ABSTRACT
One new species of Racovitzaibathynella Serban and Coineau, 1994 and one of Cteniobathynella Schminke, 1973 of the order Bathynellacea Chappuis, 1915 (family Parabathynellidae Noodt, 1065) are described from Benin in West Africa. These represent the first records of Bathynellacea species in Benin following a broad sampling throughout the country. Two species of the genus Racovitzaibathynella are known from South Africa and one is known from Chad and Israel. Specimens of Racovitzaibathynella beninensis Camacho and Lagnika sp. n. and Cteniobathynella boutini Camacho and Lagnika sp. n. have a unique combination of morphological characters and several shared generic features. Racovitzaibathynella beninensis sp. n. has a six-segmented antennule with three aesthetascs on segment five; a five-segmented antenna; concave labrum; mandible with only three teeth on the distal endite; no epipod on thoracopods I to III; plumose setae on the exopod of thoracopods I to VII; female thoracopod VIII distal-end bilobed, and two plumose setae on the endopod of the uropod. Cteniobathynella boutini sp. n. has a six-segmented antennula; five-segmented antenna; no epipod on thoracopods I to III; endopod on ThVIII of males as exopod, and five setae on the endopod of the uropod. To complement the morphological description of the two new species, a phylogenetic analysis based on the 18S ribosomal RNA (rRNA) gene was performed to infer their phylogenetic position within the family Parabathynellidae. The phylogenetic reconstruction shows an Afrotropical clade, which includes the new African species, and is clearly distinct from the European, Australian and Asiatic clades.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:7F97EBD0087C4324A601-EE8194E8E577
Introduction
Data on Bathynellacea Chappuis, 1915 of Africa are scarce (Camacho et al. Citation2016): sampling of this group has been conducted in only a few African countries and records for the continent are very limited. Nevertheless, the three families of the order are known from the African continent, including the family Leptobathynellidae Noodt, 1965, which has not been found in either Europe or Australia, areas more intensively sampled than Africa. So far, only 36 species of Bathynellacea are known from continental Africa and Madagascar, including the two new species described here (see Camacho et al. Citation2016): 26 belong to the family Parabathynellidae Noodt, 1965 (13 genera); five to the family Leptobathynellidae (four genera), and five to the Bathynellidae Grobben, 1905 (four genera). In West Africa (specifically in Ivory Coast), only four species have been recorded to date, three Parabathynellidae (Haplophallonella heterodonta Serban and Coineau, Citation1994; Lantobathynella pentodonta Serban and Coineau, Citation1982 and Acanthobathynella knoepffleri Coineau, Citation1967), and one Bathynellidae (Nunubathynella eburnea Schminke, Citation1979). Prior to the present study, the most recent discovery of Bathynellacea species from Africa was in Chad (Central Africa), where A. Brancelj collected two new species in 2014: Racovitzaibathynella dumonti Camacho et al., Citation2016 and Haplophallonella irenae Camacho et al., Citation2016. Here, we use morphological and molecular approaches to describe two new species from Benin, infer their phylogenetic position within Parabathynellidae and explore phylogenetic relationships among all genera of Parabathynellidae using sequence data from the nuclear 18S rRNA marker.
Material and methods
Study area and sampling methods
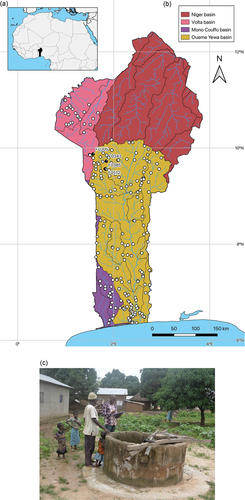
The material studied was collected during a series of sampling campaigns organised between 2015 and 2022. These campaigns were part of a project that aims to investigate the largely unknown stygofauna from the groundwater in Benin (Martin et al. Citation2017). The 335 faunistic samples were taken at 239 localities, mainly in the Ouémé and Mono Couffo catchment basins but also in the Upper Volta basin (). Samples were taken from modern wells lined with a casing (BURGÉAP Citation1981) using a modified Cvetkov phreatobiological net (funnel, 200 µm mesh size, 150 µm below the valve) (Cvetkov Citation1968; Boutin Citation2011). This net is particularly suitable for sampling animals in the water column but can also sometimes be used for sampling the sediment. The net is pulled from the bottom of the water to the surface about 10 times, causing the substrate and the animals in it to be suspended by creating an upwards current. Faunal samples were fixed in 95% ethanol immediately after collection and later sorted in a laboratory in the Zoology Department at Abomey-Calavi University. Specimens from the same sample were preserved in 95% ethanol and stored in the same vial at 5°C.
Figure 1. (a) Map of Benin in Africa. (b) Location of the wells sampled in Benin (white dots) and stations where Parabathynellidae were found (black dots). The numbers correspond to the sample identifiers. (c) Modern well (Vanhoui, Djougou, Donga department) where the new species R. beninensis sp. nov. was found.
Morphological study
Four specimens were used for the morphological study: one female and one male of the new species of Racovitzaibathynella, and one female and one male of the new species of Cteniobathynella. These specimens constitute the morphological type series of the two new species described here.
All specimens were completely dissected and permanent preparations of all anatomical parts were made following the glycerine jelly methods described by Perina and Camacho (Citation2016). Morphological examinations were performed using an oil immersion objective (100×) on a Zeiss interference microscope. Drawings were done using a drawing tube, digitalised using a WACOM tablet and retouched using Freehand and/or Adobe Illustrator drawing software. The material was deposited in the arthropod collection at the Museo Nacional de Ciencias Naturales, Madrid (MNCN).
We used the terminology proposed by Serban (1972 and following papers). Morphological and molecular descriptions are based on the type series. Anatomical terms and abbreviations used throughout the text, tables and figures (after Camacho Citation1986): AI = antennule; AII = antenna; Bsp = basipod; Ctenidia = spinule; Endp = endopod; Exp = exopod; Md = mandible; MxI = maxillule; MxII = maxilla; O.lb = outer lobe; P.lb = penial lobe; Symp = sympod; Th I–VIII = thoracopods I–VIII; and Urp = uropod. Institutional abbreviations: MNCN = Museo Nacional de Ciencias Naturales de Madrid (Spain); ARTP/MNCN = MNCN Arthropod Collection; CSIC = Consejo Superior de Investigaciones Científicas (Spain); and MNCN/ADN = MNCN Tissues and DNA Collection.
Specimen collection for DNA extraction
The segments of the abdomen that have no morphological characters used in the description of the two specimens of each new species were used for the DNA extraction. The four DNA extracts, constituting the DNA type series, were deposited in the MNCN/ADN. Voucher numbers of the specimens of the new species used in the analysis are indicated in .
Table 1. Localities (Datum WGS84) of the studied specimens of Bathynellacea. Voucher numbers of the Tissues and DNA Collection of the Museo Nacional de Ciencias Naturales (MNCN), CSIC (Spain) and GenBank accession numbers of the specimens included in the phylogenetic analysis are also provided.
DNA extraction, amplification and sequencing
The DNA extraction and amplification methods used were previously described by Camacho et al. Citation2020. Specimens were washed in distilled water for a few minutes before dissection and the abdomen of each specimen was placed in 0.5 mL digestion buffer (Gilbert et al. Citation2007) and incubated overnight at 55°C with gentle agitation. The digestion buffer consisted of 5 mM CaCl2, 2% sodium dodecyl sulphate (SDS), 40 mM dithiotreitol (DTT), 250 mg/ml proteinase K, 10 mM Tris buffer pH 8, 2.5 mM ethylene-diamine-tetra-acetic acid (EDTA) pH 8.0, and 10 mM NaCl (final concentrations). After incubation, nucleic acids were extracted from the digestion buffer using a Qiaquick Polymerasa Chain Reaction (PCR) purification kit (QIAGEN) (Alda et al. Citation2007). For nuclear 18S rRNA, three fragments of the gene were amplified using the primers 1 F and 3 R; 3 F and 5 R and 5 F and 9 R (Giribet et al. Citation1996). The PCR was performed in a final volume of 25 μL and included 3 μL of template DNA, 1× of the corresponding buffer (75 mM Tris HCl, pH 9.0; 50 mM KCl and 20 mM (NH4)2SO4), 2 mM MgCl2, 10 mM dNTPs mix, 0.1 μM of both primers, 0.02% Bovine Serum Albumin (BSA), and 0.125 units AmpliTaq Gold® DNA Polymerase (Applied Biosystems). The PCR programme consisted of an initial denaturation step at 95°C for 10 min, followed by 60 amplification cycles (95°C for 30s, 45–49°C for 45s and 72°C for 45s) and a final elongation step at 72°C for 10 min. PCRs were run on an Eppendorf Mastercycler gradient. Five microlitres of PCR product were electrophoresed through a 1.5% agarose gel and visualised with SYBR SafeTM DNA Gel Satin (Invitrogen) under ultraviolet light. PCR products were purified using ExoSAP-IT (USB Amersham, Buckinghamshire, UK) and incubated at 37°C for 45 min, followed by 80°C for 15 min to inactivate the enzyme. Purified PCR products were sequenced in both directions using the BigDye Terminator v. 3.1 sequencing kit (Applied Biosystems Inc., Foster City, USA) in a 10-μL volume, containing 15–20 ng of purified product and 3 pmol of primer (Camacho et al. Citation2016). Sequences obtained were then compared with those available from GenBank using BLAST (Altschul et al. Citation1997). We obtained 18S rRNA sequences from three of the four specimens of the new species (). All sequences were compiled and edited using Geneious v. 10.2.4 (https://www.geneious.com) (Kearse et al. Citation2012).
Phylogenetic and DNA sequence analysis
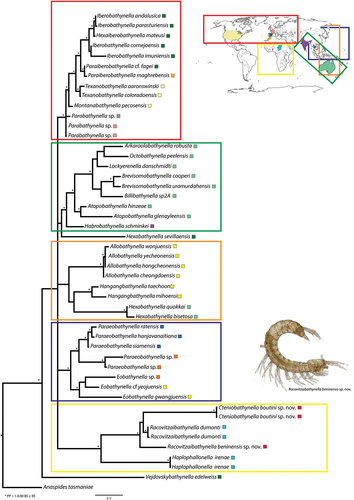
To examine the phylogenetic relationships among genera, we analysed the 18S rRNA sequences obtained in this study and selected taxa from GenBank. Voucher and GenBank accession numbers of the specimens used in the study are shown in . The following 21 genera (43 species and 46 specimens) of the family Parabathynellidae were included in the analyses: Allobathynella Morimoto and Miura, 1957 (four species), Arkaroolabathynella Abrams and King, Citation2013 (one species), Atopobathynella (two species), Billibathynella Cho, Citation2005 (one species), Brevisomabathynella Cho, Park and Ranga Reddy, Citation2006 (two species), Cteniobathynella (the new species), Eobathynella Birstein and Ljovuschkin, 1964 (three species), Habrobathynella Schminke, Citation1973 (one species), Hangangbathynella Park and Cho, Citation2013 (two species), Haplophallonella Serban and Coineau, Citation1975 (one species), Hexabathynella (three species), Hexaiberobathynella Camacho and Serban, 1998 (one species), Iberobathynella Schminke, Citation1973 (four species), Lockyerenella Little and Camacho, Citation2017 (one species), Montanabathynella Camacho, Stanford and Newell, 2009 (one species), Octobathynella Camacho and Hancock 2010 (one species), Parabathynella Chappuis, 1926 (three species), Paraeobathynella Camacho, Citation2005 (four species), Paraiberobathynella Camacho and Serban, 1998 (two species), Racovitzaibathynella (three species including the new one) and Texanobathynella Delamare Deboutteville, Coineau and Serban, 1975 (two species). Anaspides tasmaniae (Thomson, 1893) and Vejdovskybathynella Serban and Leclerc, 1989 were chosen as outgroups. We performed the analyses including all genera listed (), with 48 specimens and 1372 bp.
Sequences were aligned using MAFFT (Multiple Alignment using Fast Fourier Transform) algorithm (Katoh and Toh Citation2008), as implemented in Geneious and the final alignment was checked with Geneious and Mesquite v. 3.04 (Maddison and Maddison Citation2015). Sequence divergences (uncorrected p-distances) of 18S among genera were calculated using PAUP*4.0b 10 (Swofford Citation2002).
Phylogenetic analyses were performed using Bayesian inference (BI) approaches for the 18S datasets. The BI analyses were run in MrBayes 3.2.6 (Ronquist et al. Citation2012) as implemented in CIPRES Science Gateway v. 3 (Miller et al. Citation2010). The substitution model space was explored with the reversible-jump model (option lset nst = mixed rates = invgamma; Huelsenbeck et al. Citation2004). Two independent analyses were run with one cold and three heated chains, each chain run for 100 million generations, with the first 25% of trees discarded as burn-in. From the resulting trees, a 50% majority rule consensus tree was obtained. The consensus phylogenetic tree was then edited in FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree). We calculated the best nucleotide substitution model for both datasets using the Bayesian information criterion (BIC) and the Akaike information criterion (AIC) (Posada and Buckley Citation2004) as implemented in JModelTest v2 (Darriba et al. Citation2012).
Results
Bathynellacea were found at only three of the 239 localities sampled () with the phreatobiological net. Wells were sampled multiples times over several years and during two local climatic seasons (wet and dry) for a total sample effort of 335 samples. Despite the intensive sampling, only four samples contained bathynellaceans.
At station BEN110 (4 August 2017), a juvenile specimen of Parabathynellidae was collected; however, it was so badly deteriorated that it could not be identified or studied further. At the other two stations – BEN128, Moné (16 August 2017), and BEN147, Vanhoui (28 August 2017 and 25 August 2018) – two specimens at each station of two distinct genera were collected; these specimens belong to the two new species described below.
Systematic account
The two new species described here belong to the genera Cteniobathynella and Racovitzaibathynella, respectively, which both belong to the tribe Cteniobathynellini, in agreement with Serban and Coineau Citation1994, and to the ‘Cteniobathynella group’ in line with Schminke (Citation2011) for having five-segmented antenna (considered Type I by Schminke). Five species of the genus Cteniobathynella are distributed in Africa and two are known from Brazil. Three species of Racovitzaibathynella are distributed in South Africa and Chad.
Family PARABATHYNELLIDAE Noodt, 1965
Tribe CTENIOBATHYNELLINI Serban and Coineau Citation1975
Tribe diagnosis (according to Serban & Coineau, Citation1975 with amendments)
AI six-segmented. AII with two- or three-segmented protopod and three-segmented endopod (normally) or two-segmented (reduced). Labrum always with teeth, distal edge simple or clefted, with a convex contour. Md with four or five distal teeth on the pars incisiva and three or five claws on the pars molaris; proximal tooth of pars incisiva present or reduced. MxI with five or six teeth on distal endite. MxII, two- or three- segmented, with one or two long distal claws. Male ThVIII with exopod on distal face of basipod; outer lobe, always fused with penial region; endopod more or less reduced; penial region normally with two lobes and teeth. Lateral seta on pleotelson present.
Racovitzaibathynella Serban and Coineau, Citation1994
Amended generic diagnosis (after Schminke Citation2011; Camacho et al. Citation2016)
AI six-segmented. AII five-segmented; outer seta of terminal segment in male transformed into a long, strong fang curved inwards. Md with large tooth on the ventral edge; lobe with row of three or four claws. MxII three-segmented. Male ThVIII twice as long as wide; penial region consisting of two lobes: dentate and posterior lobes; dentate lobe with denticles; outer lobe fused with penial region; exopod located on distal face of basipod; endopod reduced to a seta. Female ThVIII 1-segmented and very reduced in size. Furcal rami with three terminal spines.
Type species
Racovitzaibathynella emilei Serban and Coineau, Citation1994. Type locality: interstitial environment of Mutlumuvi River, Kruger National Park, Transvaal, South Africa.
Other species
Racovitzaibathynella transvaalensis Serban and Coineau, Citation1994, type locality: interstitial environment of Mutlumuvi River, Kruger National Park, Transvaal, South Africa. Racovitzaibathynella dumonti Camacho et al., Citation2016, type locality: dry channel of the Oued Douar River, near Totous village, Tibesti area, Chad.
Racovitzaibathynella beninensis Camacho and Lagnika sp. n.
()
urn:lsid:zoobank.org:pub:urn:lsid:zoobank.org:act:492703A7-520A-4ED7-B793-B3ECF46E0CB6
Material examined
Type locality
Modern wells (BEN147), Vanhoui, Djougou, Donga, Benin (9.56519°N, 1.82198°E, 388 m alt.; WGS84), 28/08/2017 (one female) and 27/05/2018 (one male); collected by M. Lagnika, J. Hotepko and P. Martin.
Type material
Holotype male (MNCN 20.04/20,703), allotype female (MNCN 20.04/20,704), type series comprised of the two specimens, each on an individual slide, and two DNA samples as the DNA types (MNCN/ADN 54919 male and MNCN/ADN 54873 female).
Description
Body
Total length of holotype 1.1 mm and allotype 0.91 mm. Body elongated (), segments widening towards posterior end; approximately 9 times as long as wide. Head one-third longer than wide. All drawings are of the (male) holotype except for ThVIII, antenna, labrum and Md of the allotype (female).
Antennule
(). Six-segmented; length of first three articles 1.4 times longer than other three articles combined; sixth article as long as the third article but half as wide; inner flagellum rectangular, large, and half the height of the fourth article; setation as in ; article three with three smooth setae; article five with three terminal aesthetascs, similar in size; sixth article with three aesthetascs, one slightly shorter than the other two. AI longer than AII.
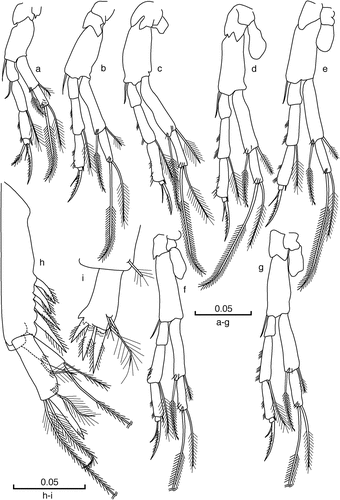
Figure 3. Racovitzaibathynella beninensis sp. n. (a, b, d, f, h, j–n) Male holotype. (c, e, g, i) Female allotype. (a) Antennule (dorsal view); (b) antenna (dorsal view); (c) antenna, female (dorsal view); (d) labrum; (e) labrum, female; (f) mandible; (g) mandible, female; (h) maxillule; (i) maxillule, female; (j) maxilla (dorsal view); (k) thoracopod VIII (latero-external view); (l) thoracopod VIII (latero-internal view); (m) thoracopod VIII (latero-external view); (n) thoracopod VIII (latero-internal view); (o) thoracopod VIII of female allotype (ventral view). Scale bars in mm.
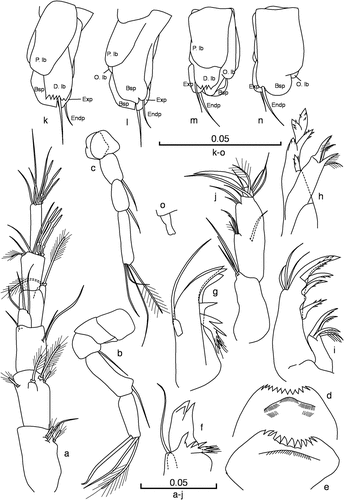
Antenna
(). Five-segmented; as long as the first four articles of AI; first two articles are similar in length; the third is the shortest, the fourth is 2.5 times longer than the first two, and the fifth is slightly shorter than the fourth one; last article with three setae: one smooth, one plumose and one outer seta transformed into a long strong fang that is curved inwards (sexual dimorphism); setal formula: 0/0/1 + 0/1 + 0/3(1).
Antenna of the allotype
(). Similar to that of the holotype but with four setae on the last article, with the outer seta smooth and not transformed; setal formula: 0/0/1 + 0/1 + 0/4(1).
Labrum
(). Distal edge concave, with 12 teeth, the lateral ones somewhat smaller than the central ones.
Labrum of the allotype
(). Similar to the male’s but with fewer teeth, only 11; the central teeth are triangular and slightly larger than the lateral ones.
Mandible
(). Pars incisiva with three teeth on the distal part, and a well-developed tooth on the ventral edge; pars molaris with four claws, the two most proximal joined and with many fine hairs; mandibular palp, one-segmented, with a distal seta that extends beyond the pars incisiva.
Mandible of the allotype
(). Similar to that of the male.
Maxillule
(). Proximal endite with two long serrulate claws and two smaller claws also with setules; distal endite with five claws with denticles, and with three unequal sub-terminal smooth setae on the outer distal margin.
Maxillule of the allotype
(). Similar to that of the male but with larger claws.
Maxilla
(). Three-segmented; basal article slightly shorter than the second article, armed with three smooth setae; second article twice as long as wide, with nine setae (3 + 1 on medial region), and a very small third article with one strong terminal claw and four setae. Setal formula: 3/9/4 + 1.
Thoracopods I to VII
(). Well developed, length gradually increasing from I to IV, last three thoracopods similar in length; epipod absent on Th I–III (), present on Th IV–VII (), almost half of the length of the basipod; basipod with one smooth seta at distal inner corner in Th I–VII. Exopod two-segmented, equal in length to the first two endopod articles of ThI and slightly shorter than the first three endopod articles of ThII–VII. All exopods with two setae, one barbed and one plumose, on each article and with one group of strong ctenidia at the base of the inner setae; article one twice as long as article two in all Ths and similar in length to the first two articles of the endopod in ThII–VII. Endopod four-segmented; first article short, similar in all Ths, half as long as articles two and three, which are identical and similar in all Ths and with clusters of strong spinules along the outer margin; fourth article small, with one claw, one smooth and one barbed seta on Th I and one barbed claw and one smooth seta on the rest of Ths; outer distal seta on second article always plumose; outer short seta on third article of Th I–VII smooth. Setal formula of endopod: Th I, 1 + 0/0 + 1/0 + 1/3(1); Th II–VII, 0 + 0/0 + 1/0 + 1/2(1).
Male thoracopod VIII
(). Rectangular; with penial lobe and outer lobe reaching to the middle of the thoracopod; dentate lobe with several big teeth; O. lb short and fused with basipod, distal end rounded; P. lb does not cover D. lb; basipod well developed on latero-external side, with seta and distal end bilobed; endopod reduced to a smooth seta located at a middle section of the posterior region of the exopod on the external part of the basipod distal edge; exopod well-developed, distinctive, with main axis (long branch) almost parallel to the distal edge of the basipod and with rounded distal end.
Thoracopod VIII of the allotype
(). Very reduced, twice as long as wide, rectangular, with dilated basis and slightly bilobed distal part.
Pleopods
Absent.
Uropods
(). Sympod 4 times as long as wide, 2 times as long as the endopod and 2.6 times as long as the exopod; sympod with four barbed spines of different sizes, located on the distal half, all smaller than the apical spine of the endopod, distal-most spine of the sympod slightly longer than the penultimate; endopod 1.5 times longer than exopod and 2.5 times as long as wide, with a long apical spine (25% shorter than the endopod), with setules and four setae, two terminal barbed and two latero-external plumose setae of similar length, with four groups of strong ctenidia on the dorsal side; exopod with terminal barbed setae of different size.
Uropod of the allotype
Similar to that of the holotype, except for the number of spines on the sympod (five).
Pleotelson
With one small, plumose ventro-lateral seta on each side close to the insertion of the furca. Anal operculum not protruded.
Furcal rami
(). Rectangular, twice as long as wide, with three strong barbed spines, similar in length, with the distal one slightly longer than others; membrane with setules is present on the distal edge of the rami, at the base of the spines; two plumose setae of different size on the dorsal side of the furcal rami.
Etymology
The species name is dedicated to Benin, where the species was collected, as the first species of Bathynellacea found in this country.
Remarks
The new species belongs to the genus Racovitzaibathynella. The main differences between this genus and all other genera belonging to the ‘Cteniobathynella tribe’ were pointed out by Serban and Coineau in 1994 when the genus was described (Camacho et al. Citation2021). The new species fits the generic diagnosis and shares characters with the other three species of the genus: R. emilei, R. transvaalensis (both from South Africa) and R. dumonti (from Chad). Differences and similarities amongst Racovitzaibathynella are summarised in , some of which are discussed below. Racovitzailbathynella. beninensis sp. n. has fewer setae on the first three articles of AI than R. dumonti, which has fewer than the South African species R. emilei and R. transvaalensis; the new species also has three aesthetasc on the fifth article. The ratio between the article size of AI and AII varies among all species; for example, the third article of AII is short in the new species but long in R. dumonti (similar in length to its last two articles), and all four species lack setae on the second article of AII. The labrum of the new species differs from the others as it is concave and has more teeth than that of R. dumonti but fewer than that of R. transvaalensis, though is similar in number to R. emilei. Md and MxI are very similar in all species, but the new species has three teeth on the pars incisiva of the Md and not four as in the rest of the species. Racovitzailbathynella beninensis sp. n. has three setae on the first article of MxII whereas the others have only two; in contrast, on the second article, it has fewer setae (17) than the South African species R. transvaalensis and R. emilei (19) but the same number as R. dumonti. The species from South Africa lack an epipod on ThI, R. dumonti lacks it on both ThI and ThII, and the new species lacks it on ThI to ThIII. As in R. dumonti, the endopod of all the thoracopods in the new species has a high number of ctenidia. The ratio between exopod and endopod article size of all thoracopods is similar in the three known species but differs in the new species as the exopod reaches almost the length of the first three articles of the endopod in ThII to ThVII; in the rest of the species the exopod is shorter. The thoracopod VIII of males of R. beninensis sp. n. is similar to those of the other species in its general appearance and the position of the lobes; however, there are some minor differences in the new species: the dentate lobe has fewer teeth, similar in number to R. dumonti; the outer lobe is small and rounded, instead of square or triangular, and, like in R. dumonti, does not cover the posterior part of the dentate lobe; and the posterior lobe is long, but not as long as in R. dumonti. In addition, R. beninensis sp. n. has a basipodal seta, which is not present in the rest of the species, and a basipod that has two lobes in the distal region, like in R. dumonti. Regarding setae on the endopod, the new species has only one, R. dumonti has two of different sizes, and the other two species have two of equal size. The female thoracopod VIII of the new species is not as oval as in the South African species, but more trapezoidal (as in R. dumonti) and with a bifurcated tip. The sympod of the uropod of the new species is inhomonomous, like in R. emilei, but with fewer spines, like in R. transvaalensis. The length to width ratios among sympod, exopod and endopod vary among the four species. The furcal rami of the new species are rectangular and as elongated as in the South African species, whereas they are thicker and less elongated in R. dumonti. None of the species of the genus Racovitzaibathynella have a protruded anal operculum. With respect to habitat, the three previously described species were found in interstitial epigean river environments; the new species was found in a modern well used for domestic water supply.
Table 2. Characters of the four species of the genus Racovitzaibathynella Serban and Coineau, Citation1994: Racovitzaibathynella emilei Serban and Coineau, Citation1994; Racovitzaibathynella transvaalensis Serban and Coineau, Citation1994; Racovitzaibathynella dumonti Camacho et al., Citation2016; Racovitzaibathynella beninensis sp. n. Camacho and Lagnika. Abbreviations: AI = antennule; AII = antenna; D.end = distal endite; H = homonomous; IH = inhomonomous; Md = mandible; MxI = maxillule; MxII = maxilla; N = number of; NPr = not pronounced; Symp = sympod; Th = thoracopod; ThI = thoracopod 1; ThVIII = thoracopod 8; Urp = uropod; XS = extra small.
Cteniobathynella Schminke, Citation1973
Amended generic diagnosis Schminke, Citation1973, pp. 72–75 (p. 77: diagnosis Schminke Citation2011)
Synonym: Parabathynella Chappuis, 1926 (partim), Thermobathynella Capart, 1951 (partim).
AI six-segmented; segments 5 and 6 with two and three aesthetascs, respectively. AII five-segmented; setal formula: 0 + 0/0,1 + 0,1/1 + 0/1 + 0/3,4(1). Md with conspicuous tooth on the ventral edge. MxI with proximal and distal endite having four and five spines, respectively. MxII three-segmented. Th I–VII with a basipodal seta; exopod of Th I–VII two-segmented, both segments with two terminal setae; second and third segment of the endopod of Th II–VII with comb spinules. Furcal rami with three spines.
Type species
Thermobathynella leleupi Delamare Deboutteville and Chappuis, 1955.
Other species
C. ahnerti Choi and Cho, Citation2005; C. bakeri (Green, 1964); C. calmani (Por, 1968); C. caparti (Fryer, 1957); C. essameuri Dumont, 1981; C. noodti Schminke, Citation1973 and C. teocchii (Coineau and Knoepffler, 1971).
Cteniobathynella boutini Camacho and Lagnika sp. n.
()
urn:lsid:zoobank.org:act:FB9D034F-E4A8-451E-81A5-695A2E9FBC1B
Material examined
Type locality
Modern well (BEN128) (9.72573°N, 1.84716°E, 387 m. alt.; WGS84), Moné, Djougou, Donga, Benin (West Africa), 16/08/2018 (one male and one female); collected by M. Lagnika, J. Hotepko and P. Martin.
Type material
Holotype male (MNCN 20.04/20,701), allotype female (MNCN 20.04/20,702), type series comprised of the two specimens, each on individual slides, and two DNA samples as the DNA types (MNCN/ADN 54871 male and MNCN/ADN 54872 female).
Description
Body
Total length of holotype 0.84 mm, and of allotype 0.90 mm. Body elongated, segments widening towards the posterior end. Head one-third longer than wide. All drawings are of the (male) holotype except one figure of the antenna of the allotype (female).
Antennule
(). Six-segmented; length of first three articles as long as the other three articles combined; sixth article as long as the first and second articles but only a third as wide; inner flagellum rectangular, large, half the height of the fourth article; setation as in ; article three with only one smooth seta; article five with two terminal aesthetascs of different length; sixth article with three aesthetascs, one shorter than the other two. AI longer than the AII.
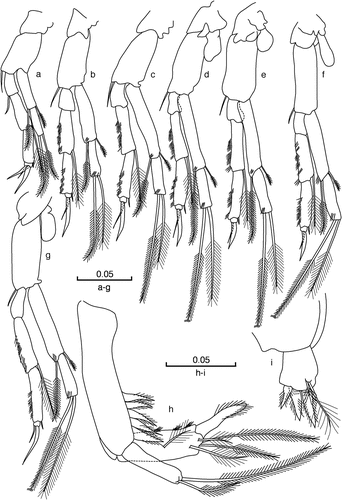
Figure 5. Cteniobathynella boutini sp. n. (a,b, d–i) Male holotype. (a) Antennule (dorsal view); (b) antenna (dorsal view); (c) antenna, female allotype (dorsal view); (d) labrum; (e) mandible; (f) maxillule; (g) maxilla (dorsal view); (h) thoracopod VIII (latero-internal view); (i) thoracopod VIII (latero-external view). Scale bars in mm.
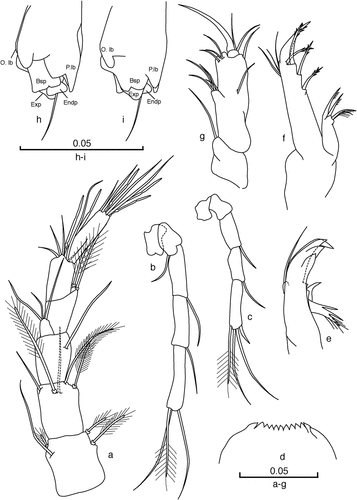
Antenna
(). Five-segmented; almost as long as the first five articles of AI; first two articles are similar and very short; the last three are long and of equal length; last article with three setae: two smooth and one plumose; setal formula: 0/0 + 1/1 + 0/1 + 0/3(1).
Antenna of the allotype
(). Similar to the holotype’s but without a seta on the second article and with four setae on the last article, the outermost one very small and thick, resembling a small spine; setal formula: 0/0/1 + 0/1 + 0/4(1).
Labrum
(). Distal edge slightly concave, with 12 teeth, the lateral ones slightly larger than the central ones.
Mandible
(). Pars incisiva with four teeth on the distal part, and a well-developed tooth on the ventral edge; pars molaris with three claws, the two most proximal claws joined and with many fine hairs; mandibular palp one-segmented, inserted very high, at the level of the tooth of ventral edge, with a distal seta that reaches the end of the pars incisiva.
Maxillule
(). Proximal endite with two long serrulate claws and two smaller claws also with setules; distal endite with five very thin claws with denticles, located on the distal third of MxI, and with three small, unequal sub-terminal smooth setae on the outer distal margin.
Maxilla
(). Three-segmented; basal article very short, armed with one smooth seta; second article three times as long as wide, with nine setae (4 + 1 on medial region) and a very small third article with one strong terminal claw and two setae. Setal formula: 1/9/2 + 1.
Thoracopods I to VII
(). Well developed, length gradually increasing from I to IV, last three thoracopods similar in length; epipod absent on Th I–III () but present on Th IV–VII (), small, one-third the length of the basipod; basipod with one smooth seta on the distal inner corner in Th I–VII. Exopod two-segmented, from ThIII to ThVI is about as long as endopod, slightly shorter in TVII; basal article about as long as the first two articles of the endopod, except in ThI and II, where it is slightly shorter; exopod with two barbed setae on the first article, one very long barbed and one plumose seta on the second article, and a group of strong ctenidia at the base of the inner setae; article one 2.5 times as long as article two in all Ths. Endopod four-segmented; first article short, similar in length in all Ths, half as long as articles two and three, which are similar in length and in appearance in all Ths and both with clusters of strong spinules along the inner margin; the fourth article small, with two claws and one smooth seta on Th I and one claw and one seta on the rest of Ths; outer distal seta on second article always plumose; outer small seta on third article of Th I-VII smooth, as a small spine. Setal formula of endopod: Th I, 1 + 0/0 + 1/0 + 1/3(1); Th II–VII, 0 + 0/0 + 1/0 + 1/2(1).
Male thoracopod VIII
(). Rectangular; with outer lobe located at the middle of the thoracopod, short and fused with the basipod and with a small rounded distal extension; penial lobes with an extension that reaches the distal end of the basipod with which it is fused; rounded exopod that protrudes beyond the distal end of basipod; the basipod is well developed on the latero-external face, without setae, and with distal end bilobed; endopod similar to exopod, with a smooth long seta.
Pleopods
Absent.
Uropods
(). Sympod nearly 5 times as long as wide, 2 times as long as the endopod and exopod, which are similar in length; five barbed spines of similar length located in the distal third of the sympod, half the size of the apical spine of the endopod; endopod 3 times as long as wide, with a long apical spine (20% shorter than the endopod) with setules and five setae, two terminal barbed and three latero-external plumose setae, with four groups of strong ctenidia on the dorsal side; exopod with two terminal barbed setae of different lengths.
Pleotelson
A ventro-lateral seta on each side. Anal operculum not protruded.
Furcal rami
(). Almost square, with three thick barbed spines, the largest of which is the distal one, followed by the middle and then the most proximal one; a membrane with setules at the base of the spines along the distal edge; unequal plumose setae on dorsal side of furcal rami.
Etymology
The species name ‘boutini’ is dedicated to our colleague Claude Boutin, who was a pioneer in the study of the underground aquatic environment in Africa, particularly in Benin, where stygofauna were completely unknown before collaborations were initiated under his impetus.
Remarks
The new species Cteniobathynella boutini is, together with C. caparti, one of the smallest species of the genus, measuring less than 1 mm. However, C. boutini sp. n. differs from the other species of the genus in the traits detailed in . The new species may or may not have a seta on the second article of AII, which is always present in the rest of the African species, and it has three or four setae on the last article, whereas the other species always have three setae. The new species has a single claw on the last article of MxII, in contrast to the two on the other species. In addition, it has a single seta on the first article, whereas the others have two or none. All species lack an epipod on ThI, but C. boutini sp. n. also does not have one on ThII and ThIII. Regarding the proportions of the articles of thoracopod exopod, the first article is always twice as long as the second in all species. Exopod to endopod article proportions reveal two types of ThI: (1) the first article of the exopod is shorter than the first two articles of the endopod, and the exopod reaches the middle of the third article of the endopod (C. teocchii, C. calmani, C. capparti and the new species); and (2) the first article of the exopod is the same length as the first two articles of the endopod, and the exopod reaches the middle of the third endopod article (type 2a, C. leleupi) or is the same length as the first three endopod articles (type 2b, C. bakeri) (ThI of C. essaumeri has not been described to date). The second type with the two alternative configurations is the one that is repeated in all the other thoracopods: ThII to ThVII of C. teocchii and C. calmani present type 2a, and the rest of the species, including the new one, present the most frequent type, 2b. Males of the new species have a small endopod on ThVIII; in C. leleupi and C. teocchii (the only other species for which ThVIII has been described), the endopod is reduced to two setae. Cteniobathynella boutini sp. n. lacks pleopods like all species of the genus. In the new species, the sympod of the uropod is homonomous with few spines; this is similar to almost all the other species except C. caparti, which has a longer distal spine, and C. essaumeri, which has more than double the number of spines (10–12), all equal in size. The new species has five setae on the endopod of the uropod, whereas the rest of the species only have three or four. The exopod and endopod of the uropod are almost the same length in the new species, as in C. calmani, C. caparti and C. teocchii; in the other species, the endopod is longer than the exopod. With respect to habitat, most of the African species were found in the interstitial environment of rivers. Only the new species and C. calmani were found in manufactured water well supply.
Table 3. Characters of the species of the genus Cteniobathynella Schminke, Citation1993 found in Africa: C. bakeri (Green, 1964); C. calmani (Por, 1968); C. caparti (Fryer, 1957); C. essaumeri Dumont, 1981; C. leleupi Delamare Deboutteville and Chappuis, 1955; C. teocchii (Coineau and Knoepffler, 1971); Cteniobathynella boutini sp. n. Abbreviations: AI = antennule; AII = antenna; D.end = distal endite; H = homonomous; IH = inhomonomous; Md = mandible; MxI = maxillule; MxII = maxilla; N = number of; NPr = not pronounced; St = setae; Symp = sympod; Th = thoracopod; ThI = thoracopod 1; ThVIII = thoracopod 8; Urp = uropod; XS = extra small.
Molecular results
We obtained 18S rRNA gene sequences for one specimen of R. beninensis sp. n. and two specimens of C. boutini sp. n. (). They were compared with 18S sequences of representatives of other taxa of the Parabathynellidae (19 genera and 41 species) from the USA, Spain, Slovenia, Morocco, Chad, India, Thailand, Vietnam, South Korea and Australia. With these data, we analysed the genetic divergence among genera and species and the phylogenetic position of both new species in relation to 19 other genera of the family Parabathynellidae.
Genetic divergences
The uncorrected sequence divergence values of 18S rRNA among all the studied genera are shown in . The highest level of genetic divergence (17.8% and 17.5%) is observed between the new species Cteniobathynella boutini and Octobathynella and the two species of Brevisomabathynella, respectively. This new species shows very high divergence values with almost all the genera included in the analysis; the lowest values are show with Racovitzaibathynella dumonti Camacho et al., Citation2016 and with the other new species, R. beninensis (5.0% and 6.0%, respectively). The other new species, R. beninensis, has the highest level of genetic divergence with Brevisomabathynella (14.1%) and the lowest (2.2%) with the other species of the genus Racovitzaibathynella: R. dumonti from Chad. The lowest value of genetic divergence, with respect to the outgroup, is observed with the three specimens of Parabathynella spp from Europe (6.2–6.4%). The Australian genera show higher levels of genetic divergence with the genera of the new species Racovitzaibathynella (11.9–14.1%) and particularly with Cteniobathynella (15.2–17.8%). The two new species show a genetic divergence of 6% between them. Cteniobathynella boutini sp. n. shows less genetic divergence with Racovitzaibathynella dumonti (5.1%) than with the new species R. beninensis. See for a comprehensive comparison.
Table 4. Data set of 18S p-distances of all taxa, including Australian taxa with short fragments, between genera.
Phylogenetic analyses
The 18S phylogeny inferred for the Parabathynellidae using a Bayesian approach is shown in (all genera). The phylogeny recovered four clades corresponding to the following regions: Holarctic (red), East Asian (blue), Afrotropical (yellow), Australian–East Asian (orange) and Australasian (green) but the Australian–East Asian clade had low support. One clearly divergent Afrotropical clade with long branches includes the new Benin species of Racovitzaibathynella and Cteniobathynella and the Racovitzaibathynella species from Chad; Cteniobathynella is included in the Racovitzaibathynella clade. Paraeobathynella and Eobathynella are grouped together in the East Asian clade. The Australasian clade includes most Australian species of Arkaroolabathynella, Octobathynella, Lockyerenella, Brevisomabathynella, Billibathynella, Atopobathynella and the Indian species of Habrobathynella; and Hexabathynella sevillaensis forms a lineage on its own. The Holarctic clade includes the Iberian and Maghrebian species of Iberobathynella, Paraiberobathynella and Hexaiberobathynella; the American species of Texanobathynella and Montanabathynella; and three Parabathynella species.
General discussion and conclusion
In this study, although there are still many gaps due to the lack of molecular data, the fact that the two new species belong to the genera Cteniobathynella and Racovitzaibathynella, respectively, seems clear from our detailed morphological study.
Serban and Coineau (Citation1994) questioned whether all Cteniobathynella species – five African, one Middle Eastern (Cteniobathynella calmani) and two South American (Cteniobathynella ahnerti and C. noodti) – really belong to this genus. Therefore, we analysed similarities and differences among the African species of the genus only.
The two new species described here belong to Cteniobathynella and Racovitzaibathynella (see Serban and Coineau Citation1975, Citation1994 for details), which both belong to the tribe Cteniobathynellini, in agreement with Serban and Coineau (Citation1994), and to Schminke’s ‘cteniobathynella group’, owing to having a Type I five-segmented antenna (Drewes and Schminke Citation2007). The main differences between these two genera and all the other genera belonging to the ‘Cteniobathynella tribe’ were highlighted by Serban and Coineau when they described Racovitzaibathynella (1994) and by Drews and Schminke (Citation2007) in their study summarising the differences between the genera of the families Parabathynellidae and Leptobathynellidae (the latter family now supported by morphological and molecular data: Camacho et al. Citation2021), which are included in the ‘Cteniobathynella group’.
Regarding secondary traits, all African genera of the ‘Cteniobathynellini tribe’ (Cteniobathynella; Ctenophallonella Coineau and Serban, 1978; Haplophallonella; Habrobathynella; and Racovitzaibathynella) are very similar (Camacho et al. Citation2016). Differences can be observed in the male Th VIII and in the chaetotaxy of the antenna that represent sexual dimorphism, which is only present in Racovitzaibathynella. In contrast to most of the African species of Cteniobathynella (see ), the male ThVIII of Racovitzaibathynella species has been well described (see ), allowing for a detailed comparison. For instance, R. boutini sp. n. does not share the presence of teeth in the penial region (absent also in Cteniobathynella), but it shares the presence of a posterior lobe and the specific position of the seta on the endopod, which is all that remains of this segment (there is a small endopod that is merged with the basipod in Cteniobathynella). All these features together with the sexual dimorphism in the chaetotaxy of AII place the new species within Racovitzaibathynella (Serban and Coineau, Citation1994) is also a defining character of the genus. Unfortunately, the characters of the male ThVIII could only be compared among the new species, C. leleupi and C. teocchii (). This appendage has not been described in C calmani and, in the other species, ie C. bakeri, C. caparti and C. essaumeri, the description is not detailed enough to allow for an adequate comparison.
Given that most of the sampling for Bathynellacea in Africa was carried out between 1949 and 1980 and focused on parts of central and eastern Africa, Madagascar and especially South Africa (Camacho et al. Citation2016), new knowledge of the group, particularly for other regions, is long overdue. Regarding the other genera, Abrams et al. (Citation2012) obtained partial 18S rRNA sequences (about 700 bp) of several species belonging to three Australian genera of Parabathynellidae. Moreover, more sequences have become available from GenBank, including for the following: Octobathynella peelensis Camacho and Hancock, 2010, Lockyerenella and Arkaroolabathynella from Australia; and Eobathynella and Hangangbathynella from South Korea and Vietnam. Although there are a large number of other sequenced specimens, some of which are available in GenBank, these specimens have not been assigned to nominal species or morphotypes (Matthews et al. Citation2020). Therefore, these sequences were not included in our analyses as some probably correspond to new undescribed genera. Two new Hexabathynella species (Perina et al. Citation2023a) have been recently described from Australia; they have long 18S fragments and thus were included in our analysis. In a previous study of genetic divergence of Australian Parabathynellidae, Abrams et al. (Citation2012) observed average uncorrected sequence divergences between 3.1% and 8.8% for 18S rRNA among the genera studied, and an average divergence among the Australian parabathynellid species of 4%. In our study, we observed similar and higher genetic divergences ranging from 3.0% to 17.5% among genera of Parabathynellidae.
The molecular results obtained from sequences of the Cteniobathynella genera are particularly interesting: despite being included in the Racovitzaibathynella clade, Cteniobathynella boutini sp. n. forms a long branch, highlighting the substantial genetic divergence between the two genera. It would be interesting to obtain molecular data for other species and see whether they form a monophyletic clade with C. boutini sp. n., given the morphological similarity among all the species of this genus, as described above. Molecular information is now available for four African species of three closely related genera: Racovitzaibathynella, Cteniobathynella and Haplophallonella. This information is valuable for comparing genera around the world, and when other markers (COI, for example) are available for different species, it will be possible to undertake a detailed study of the interspecific relationships within each of these African genera and species.
The three Hexabathynella species, one from Spain and two from Australia, do not form a monophyletic clade in our analysis. However, we do not have molecular data for described species (25) of this cosmopolitan genus. The genus has well-defined diagnostic characters, shared by all species included in it; however, it is possible that the consistent morphology is the product of convergent evolution, which could explain the separation into two different clades of the Australian and Spanish Hexabathynella species. The number of described species with genetic data, the number of undescribed species not included and the paucity of sampling records all affect the results. In this case, a preliminary phylogeny has been obtained with only 18S and selected species with the aim to place the new taxa. A more comprehensive phylogeny with additional loci (COI, 16S, or 12S) is needed to better understand the relationships between and within genera.
The application of traditional taxonomic tools, together with molecular techniques, helps guide taxonomic decisions. Incorporating various approaches and sources of data has become increasingly important (Camacho et al. Citation2011; Abrams et al. Citation2012; Little and Camacho Citation2017; Perina et al. Citation2019, Citation2023a, Citation2023b), particularly given the discoveries of cryptic species in Bathynellacea (Camacho et al. Citation2011, Citation2012, Citation2013; Cook et al. Citation2012). Morphology-based studies that include these species would result in an underestimation of the overall diversity of this crustacean order.
Distribution of species of Ctenibathynella and Racovitzaibathynella genera
Thirty-six species of Bathynellacea are currently known for the African continent and Madagascar, including the two new ones described here from Benin, which extend our knowledge of the order in Africa. These species belong to 21 genera; 26 species belong to the family Parabathynellidae (13 genera); five species to the family Leptobathynellidae (four genera), and five species to the family Bathynellidae (four genera) (Camacho et al. Citation2016). Below, we list the type locality, collection date and name(s) of the collector(s) for all known species of the genera Racovitzaibathynella (four species) and Cteniobathynella (nine species) (see map in ).
Figure 8. Map showing the global distribution of species of the genera Cteniobathynella and Racovitzaibathynella.
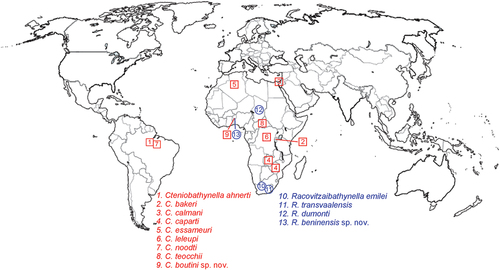
Cteniobathynella ahnerti Choi and Cho, Citation2005. Tributary of Para River, 85 km South Belo Horizonte, 12 km before Para River, Corrego, Itaguara, Brazil. Leg.: W. Noodt (1968).
Cteniobathynella bakeri (Green, 1964). Kaiso Spit, Lake Albert, Uganda (East Africa). Leg.: J. Green (10/1962).
Cteniobathynella calmani (Por, 1968). En Nur, Spring 6 in the Tabgha group of springs, Heptapegon, En Nur, near the northern shore of Lake Tiberias, Lower Galilee, Israel. Leg.: M. Tsurnamal (1967).
Cteniobathynella caparti (Fryer, 1957). Sandy beach at Samfya, near the south-west corner of Lake Bangweulu, Zambia; several rivers (Shewanoe River, streams of Matopo Hills, Bembezi River, Nuanetzi River) found by wells, 1964, Zimbabwe (Oriental Africa). Leg.: G. Fryer (3/1956, 9/1956).
Cteniobathynella essameuri Dumont, 1981. Spring Ain Essameur, on the flanks of Djebel Idjerane, which is part of the Adrar Ahnet, about 150 km south-east of In Salah, Central Algeria and Central Sahara, Algeria (North Africa). Leg.: H.J. Dumont? (biological expedition 13/9/1979).
Cteniobathynella leleupi Delamare Deboutteville and Chappuis, 1955. Uvira, north-west corner of Tanganyika Lake, Democratic Republic of Kongo (Central Africa). Leg.: N. Leleup (7/7/1954).
Cteniobathynella noodti Schminke, Citation1973. Cubatao River, about 2.5 km above oil refinery at motor highway Sao Paulo-Santos, Brazil. Leg.: W. Noodt (28/9/68).
Cteniobathynella teocchii (Coineau and Knoepffler, 1971). rapid stream at the exit of a cave in the savanna Bébé about 3 km from Boukoko, near M’Baiki in the Lobaye valley, Central African Republic (Central Africa). Leg.: L.-Ph. Knoepffler (23/9/1969).
Cteniobathynella boutini sp. n. Camacho and Lagnika. Modern well (9.72573°N, 1.84716°E, WGS84), Moné, Djougou, Donga, Benin (West Africa). Leg.: M. Lagnika (16/08/2017).
Racovitzaibathynella emilei Serban and Coineau, Citation1994. Mutlumuvi River, tributary of the Sabie River, Kruger National Park (16 miles from Skukuza), Transvaal, South Africa (West Africa). Leg.: Y. Coineau and L.-Ph. Knoepffler (3/02/1972).
Racovitzaibathynella transvaalensis Serban and Coineau, Citation1994. Mutlumuvi River, tributary of the Sabie River, Kruger National Park (16 miles from Skukuza), Transvaal, South Africa (Austral Africa). Leg.: Y. Coineau and L.-Ph. Knoepffler (3/02/1972).
Racovitzaibathynella dumonti Camacho, Brancelj, Dorda, Casado and Rey, Citation2016. Ouet Douar River, near Totous village, Tibesti Mountains, Chad (Central Africa). Leg.: A. Brancelj (14/03/2014).
Racovitzaibathynella beninensis sp. n. Camacho and Lagnika. Modern well (9.56519°N, 1.82198°E, WGS84) Vanhoui, Djougou, Donga, Benin (West Africa). Leg.: M. Lagnika (28/08/2017)
Previous sampling effort in Africa has been very limited and opportunistic: only 13 of the 54 countries have been sampled to date. So far, in Benin, 250 wells have been sampled across the entire Ouémé Basin. Despite the broad sampling effort carried out for this study, the number of specimens of Bathynellacea collected were surprisingly low, with four specimens of Parabathynellidae found in only three wells. Our results suggest Parabathynellidae might be rare in the country. This may be due to historical constraints: as this is the only area in Benin where other groups with a marine origin (amphipods, Lagnika et al. Citation2016 and oligochaetes, Martin unpubl. data) have been found, it is possible that there was a past marine introgression into a narrow strip of present-day Beninese territory. Benin experienced remarkable denudation during the Mesozoic and Cenozoic, and erosive processes related to marine transgressions that date to the late Cenozoic (Wildman et al. Citation2019). Perhaps marine transgressions and/or other palaeobiogeographic events, at different scales, could help explain the presence of two species belonging to different genera in two nearby localities, but such a study cannot be adequately addressed until more data is available and is beyond the scope of this paper.
Acknowledgements
We gratefully acknowledge C. Puch for the help with sampling, among other things. We thank the colleagues who sent us specimens collected in different parts of the world so that these papers are possible and we can learn about the real diversity of this group of stygobiont crustaceans: K. Abrams, C. Bou, A. Brancelj, D. Jaume, P Hancock, B. Hutchins, P. Leclerc, J. Little, B. Newell, V. Ortuño, Y. Ranga Reddy, J. Rodriguez, A. Tinaut and S. Watiroyram. We thank Melinda Modrell who helped us with the English translations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abrams KM, Guzik MT, Cooper SJB, Humphreys WF, King RA, Cho J-L, Austin AD. 2012. What lies beneath: molecular phylogenetics and ancestral state reconstruction of the ancient subterranean Australian Parabathynellidae (Syncarida, Crustacea). Mol Phylogenet Evol. 64:130–164. doi: 10.1016/j.ympev.2012.03.010.
- Alda F, Rey I, Doadrio I. 2007. An improved method of extracting degraded DNA samples from birds and other species. Ardeola. 54:331–334.
- Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. doi: 10.1093/nar/25.17.3389.
- Boutin C. 2011. Réflexion et rappels sur la diversité des recherches concernant “la Biodiversité” et sur leur importance relative, notamment au Maghreb et en Afrique Subsaharienne. In: Université Kasdi MerbahUniversité Kasdi Merbah, editor. Séminaire international sur la biodiversité faunistique en zones arides et semi-arides, 22-24 novembre 2009. Ouargla (Algérie): Actes du Séminaire international sur la biodiversité faunistique en zones arides et semi-arides, Université Kasdi Merbah; p. 33–55.
- BURGÉAP. 1981. La construction des puits en Afrique tropicale. Paris: Ministère de la Coopération au Développement, République française; p. 204.
- Camacho AI. 1986. A new species of the genus Hexabathynella (Syncarida, Bathynellacea, Parabathynellidae) from Spain. Bijd Dierk. 56:123–131. doi: 10.3897/zookeys.386.6296.
- Camacho AI, Brancelj A, Dorda BA, Casado A, Rey I. 2016. New Parabathynellidae species in Africa: the first bathynellids from Chad and assay of their phylogenetic position in the order Bathynellacea (Crustacea, Malacostraca) based on 18S sequences. J Nat Hist. 50(43–44):2691–2726. doi: 10.1080/00222933.2016.1210260.
- Camacho AI, Dorda BA, Rey I. 2011. Identifying cryptic speciation across groundwater populations: first COI sequences of Bathynellidae (Crustacea, Syncarida). Graellsia. 67(2):7–12. doi: 10.3989/graellsia.2011.v67.031.
- Camacho AI, Dorda BA, Rey I. 2012. Undisclosed taxonomic diversity of Bathynellacea (Malacostraca: Syncarida) in the Iberian Peninsula revealed by molecular data. J Crust Biol. 32(5):816–826. doi: 10.1163/193724012X638473.
- Camacho AI, Dorda BA, Rey I. 2013. Integrating DNA and morphological taxonomy to describe a new species of the family Bathynellidae (Crustacea, Syncarida) from Spain. Graellsia. 69(2):179–200. doi: 10.3989/graellsia.2013.v69.086.
- Camacho AI, Mas-Peinado P, Iepure S, Perina G, Dorda BA, Casado A, Rey I. 2020. Novel sexual dimorphism in a new genus Bathynellidae from Russia, with a revision of phylogenetic relationships. Zool Scr. 49:47–63. doi: 10.1111/zsc.12387.
- Camacho AI, Mas-Peinado P, Ranga Reddy Y, Bandari E, Shaik S, Perina G, Dorda BA, Casado A, Rey I. 2021. An integrated approach to re-evaluate the validity of the family Leptobathynellidae (Crustacea, Bathynellacea). Zooll J Linn Soc. 192:853–895. doi: 10.1093/zoolinnean/zlaa121/6144087.
- Cook BD, Abrams KM, Marshall J, Oerna CN, Choy S, Guzik MT, Cooper SJB. 2012. Species diversity and genetic differentiation of Stygofauna (Syncarida: Bathynellacea) across an alluvial aquifer in north-eastern Australia. Aust J Zool. 60:152. doi: 10.1071/ZO12061.
- Cvetkov L. 1968. Un filet phréatobiologique. BullInst Zool Mus Acad Bulg Sci Sect Biol. XXVII:215–218.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and high-performance computing. Nat Methods. 9:772. doi: 10.1038/nmeth.2109.
- Drewes J, Schminke HK. 2007. Number of families within Bathynellacea (Malacostraca) and year of publication of their names, with redescriptions of Baikalobathynella magna (Bazikalova, 1954) from Lake Baikal. Crustaceana. 84(11):1377–1401. doi: 10.1163/001121611X590120.
- Gilbert MTP, Moore W, Melchior L, Worobey M. 2007. DNA extraction from dry museum beetleswithout conferring external morphological damage. PLOS ONE. 2:e272. doi: 10.1371/journal.pone.0000272.
- Giribet G, Carranza S, Baguña J, Riutort M, Ribera C. 1996. First molecular evidence for the existence of a tardigrada+ arthropoda clade. Mol Biol Evol. 13(1):76–84. doi: 10.1093/oxfordjournals.molbev.a025573.
- Huelsenbeck JP, Larger B, Alfaro ME. 2004. Bayesian Phylogenetic Model Selection Using Reversible Jump Markov Chain Monte Carlo. Mol Biol Evol. 21:1123–1133. doi: 10.1093/molbev/msh123.
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 9:286–298. doi: 10.1093/bib/bbn013.
- Kearse M, Moir R, Wilson A, Stones-Haves S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Ganeious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649. doi: 10.1093/bioinformatics/bts199.
- Lagnika M, Messouli M, Ibikounlé M, Sakiti NG, Boutin C, Coineau N. 2016. First record of groundwater amphipods (Crustacea) from Benin; range extension of the genus Pseudoniphargus to South of the Sahara, in western Africa. Bull Soc Hist Nat Toulouse. 152:21–30.
- Little J, Camacho AI. 2017. Morphological and molecular characterisation of a new genus and new species of Parabathynellidae (Crustacea: Syncarida) in Queensland, Australia. Invertebr Syst. 31:208–219. doi: 10.1071/IS16054.
- Maddison WP, Maddison DR. 2015. Mesquite: a modular system for evolutionary analysis. Version 3.04 http://mesquiteproject.org.
- Martin P, Lagnika M, Sonet G, Ibikounle M. 2017. DNA barcoding and diversity of groundwater oligochaetes in Benin (West Africa). Scientific abstracts of the 7th international barcode of life conference. Genome. 60:11, 971.
- Matthews EF, Abrams KM, Cooper SJB, Huey JA, Hillyer MJ, Humphreys WF, Austin AD, Guzik MT. 2020. Scratching the surface of subterranean biodiversity: molecular analysis reveals a diverse and previously unknown fauna of Parabathynellidae (Crustacea: Bathynellacea) from the Pilbara, Western Australia. Mol Phylogenet Evol. 142:106643. doi: 10.1016/j.ympev.2019.106643.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 gateway computing environments workshop (GCE). 2010. Ieee; 1–8.
- Perina G, Camacho AI. 2016. Permanent slides for morphological studies of small crustaceans: serban’s method and its variation applied on Bathynellacea (Malacostraca). Crustaceana. 89(10):1161–1173. doi: 10.1163/15685403-00003576.
- Perina G, Camacho AI, Cooper S, Floeckner S, Blyth AJ, Sacco M. 2023b. An integrated approach to explore the monophyletic status of the cosmopolitan genus Hexabathynella (Crustacea, Bathynellacea, Parabathynellidae): two new species from Rottnest Island (Wadjemup), Western Australia. Syst Biodivers. 21(1):2151662. doi: 10.1080/14772000.2022.2151662.
- Perina G, Camacho AI, Danks M, White N, Guzik M. 2023a. Two new species of Atopobathynella (Parabathynellidae, Bathynellacea) from the Pilbara region, Australia. Syst Biodivers. 21(1):2228326. doi: 10.1080/14772000.2023.2228326.
- Perina G, Camacho AI, Huey J, Horwitz P, Koenders A. 2019. The role of allopatric speciation and ancient origins of Bathynellidae (Crustacea) in the Pilbara (Western Australia): two new genera from the De Grey River catchment. Contrib Zool. 88(4):452–497. doi: 10.1163/18759866-20191412.
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 53:5):793–808. doi:10.1080/10635150490522304.
- Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck ,JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. doi: 10.1093/sysbio/sys029.
- Schminke HK. 2011. Arthropoda: crustacea: malacostraca: bathynellacea: parabathynellidae. In: Flora and Fauna of Korea. Invertebrate fauna of the World. Vol. 21. Republic of Korea: Chon-chun Kim; National Institute of Biological Resources Environmental Research Complex.
- Serban E, Coineau N. 1975. Haplophallonella heterodonta n.g. n. sp. Parabathynellidé (Podophallocarida, Bathynellacea) d’Afrique (Côte d’Ivoire). Trav Inst Spéléol «E Racovitza». 14:51–70.
- Serban E, Coineau N. 1994. Racovitzaibathynella emilei n. g. n. sp. et R. transvaalensis n. sp. (Bathynellacea, Podophallocarida). Trav Inst Spéléol «E Racovitza». 33:11–30.
- Swofford DL. 2002. PAUP*: phylogeny analysis using parsimony (*and other methods), version 4.0b10. Sunderland (Massachusetts): Sinauer Associates Inc.
- Wildman M, Webster D, Brown R, Chardon D, Rouby D, Ye J, Dall’Asta M. 2019. Long-term evolution of the West African transform margin: estimates of denudation from Benin using apatite thermochronology. J Geol Soc. 176(1):97–114. doi: 10.1144/jgs2018-078.