratton Collection Acc. No. 1894, H = 19 mm. New Zealand, Northland, Hokianga, 3 specimens (2 dissected), AM 79176, H = 11–14 mm. New Zealand, South Island, Awarua, 1 shell, AMS c.457260, H = 27 mm.
External morphology (–c))
Animal light grey or brown to nearly black. Cephalic shield, squarish, broad; shallowly bilobed, posterior part extending over shell. Eyes visible, periocular areas small, may be pigmented or unpigmented. Hancock’s organ elongate, horizontal ridge with prominent dorsal and ventral branches. Parapodial lobes large, covering shell anteriorly and laterally. Rounded pallial lobe extends beyond apex.
Shell (–d))
Shell rounded, bulbous; smooth with faint growth lines. Whitish in colour; periostracum transparent to yellowish. Aperture wide, tapering posteriorly, columella thick, separated from last whorl by short furrow, furrow covered by callus posteriorly. Outer lip rounded; shoulder rounded.
Jaws ())
Semi-circular; shape of rods rounded with serrated edge.
Radula (, f))
Radular formula 28 × 25.1.1.1.25 (AM112090, H = 6 mm), 16 x 27–22.1.1.1.22–27 (Rudman Citation1971a, Citation1971b). Rachidian tricuspid; central cusp triangular, narrow, pointed; lateral cusps reduced, barely raised form base.
Gizzard plates (, h) and )
Rachis present, 8–16 transverse ridges; minute rods present along upper inner part of posterior side of ridges and rachis only.
Male reproductive system (, d))
Male reproductive system with oblong, tapering, annulated prostate, fundus semi-enclosed with glandular epithelium. Two retractor muscles; one connecting upper part of fundus to body cavity, the other attached to seminal duct and lower part of atrium.
Distribution
The species is most frequently recorded around New Zealand’s North Island and the northern parts of the South Island (Rudman Citation1971a; Rudman Citation1971b, Citation1999, Citation2003, Citation2006; Powell Citation1979; Furneaux Citation2003; Morley and Hayward Citation2015). However, the species also can be found on the southern coast of the South Island (based on one shell housed at the AMS c.457260), but to our knowledge, it has not been observed alive so far south.
Remarks
This species occurs intertidally in sheltered areas in sandy-mud bottoms, seagrass and coralline algae turfs on rocky platforms, and has been reported to feed on green algae such as Ulva, Enteromorpha (= Ulva), Cladophora and Rhizoclonium and to scrape diatoms from seagrass leaves and sediment (Rudman Citation1971b, Citation2003; Hayward Citation1979; Willan Citation1979). During this study, we found the gut of one specimen filled with filamentous algae.
Papawera maugeansis (Burn, 1966)
(b), , , ,
Figure 6. Papawera maugeansis (Burn, Citation1966a). Detail of left lateral Hancock’s organ, dorsal branches partially covered by cephalic shield (ZMBN 125458, H = 8 mm). Abbreviations: cs, cephalic shield. db, dorsal branch. ho, Hancock’s organ. lo, labial organ. pl, parapodial lobe. vb, ventral branch. vm, visceral mass. Scale bar = 0.3 mm
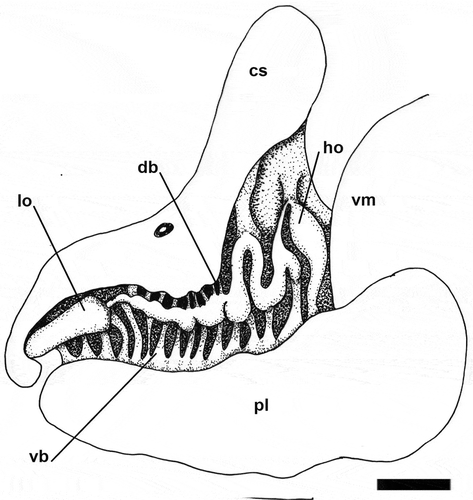
Figure 7. Papawera maugeansis (Burn, Citation1966a). (a) adpertural view of holotype (NMV F26134, H = 7 mm; Australia). (b) apertural view of shell (ZMBN 125458, H = 8 mm; Australia). (c) apertural view of shell (AMS c.105854, H = 5 mm; Australia). (d) apertural view of shell, (NHMUK MacAndrew col. 1563, H = 7.5 mm; Australia). (e) SEM, whole radula (AMS c.105854, H = 5 mm; Australia). (f) SEM, detail of rachidian and inner laterals in central part of radula (ZMBN 125458, H = 8 mm; Australia). (g) Right lateral view of whole gizzard plate (ZMBN 125458, H = 8 mm; Australia). (h) SEM, dorsal surface of whole gizzard plate (ZMBN 125458, H = 8 mm; Australia). Scale bars: e = 100 µm. f = 50 µm. g, h = 30 µm
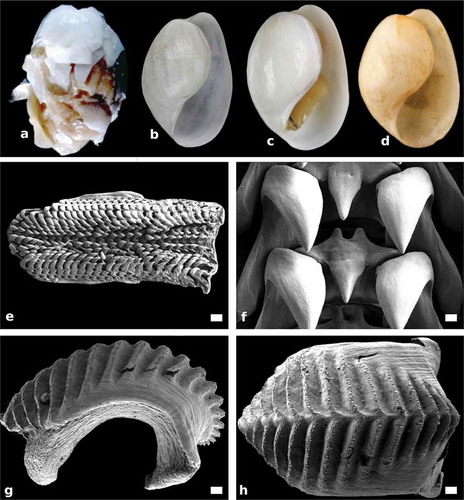
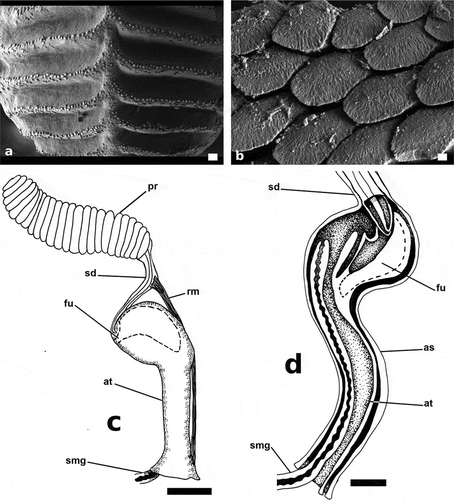
Figure 8. Papawera maugeansis (Burn, Citation1966a). (a) SEM, detail of ridges of gizzard plate (ZMBN 125458, H = 8 mm). (b) SEM, Detail of jaws (AMS c.105854, H = 5 mm). (c) detail of external male reproductive system (AMS c.105854, H = 5 mm). (d) detail of interior of ventrally opened atrium and fundus (ZMBN 125458, H = 8 mm). Abbreviations: as, atrium sheet. at, atrium. fu, fundus. pr, prostate. sd, seminal duct. smg, seminal groove. rm, retractor muscles. Scale bars: a = 20 µm. b = 2 µm. c = 0.5 mm, d = 0.25 mm
Haminoea maugeansis Burn Citation1966a, p. 330, Citation1969, p. 68, Citation1974, p. 8, Citation2006, p. 8 Eichler Citation2007. Rudman Citation2007. Hales Citation2010. p/ 249. Burn and Wilson Citation2011. Burn Citation2015, p. 65. Grove Citation2018.
Papawera maugeansis – Oskars and Malaquias Citation2019, p. 6.
Haminea tenera (Adams Citation1850) – Pritchard and Gatliff Citation1903, p. 217.
Haminoea tenera (Adams Citation1850) (in part) – Jansen Citation1995, p. 86.
Haminoea tenera (Adams Citation1850) – MacPherson and Gabriel Citation1962, p. 243. Burn Citation1966b, p. 266.
Diagnosis
Animal light yellowish-grey or light brown to densely black; orange blotches of variable size present on mantle; opaque white dots may be present on cephalic shield and parapodial lobes. Cephalic shield, broad, deeply bilobed; periocular area unpigmented. Hancock organ ridge-like, with prominent dorsal and ventral branches. Shell oval; smooth with faint growth lines; whitish-translucent in colour; periostracum transparent; aperture wide, tapering posteriorly, columella narrow, separated from last whorl by short furrow; furrow covered by broad, flat, callus posteriorly. Radula formula 24–30 x 7–5.1.1.1.5–7; rachidian tricuspid; central cusp triangular, narrow, pointed; lateral cusps reduced, barely visible. Gizzard plates with 14–20 transverse ridges; single row of rods present along top of ridges; pseudo-rachis present. Male reproductive system with oblong, oval, annulated prostate; fundus open, pocket-like. Distribution restricted to southeastern Australia.
Type locality
Port MacDonnell, South Australia, Australia (Burn Citation1966a).
Material examined
Australia, South Australia, Port MacDonnell, Holotype, NMV F26134, H = 7 mm (image seen, https://ozcam.ala.org.au/occurrences/e789bb90-d64b-4e07-ad0b-89aed83c5a5b). Australia, Victoria, Shoreham, Westernport, 10 specimens (2 dissected), AMS c.105854, H = 3–8 mm. Australia, Victoria, Shoreham, Shoreham Beach, 1 specimen (sequenced), NMV F209129, H = 5 mm. Australia, Victoria, Inverloch, Town Area, 1 specimen (sequenced, dissected), ZMBN 125459 ex NVM F194630, H = 5.7 mm. Australia, Victoria, Clifton Springs, Curlewis Reef, 1 specimen (sequenced, dissected), (ZMBN125458 ex NVM F112423, H = 8 mm). Australia, Victoria, 1 shell, Haminea tenera, NHMUK unnumbered, MacAndrew Collection Acc. No. 1563, H = 7.5 mm.
External morphology (–h), )
Animal light grey or brown to completely black; orange blotches of variable size present on mantle; opaque white dots may be present on cephalic shield and parapodial lobes. Cephalic shield squarish, broad; deeply bilobed, posterior part extending over shell. Periocular area unpigmented. Hancock’s organ elongate, horizontal ridge with long dorsal and ventral branches, dorsal branches may be partially hidden by cephalic shield. Parapodial lobes small, partially covering anterior part of shell, do not meet dorsally. Rounded pallial lobe extends beyond apex.
Shell (–d))
Shell oval; smooth with faint growth lines. Whitish-transparent in colour; periostracum transparent. Aperture wide, tapering posteriorly, columella narrow, separated from last whorl by short furrow; furrow covered by broad, flat, callus posteriorly. Outer lip gently curved; shoulder rounded.
Jaws ())
Semi-circular; shape of rods rounded with serrated edge, serration seems slightly worn.
Radula (, f))
Radular formula 25 × 6.1.1.1.6 (AMS c.105854, H = 8 mm), 30 × 7.1.1.1.7 (ZMBN 125458, H = 8 mm), 24 × 5.1.1.1.5 (as 6.1.6, NMV F26134, H = 7 mm; Burn Citation1966a). Rachidian tricuspid; central cusp triangular, narrow, pointed; lateral cusps reduced, barely visible.
Gizzard plates (, h) and )
Fourteen to 20 transverse ridges. Pseudo-rachis present. Ridges with a single row of minute rods along top edge.
Male reproductive system (, d))
Male reproductive system with oblong, oval, annulated prostate, fundus open, pocket-like. Retractor muscle connects seminal duct to lower part of atrium.
Distribution
This species is endemic to the Maugean cool-temperate province (Burn Citation1966a) and has been recorded from Victoria, South Australia and Tasmania (Burn Citation1966a, Citation2006).
Remarks
The species P. maugeansis has been historically misidentified as several other haminoid species. Pritchard and Gatliff (Citation1903) identified specimens from around Victoria as Haminea tenera, and Jansen (Citation1995) reported the occurrence of Haminoea tenera from mid-New South Wales to South Australia and Tasmania. However, as previously mentioned herein, H. tenera is a distinct species although its taxonomic status remains elusive, but is under investigation by Oskars and Malaquias (work in progress). Janssen (Citation1995) gave only a general description of the shells and noted that they were ‘translucent white brown’, but did not mention any feature of the sculpture. Thus, her records from New South Wales could be any tropical species of Haloa (see Nimbs and Smith Citation2016). On the other hand, records from South Australia and Tasmania are likely of P. maugeansis, which is the only species known to occur in Victoria, South Australia and Tasmania (Burn Citation1966a, Citation1966b, Citation2006; Burn and Wilson Citation2011; Grove Citation2018).
Papawera maugeansis occurs in intertidal and shallow subtidal seagrass beds in sheltered areas and in rocky platforms (Rudman Citation2007). The species feeds on algae and diatom films that it scrapes from the substrate (Burn Citation1966a, Citation1966b, Citation1974; Rudman Citation2007). Burn (Citation1974) reported that P. maugeansis is preyed on by the headshield sea slug, Philinopsis taronga (Allan, Citation1933).
Discussion
Papawera is a genus recently introduced by Oskars and Malaquias (Citation2019) for two species with a temperate Australasian distribution, P. maugeansis and P. zelandiae. Both inhabit intertidal and shallow sheltered waters where they can be locally common (Powell Citation1979; Burn Citation2006; Morley and Hayward Citation2015; Oskars and Malaquias Citation2019). The sister relationship of the genus is still not resolved, but the phylogenetic hypothesis by Oskars and Malaquias (Citation2019; ) suggested a possible sister relationship with the tropical IWP genus Smaragdinella. Yet, this was only marginally supported by maximum likelihood analysis (bootstrap support = 71), and did not receive support in Bayesian analysis (posterior probability = 0.74).
Several previous works have included details about the morphology and ecology of P. maugeansis and P. zelandiae (Burn, Citation1966a, Citation1969, Citation1974; Rudman, Citation1971a, Citation1971b, Citation2003; Burn and Wilson, Citation2011), but the current contribution is the first to study these snails using SEM methods in order to provide ultrastructural data on the radula, jaws and gizzard plates. It is also the first to describe the male reproductive system of P. maugeansis and the female reproductive system of both species. The two species are externally quite distinct, with P. zelandiae bearing a large rounded shell, a cephalic shield with a minute posterior indentation, and large parapodial lobes, whereas, P. maugeansis is small with an elongate shell and a deeply bilobed cephalic shield with short parapodial lobes. Chromatically, P. maugeansis has orange blotches on the mantle, which are absent in P. zelandiae (; see for a synopsis).
Table 3. Synopsis of the most useful morphological characters for diagnosis of species in the genus Papawera.
Despite the sharp external differences, these species share several unique morpho-anatomical traits supporting their close relationship. These include the morphology of the Hancock’s organ with its prominent upper and lower vertical branches, the rachidian tooth with an elongated and narrow central cusp combined with inconspicuous lateral cusps, the male reproductive system with an annulated prostate, and the female part of the reproductive system with a blunt posterior mucous gland and a globular albumen gland with an irregular surface (see Taxonomy for details).
The shells of these snails are smooth with few growth lines, although some authors have reported the presence of faint spiral striae in P. zelandiae (Suter, Citation1913) and P. maugeansis (as H. tenera; MacPherson and Gabriel Citation1962), but we could not observe this feature, which was also not mentioned by Burn (Citation1966a, Citation1966b) or Rudman (Citation1971b).
According to Clemens-Seely and Phillips (Citation2011), P. zelandiae is poecilogonous laying egg masses from wherein both free swimming lecithotrophic veligers and fully developed juveniles hatch, a developmental mode among haminoeids only known in Haloa japonica (Gibson and Chia, Citation1991).
The shallow water molluscan fauna of Australasia is well known and the fact that only two species were detected to be part of the genus Papawera is most likely not a matter of undersampling but reflects, instead, a low diversity genus. Understanding the evolutionary history of the genus is hampered by ambiguity about its sister relationship, yet, the temperate distribution of these two species points to adaptation to colder waters, whereas all closer relatives (e.g., Bakawan, Haloa, Lamprohaminoea, Smaragdinella) have evolved and diversified largely in tropical waters. New Zealand split away from Australia around 80–60 million years ago (Mya) and full separation took over 20 Mya, with the Tasman Sea reaching its present width of 2000 km around 55 Mya (Knox Citation1980). Due to the age of this separation together with the fact that fossils of these snails are only known in Australia from the Pleistocene (ca 2.58 Mya to 11,700 years ago; Kendrik, Citation1960; Semeniuk Citation1995), the allopatric distribution of P. maugeansis and P. zelandiae is more parsimoniously explained by recent dispersal across the Tasmanian passage followed by speciation on both sides, rather than the outcome of vicariance associated with the separation of New Zealand from Australia.
Acknowledgements
We are thankful to A. Miller, B. Rudman and I. Loch (Australian Museum, Sydney) for providing the samples that have made this work possible, and to P. Crab and H. Taylor (Natural History Museum, London) for images of shells. Photographs of live animals were kindly provided by L. Altoff, J. Hales (Australia), J. Geux, D. Wilson and D. Crisp (New Zealand via iNaturalist.org).
At the University of Bergen, Norway we are indebted to L. Lindblom and K. Meland for help with molecular work, and to I. Heggstad for help with scanning electron microscopy. This research received support from The Malacological Society of London and the Meltzers Foundation, University of Bergen, through research grants attributed to the first author.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams A. 1850. Monograph of the family Bullidae. In: Sowerby GB, editor. Thesaurus conchyliorum, or monographs of genera of shells. Vol. II. London (UK): Sowerby; p. 553–608, pl. 119–125.
- Allan JK. 1933. Opisthobranchs from Australia. Rec Aust Mus. 18(9):443–450. doi:10.3853/j.0067-1975.18.1933.747.
- Angas GF. 1871. A list of additional species of marine Mollusca to be included in the fauna of Port Jackson and the adjacent coasts of New South Wales. Proc Zool Soc London. 1871:87–101.
- Burmeister H. 1837. Handbuch der Naturgeschichte. Vol. 2. Berlin (DEU): Enslin; p. 369–858. Zoologie.
- Burn R. 1966a. Notes on some opisthobranchs mainly from South Australia. Rec Sou Aust Mus. 15(2):329–352.
- Burn R. 1966b. Opisthobranchia. Port Phillip Survey 1957-1963. Mem Nation Mus Victoria. 27:265–288. doi:10.24199/j.mmv.1966.27.15.
- Burn R. 1969. A memorial report on the Tom Crawford collection of Victorian Opisthobranchia. J Malacol Soc Aust. 1(12):64–72. doi:10.1080/00852988.1969.10673833.
- Burn R. 1974. Notes on some benthonic opisthobranchs from Port Philip Bay, Victoria. J Malacol Soc Aust. 3(1):43–57. doi:10.1080/00852988.1974.10673877.
- Burn R. 2006. A checklist and bibliography of the Opisthobranchia (Mollusca: Gastropoda) of Victoria and the Bass Strait area, south-eastern Australia. Mus Victoria Sci Rep. 10(142):7–13.
- Burn R. 2015. Nudibranchs and related molluscs. Melbourne (AUS): Museum Victoria; p. 256.
- Burn R, Wilson R 2011. Bubble Shell, Haminoea maugeansis, in Taxonomic Toolkit for marine life of Port Phillip Bay, Museum Victoria. [accessed 2020 Feb 22. http://136.154.202.208:8098/species/4183
- Clemens-Seely K, Phillips NE. 2011. Effects of temperature on hatching time and hatchling proportions in a poecilogonous population of Haminoea zelandiae. Biol Bull. 221(2):189–196. doi:10.1086/BBLv221n2p189.
- Cuvier G. 1795. Second Mémoire sur l’organisation et les rapports des animaux à sang blanc, dans lequel on traite de la structure des Mollusques et de leur division en ordre, lu à la société d’Histoire Naturelle de Paris, le 11 prairial an troisième [30 May 1795]. Mag Encyclo J Sci Let Arts. 2:433–449.
- Eichler JG 2007. Haminoea maugeansis from Victoria. In: Sea Slug Forum, Jun 14. Sydney; Australian Museum. [accessed 2020 Feb 22]. http://www.seaslugforum.net/find/19408
- Fischer P. 1883. Manuel de Conchyliologie et de Paléontologie Conchyliologique, Histoire naturelle des mollusques vivants et fossiles, Tome 31. Paris (FR): Crosse; p. 1369.
- Furneaux P 2003. Haminoea zelandiae from Tauranga, New Zealand. In: Sea Slug Forum, Jun 6. Sydney: Australian Museum. [accessed 2020 Feb 22]. http://www.seaslugforum.net/find/10149
- Gibson GD, Chia FS. 1991. Contrasting reproductive modes in two sympatric species of Haminaea (Opisthobranchia: Cephalaspidea). J Mollu Stud. 57(Sup 4):49–60. doi:10.1093/mollus/57.Supplement_Part_4.49.
- Gray JE. 1843. Catalogue of the species of Mollusca and their shells which have hitherto been recorded as found at New Zealand, with the description of some lately discovered species. In: Dieffenbach E, editor. Travels in New Zealand with contributions to the geography, botany and natural history of that country. Vol II. Fauna of New Zealand. London (UK): John Murray; p. 228–263.
- Grove SJ 2018. Haminoea maugeansis Burn 1966, A Guide to the Seashells and other Marine Molluscs of Tasmania web-site. [accessed 2020 Feb 22]. https://molluscsoftasmania.org.au/project/haminoea-maugeansis/
- Habe T. 1952. Atyidae in Japan. Illustrated Catalogue of Japanese shells. Vol. 20. Tokyo (JP): Malacological Society of Japan.
- Hales J. 2010. A list of the intertidal Opisthobranchs of Harmers Haven, South Gippsland. Vic Nat. 127:248–254.
- Hayward BW. 1979. An intertidal Zostera pool community at Kawerua, Northland and its foraminiferal microfauna. Tane Auckland. 25:173–186.
- Holznagel WE. 1998. Research note: A nondestructive method for cleaning gastropod radulae from frozen, alcohol-fixed, or dried material. Am Malacol Bull. 14:181–183.
- Inkscape Team. 2015. Inkscape 0.92. [accessed 2018 Jun 01]. www.inkscape.org
- Jansen P. 1995. Seashells of Central New South Wales: a survey of the shelled marine molluscs of the Sydney Metropolitan Area and adjacent coasts. Belgian Gardens (AUS): Patty Jansen (Self Published); p. 129.
- Kendrick GW. 1960. The fossil Mollusca of the Peppermint Grove Limestone, Swan River district or Western Australia. West Aust Nat. 7(3):53–66.
- Knox GA. 1980. Plate tectonics and the evolution of intertidal and shallow-water benthic biotic distribution patterns of the southwest Pacific. Palaeogeogr Palaeocl. 31:267–297. doi:10.1016/0031-0182(80)90022-X.
- Kobelt W. 1896. Die Familie Bullidae. In: Martini FHW, Chemnitz JH, editors. Systematisches Conchylien-Cabinet 1 (9). Nürnberg (GER): Bauer Raspe; p. 190.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. doi:10.1093/molbev/msw054.
- Kuroda T, Habe T. 1952. Check list and bibliography of the Recent Marine Mollusca of Japan. Tokyo (JP): Leo W. Stach; p. 210.
- MacPherson JH, Gabriel CJ. 1962. Marine molluscs of Victoria. Melbourne (AUS): Melbourne University Press; p. 475.
- Marcus E, Burch JB. 1965. Marine euthyneuran Gastropoda from Eniwetok Atoll, Western Pacific. Malacologia. 3(2):235–262.
- Mattis P, Kimball S, Singh M 1995. GIMP: GNU image manipulation program. [accessed 2018 Oct 01]. www.gimp.org
- Morley MS, Hayward BW. 2015. Intertidal records of ‘sea slugs’ (nudibranchs and allied opisthobranch gastropods) from northern North Island, New Zealand. Rec Auck Mus. 50:33–75.
- Natterer M. 2018. GIMP 2.10.0. [accessed 2018 Aug 01]. https://www.openhub.net/p/gimp/contributors/summary
- Nimbs MJ, Smith SD. 2016. An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). Proc Roy Soc Victoria. 128(2):44–113. doi:10.1071/RS16011.
- Oskars TR, Malaquias MAE. 2019. A molecular phylogeny of the Indo-West Pacific gastropods Haloa sensu lato (Cephalaspidea: Haminoeidae): tethyan vicariance, higher generic diversity, and ecological specialization. Mol Phylo Evol. 139. doi:10.1016/j.ympev.2019.106557.
- Pilsbry HA. 1895. Polyplacophora. Acanthochitinidae, Cryptoplacidae and appendix. Tectibranchiata. Man Conch. 15(60):181–436.
- Pilsbry HA. 1921. Marine molluscs of Hawaii, XIV, XV. Proc Acad Nat Sci Phil. 72:360–382.
- Powell AWB. 1979. New Zealand Mollusca. Auckland (NZ): Collins; p. 500.
- Pritchard GB, Gatliff JH. 1903. Art XIX, Catalogue of the Marine Shells of Victoria Part VI Roy Soc Victoria. 15:176–223.
- Puillandre N, Lambert A, Brouillet S, Achaz G. 2012. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 21(8):1864–1877. doi:10.1111/j.1365-294X.2011.05239.x.
- Rudman WB. 1971a. On the opisthobranch Genus Haminoea Turton & Kingston. Pac Sci. 25:545–559.
- Rudman WB. 1971b. Structure and functioning of the gut in the Bullomorpha (Opisthobranchia) Part 1. Herbivores J Nat Hist. 5(6):647–675. doi:10.1080/00222937100770491.
- Rudman WB 1999. Gizzard plates of Haminoea zelandiae. In: Sea Slug Forum, Dec 23. Sydney: Australian Museum. [accessed 2018 Oct 15]. http://www.seaslugforum.net/factsheet/hamizela
- Rudman WB 2003. Haminoea zelandiae (Gray, 1843). In: Sea Slug Forum, Jun 6. Sydney: Australian Museum. [accessed 2020 Feb 22]. http://www.seaslugforum.net/find/hamizela
- Rudman WB 2006. Colour variability in Haminoea zelandiae. In: Sea Slug Forum, Oct 27. Sydney: Australian Museum. [accessed 2020 Feb 22]. http://www.seaslugforum.net/find/18126
- Rudman WB 2007. Haminoea maugeansis Burn, 1966. In: Sea Slug Forum, Jun 13. Sydney: Australian Museum. [accessed 2020 Feb 22]. http://www.seaslugforum.net/factsheet/hamimaug
- Semeniuk V. 1995. New Pleistocene and Holocene stratigraphic units in the Yalgorup Plain area, southern Swan Costal Plain. J Roy Soc West Aust. 78:67–79.
- Smith EA. 1873. Mollusca. In: Richardson J, Gray JE, editors. The Zoology of HMS Erebus and Terror under the command of Captain Sir James Clark Ross, during the years 1839 to 1843 Vol. 2. London (UK): By authority of the Lords Commissioners of the Admiralty; p. 1–7.
- Sowerby GB. 1868. Monograph of the genus Haminea. In: Reeve LA, editor. Conchologia iconica, or, illustrations of the shells of molluscous animals Vol. 16. London (UK): L. Reeve and Co; p. 1843–1878.
- Suter H. 1913. Manual of the New Zealand Mollusca: with an atlas of quarto plates. Wellington: J. Mackay, Government Printer; p. 1120.
- Thompson TE. 1976. Biology of Opisthobranch Molluscs. Vol. I. London (UK):Ray Society; p.205.
- Turton W, Kingston JF. 1830. Part II: the natural history of the District or, lists of the different species of animals, vegetables and minerals. In: Carrington NT, editor. The Teignmouth, Guide, 2, Conchology, nº 63. London (UK): E. Croydon at the Public Library; C. and J. Rivington, Baldwin and Co. Whittaker and Co.; Gore: Dawlish; Torquay: Cole and Lascombe; Exter: Exter Booksellers.
- Vogler RE. 2013. The radula of the extinct freshwater snail Aylacostoma stigmaticum (Caenogastropoda: Thiaridae) from Argentina and Paraguay. Malacologia. 56:329–332. doi:10.4002/040.056.0221.
- Willan RC. 1979. The ecology of two New Zealand opisthobranch molluscs [Thesis (PhD–Biological Sciences)]. Auckland (NZ): University of Auckland. [accessed 2020 Feb 22]. https://researchspace.auckland.ac.nz/handle/2292/1455
