ABSTRACT
The provisioning of trophic eggs is exceedingly rare in fish, with only a single example of a species exhibiting this behaviour reported in the scientific literature: the Lake Malawi catfish Bagrus meridionalis. However, observations in captivity suggest that the behaviour is widespread and likely universal among all species within the most species-rich lineage of Channa. The evolution of trophic eggs could have led to the large adaptive radiation found in the C. gachua Group, which comprises almost half of the species diversity within the genus.
Introduction
Parental care is relatively widespread among the teleost fish and occurs in some form in 20–25% of the 453 currently recognised families (Wootton and Smith Citation2014). It is scattered in different forms throughout the evolutionary tree of fish and is dominated by male only-, followed by biparental, and finally female-only care (Blumer Citation1982; Mank et al. Citation2005; Wootton and Smith Citation2014).
Despite the diversity of forms of parental care in fish, the direct provisioning of food from parents to offspring is rare. Food provisioning is known to have evolved in a handful of cichlids in the genera Amphilophus, Symphysodon, Uaru, Perissodus and Etroplus, as well as damselfish of the genus Acanthochromis, all of which produce supplemental nutrition in the form of a nutrient-rich skin mucus that is grazed upon by the fry (Kavanagh Citation1998; Buckley et al. Citation2010; Satoh et al. Citation2018, Citation2019). So far among the teleost fish, the critically endangered lake Malawi catfish, Bagrus meridionalis, appears to be the only species where the production of unfertilised (or ‘trophic’) eggs as nutrient provisioning has been described in the scientific literature (McKaye Citation1986). This type of provisioning has been more widely documented in insects and anurans (Perry Citation2004; Perry and Roitberg Citation2006; Vági et al. Citation2019) than in fish.
The reason why nutrient provisioning is so scarce in fish (and other ectothermic vertebrates) while almost universal in mammals and birds, has been explained by the thermodynamic constraints imposed on endotherms, to wit, their vulnerability to heat loss is most significant during the juvenile stage due to the high surface-to-volume ratio of their smaller bodies, often combined with the lack of an insulating layer of hair or feathers. Parental provisioning is thus vital in helping offspring grow past this vulnerable stage as quickly as possible (Beekman et al. Citation2019). In frogs, the provisioning of trophic eggs is thought to evolve under conditions of food scarcity (Dugas et al. Citation2016).
The family Channidae Fowler, 1934 is a moderately diverse assemblage of predatory, airbreathing, freshwater fishes within the order Anabantiformes. The family includes two genera and 46 currently recognised species (Britz et al. Citation2019; Rüber et al. Citation2020), but this is likely an underestimation of true species diversity (Conte-Grand et al. Citation2017). Parachanna Teugels & Daget, 1984 includes three species distributed in tropical Africa (Rüber et al. Citation2020). Forty-three species of Channa Scopoli, 1777 are distributed from Iran, in the west, to the Amur region of Russia in the east and Java in the south (Courtenay and Williams Citation2004; Rüber et al. Citation2020). Introduced populations of several species thrive far outside this range in the United States and Europe (Courtenay and Williams Citation2004). Channa is divided into seven species groups (asiatica, gachua, lucius, marulius, micropeltes, punctata and striata groups) containing between 2 and 24 species each (Rüber et al. Citation2020). All members of Channa share a similar elongated body plan but range in size from 10 cm to 120 cm (Courtenay and Williams Citation2004; Rüber et al. Citation2020).
All species of Channa for which reproductive data are available produce floating eggs in simple nests that are guarded either by the male or by both parents, and parental care extends for some time (often several weeks) after the fry becomes free swimming (Rüber et al. Citation2020). This is almost certainly the ancestral form of parental care in the family as it is universal in Channa (with the exception presented below) and its sister genus Parachanna.
A number of reports by amateur fish keepers (Ettrich Citation1986; Schnieder Citation2001) have shown that several species in the C. gachua group are paternal mouthbrooders and that females of these species produce infertile eggs as a food source for the fry. This behaviour was briefly mentioned by Zworykin (Citation2017) but has otherwise gone unnoticed in the scientific literature. The purpose of this paper is to present new observations made in captivity on the use of trophic eggs in Channa, by myself and other aquarists, and to discuss this behaviour in a phylogenetic and evolutionary context.
Methods
New behavioural data were obtained in 2019 when groups of five individuals each of three species of wild-caught Channa (C. andrao, C. gachua and C. pulchra) were obtained by the author. The fish measured 10–15 cm (C. andrao, C. gachua) or 15–20 cm (C. pulchra) cm in length. The species were kept separately in heavily planted tanks (200 to 600 L in size) at temperatures ranging from 18 to 25 C, fed every other day with live insects and earthworms, and kept on a 14/10 hour light/dark cycle with artificial lights and timer. Pair bonding occurred over a period of 1–2 weeks and once a pair had formed they attacked the remaining individuals, which were then removed to separate tanks. This procedure resulted in one bonded pair each of C. andrao and C. gachua, and two bonded pairs of C. pulchra. All pairs bred several times over the following year, during which notes were taken on breeding behaviour, and the feeding of trophic eggs was opportunistically filmed. Growth rate over the first 45 days was recorded in a clutch of C. gachua for which the fry were raised solely on tropic eggs. Every other day 4–6 fry were removed, euthanised with MS222, fixed in formalin, photographed with a Canon EOS 7D digital camera attached to an Olympus SZX16 stereomicroscope, and measured digitally in QuickPHOTO MICRO 3.1. Attempts were also made to raise fry of all three species separate from the parents in order to test whether nutrient provisioning is supplementary or obligate.
Results
After the pairs had bonded, typical anabantoid mating embracing near the surface was regularly observed among all pairs. Breeding was confirmed by the presence of eggs floating under the water surface in C. pulchra () or from the distended gular region of the mouthbrooding males of C. andrao and C. gachua ().
In C. gachua, the male was first observed carrying eggs on 27 March 2019 (), the fry were released on 30 March and the first feeding with trophic eggs was observed and filmed the same day (Supplementary video 1). The fry started swimming 3 April. The pair bred a second time in December the same year.
In C. andrao, the male was first observed carrying eggs 17 March 2019 with fry being released two days later. Some of the fry were then moved to a separate tank for an attempt at artificial rearing; the remaining fry disappeared within a couple of days and were presumed to have been eaten by the parents.
The male was observed carrying eggs again 28 March with fry being released 31 March. The fry were kept in a secluded location of the aquarium and guarded by the male until becoming free swimming on 3 April.
In both C. andrao and C. gachua, females initiated feeding the newly hatched larvae with trophic eggs almost immediately after the male first released them from his mouth, which was several days prior to their becoming free swimming. The exact frequency of feedings was not determined but appeared to be at least twice per day, based on the visibly white, extended, stomachs of the fry.
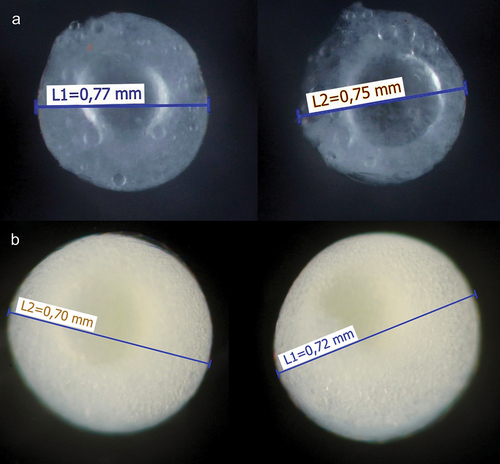
Prior to the release of trophic eggs, females would extend their fins and intensify their body colours, which would induce foraging behaviour in the newly hatched larvae (Supplementary video 1), whereas older free-swimming fry gathered around their mother waiting to be fed (Supplementary video 2). The female then circled above the fry, feeding them with unfertilised eggs (Supplementary videos 1 & 2). In contrast to fertile eggs ()), trophic eggs ()) sank (Supplementary videos 1 & 2), as they lack the large oil globule that normally makes anabantoid eggs buoyant. The female continued to feed the fry for several weeks, during which time they grew rapidly (). Clutch size (fertilised eggs) in C. andrao was approximately 250–300, and 500–1200 in C. gachua estimated from a total of 2 + 2 clutches.
Figure 4. Channa gachua fry at 1, 7, 14, 20, 28, 34 and 42 days of age display quick growth until about day 30 on a diet composed solely of trophic eggs. The growth curve is based on the average length of 4–6 fry measured every other day (lower right).
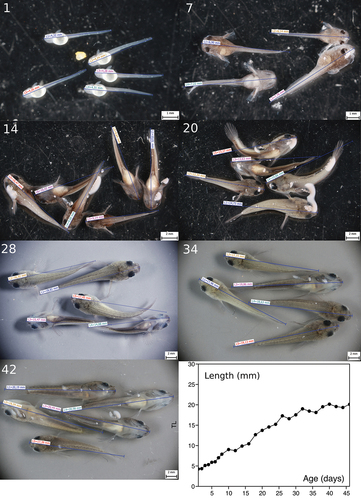
Fry of both C. gachua and C. andrao experimentally removed prior to becoming free swimming and raised isolated from the mother inevitably starved to death. The failure to recognise alternative food sources of suitable size, such as live Artemia nauplii, suggests that they are obligate egg feeders for at least their first week of life.
One pair of C. pulchra spawned 17, 27 and 31 March, and 4 April 2019 but these clutches failed to hatch. The pair continued to spawn on 28 April, 4, 11, 18, 23, 30 May and 6 June, with eggs hatching approximately 24 hours after being laid. An estimated 50–60 eggs were produced each time. A second pair of C. pulchra only spawned once on 7 April with eggs hatching on 8 and 9 April.
In C. pulchra the relatively larger eggs were left floating under the water surface (), guarded by both parents, with the male taking the more active role. Parental care continued once the fry hatched, and since the pair continued to produce a new clutch of eggs about once per week for several weeks, the shoal of fry contained siblings of different ages and sizes. Despite this disparity in sibling size, cannibalism was not observed. The female did not produce trophic eggs and the fry started foraging small live food once they became free swimming. Fry that were moved to a separate tank accepted Artemia nauplii once they became free-swiming and could be raised successfully without their parents.
Discussion
The provisioning of trophic eggs appears to be restricted to a single lineage within Channa () and has thus far been well documented in C. andrao (this study), C. orientalis (Ettrich Citation1986), C. bleheri (Harz Citation2001), and C. gachua (Schnieder Citation2001, this study). However, the behaviour has also been observed in other species within the C. gachua Group that have bred in captivity, including C. aurantimaculata, C. brunnea, C. pardalis, C. quinquefasciata, and C. stewarti (C. Dunlop, pers. com., videos on youtube), and appears to be a synapomorphy of the more diverse of the two clades that comprise the C. gachua Group (). All of the species with trophic egg provisioning (for which breeding behaviour is known) are paternal mouthbrooders, with the exceptions of the two sister species C. bleheri and C. brunnea, which have reverted to guarding their floating eggs at the water surface (Harz Citation2001, C. Dunlop, pers. com.).
Figure 5. Time-tree of Channidae adopted from Rüber et al. (Citation2020). Species groups follow Rüber et al. (Citation2020). The square indicate the hypothesised advent of trophic egg provisioning.
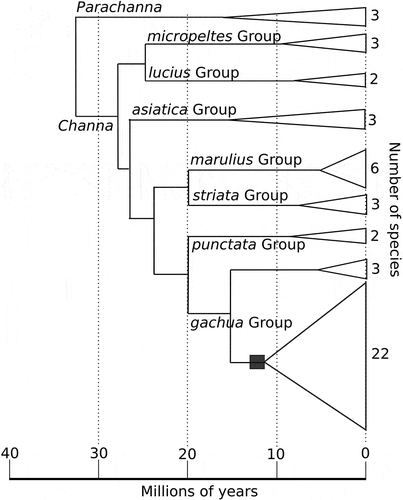
Figure 6. Phylogeny of the Channa gachua Group adopted from Rüber et al. (Citation2020) with known captive breeding behaviour indicated. The square indicate the hypothesised advent of trophic egg provisioning.
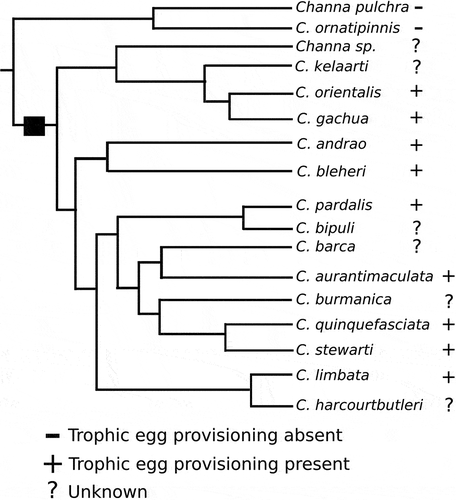
In the three-species clade within the C. gachua Group that lack the use of trophic eggs (), the production of multiple clutches of eggs separated by several days to a week was documented in C. pulchra (this study) and has also been observed in the closely related C. ornatipinnis (C. Dunlop, pers. com.). It is possible that the evolution of increased spawning frequency – as observed in C. pulchra and C. ornatipinnis – would have been a first step towards the even quicker production of eggs required for trophic use. While there is little data on breeding behaviour in Channa, there is no documentation of repeated spawning in species from any of other species groups of Channa.
The lack of ecological data for other members of the C. gachua Group makes it difficult to interpret with certainty the environmental circumstances that favour the use of trophic eggs, but in frogs the behaviour is thought to evolve in environments where there is routinely a shortage of food for the tadpoles (Dugas et al. Citation2016). Clearly, wherever small prey suitable for fry are scarce, trophic eggs would be highly advantageous.
The lineage of Channa in which trophic eggs evolved is, despite its relatively recent origin, estimated at approximately 12 million years, by far the most species-rich group within Channa () (Rüber et al. Citation2020). It contains almost half the species diversity of the genus, and includes the greatest size variation (notably several dwarf species) of any of the species groups (Rüber et al. Citation2020). While it is difficult to prove a direct causal relationship between the high diversity of this lineage and the evolution of trophic eggs, it is possible that trophic eggs provided novel ecological opportunities, which led to increased lineage diversification in the clade containing this trait compared to other lineages of Channa ().
The rarity of trophic eggs in fish may be partly due to the apparent preconditions for such a behaviour to evolve: (1) in teleost fish, food provisioning has evolved exclusively in species with biparental care. In the species of Channa studied here the male plays a vital role in guarding the fry, which liberates the female to forage and cover the caloric expenditure associated with the production of trophic eggs, and (2) the large size of the trophic eggs (compared to newly hatched fry), and their holotrophic consumption, could impose a mechanical constraint to their use. Channa may have been able to evolve trophic egg provisioning because of the prior existence of biparental care in the genus as well as the large gape, which gives them the ability to swallow large food items from a young age.
Although the fragmentary biological data currently available make these interpretations speculative, it is clear that this group of fishes offers some exciting and hitherto unexplored opportunities for studies into the evolution of nutrient provisioning in fish. As the use of trophic eggs is hitherto only known from observations in captivity, comparative field studies on the biotopes and environmental conditions where they live and reproduce, would be particularly valuable to further our understanding of the factors that favours such behaviour.
TNAH-OA_21-195-File009.mp4
Download MP4 Video (12.7 MB)TNAH-OA_21-195-File008.mp4
Download MP4 Video (11 MB)Acknowledgements
I thank the Swedish Cultural Foundation and Sakari Alhopuro Foundation for postdoctoral grants, Professor Kai Lindström and Dr. Varpu Vahtera for interesting discussions, Dr. Fred Kraus and two anonymous reviewers for commenting on earlier drafts of the manuscript, and Colin Dunlop (Anabantoid Association of Great Britain) for generously sharing his observations and experience of keeping Channa in captivity.
Disclosure statement
No potential conflict of interest was reported by the author.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Beekman M, Thompson M, Jusup M. 2019. Thermodynamic constraints and the evolution of parental provisioning in vertebrates. Behav Ecol. 30(3):583–591. doi:https://doi.org/10.1093/beheco/arz025.
- Blumer LS. 1982. A bibliography and categorization of bony fishes exhibiting parental care. Zool J Linn Soc. 75(1):1–22.
- Britz R, Dahanukar N, Anoop VK, Ali A. 2019. Channa rara, a new species of snakehead fish from the Western Ghats region of Maharashtra, India (Teleostei: Labyrinthici: Channidae). Zootaxa. 4683(4):589–600. doi:https://doi.org/10.11646/zootaxa.4683.4.8.
- Buckley J, Maunder RJ, Foey A, Pearce J, Val AL, Sloman KA. 2010. Biparental mucus feeding: a unique example of parental care in an Amazonian cichlid. J Exp Biol. 213(22):3787–3795. doi:https://doi.org/10.1242/jeb.042929.
- Conte-Grand C, Britz R, Dahanukar N, Raghavan R, Pethiyagodab R, Hui Tan H, Hadiaty RK, Yaakob NS, and Ruber L. 2017. Barcoding snakeheads (Teleostei; Channidae) revisited: Discovering greater species diversity and resolving perpetuated taxonomic confusions. PloS One 12(9):e0184017. doi:https://doi.org/10.1371/journal.pone.0184017.
- Courtenay WR, and Williams JD. 2004. Snakeheads (Pisces, Channidae): a biological synopsis and risk assessment. Vol. 1251. Reston, Virginia: US Geological Survey.
- Dugas MB, Wamelink CN, Killius AM, Richards-Zawacki CL. 2016. Parental care is beneficial for offspring, costly for mothers, and limited by family size in an egg-feeding frog. Behav Ecol. 27(2):476–483. doi:https://doi.org/10.1093/beheco/arv173.
- Ettrich G. 1986. Fische voller überraschungen. Die Aquarien Und Terrarien Zeitschrift. 39:289–293.
- Harz W. 2001. Die Nachzucht des Regenbogenschlangenkopffisches, Channa bleheri. Aquaristik Fachmagazin. 31(162):26–27.
- Kavanagh K. 1998. Notes on the frequency and function of glancing behavior in juvenile Acanthochromis (Pomacentridae). Copeia. 1998(2):493–496. doi:https://doi.org/10.2307/1447449.
- Mank JE, Promislow DE, Avise JC. 2005. Phylogenetic perspectives in the evolution of parental care in ray‐finned fishes. Evolution. 59(7):1570–1578. doi:https://doi.org/10.1111/j.0014-3820.2005.tb01806.x.
- McKaye KR. 1986. Trophic eggs and parental foraging for young by the catfish Bagrus meridionalis of Lake Malawi, Africa. Oecologia. 69(3):367–369. doi:https://doi.org/10.1007/BF00377058.
- Perry JC. 2004. The behavioural ecology of trophic egg laying. MSc thesis. Department of Biology Science, Simon Fraser University.
- Perry J, Roitberg D. 2006. Trophic egg laying: hypotheses and tests. Oikos. 112(3):706–714. doi:https://doi.org/10.1111/j.0030-1299.2006.14498.x.
- Rüber L, Tan HH, Britz R. 2020. Snakehead (Teleostei: Channidae) diversity and the Eastern Himalaya biodiversity hotspot. J Zool Syst Evol Res. 58(1):356–386. doi:https://doi.org/10.1111/jzs.12324.
- Satoh S, Awata S, Tanaka H, Jordan LA, Kakuda U, Hori M, Kohda M. 2019. Bi-parental mucus provisioning in the scale-eating cichlid Perissodus microlepis (Cichlidae). Biol J Linn Soc. 128(4):926–935.
- Satoh S, Tanoue H, Mohri M. 2018. Costs and benefits of biparental mucus provisioning in discus fish (Symphysodon aequifasciatus). Ichthyological Res. 65(4):510–514. doi:https://doi.org/10.1007/s10228-018-0636-5.
- Schnieder M. 2001. Eier fütternde Schlangenkopffische. Aquaristik Fachmagazin. 31:38–43.
- Vági B, Végvári Z, Liker A, Freckleton RP, Székely T. 2019. Parental care and the evolution of terrestriality in frogs. Proc R Soc B. 286(1900):20182737. doi:https://doi.org/10.1098/rspb.2018.2737.
- Wootton RJ, and Smith C. 2014. Reproductive biology of teleost fishes. Chichester, West Sussex: John Wiley & Sons.
- Zworykin DD. 2017. Phylogenesis of reproductive strategies in labyrinth fishes (Anabantoidei) and their sister groups. Biol Bull Rev. 7(5):428–441. doi:https://doi.org/10.1134/S2079086417050085.