ABSTRACT
Mutualistic and similar ecological associations between tarantulas (Mygalomorphae: Theraphosidae) and other animals are reviewed. Such associations are found to occur in at least nine theraphosid subfamilies. We present 63 new cases of theraphosid–anuran associations from 10 countries, documenting these interactions for the first time in Brazil, Chile, the Dominican Republic, and the Philippines. These findings are the first documentation of such associations for 13 theraphosid and 20 anuran taxa. Additionally, we report, for the first time, associations potentially of mutualistic nature between tarantulas and snakes, whip spiders, and harvestmen. Furthermore, we provide new reports of associations with ants and termites. While some of these interactions appear to be merely tolerated cohabitations, those involving anurans and ants seem to be more prevalent and complex, clearly offering benefits for both organisms. Additionally, based on multiple observations and field experiments, we propose a new hypothesis regarding the evolution of hirsuteness in theraphosids as a defensive strategy against predatory ants. This hypothesis supports previous findings that suggest a similar function for urticating setae incorporated into egg sacs and moulting mats. We further document a unique escape strategy against ants in New World arboreal theraphosids. Finally, the possibility of a chemical defence mechanism through specialised epidermal glands in theraphosids is briefly discussed.
Introduction
Symbiosis is typically defined as any type of close and long-term ecological interaction between two organisms of different species. This interaction can take several forms: mutualism, where both species benefit; commensalism, where one species benefits while the other neither benefits nor is harmed; antagonism, where one species benefits at the expense of the other; and amensalism, where one species is harmed while the other is neither benefited nor harmed (Langerhans Citation2008; Leung and Poulin Citation2008). In a stricter sense, some authors consider an interaction symbiotic only if at least one species benefits from the relationship.
Symbiotic relationships play crucial roles in all ecosystems and have long been recognised as important drivers of evolutionary novelty. Extensive research has been conducted to understand the conditions that favour these interactions and their evolutionary outcomes, as well as the adaptive mechanisms of the organisms involved (Margulis and Fester Citation1991; Yamamura et al. Citation2004; Nguyen and van Baalen Citation2020). Particularly in mutualistic and antagonistic interactions, the associated organisms may produce reciprocal adaptations through coevolutionary dynamics (Dimijian Citation2000).
While interspecific relationships involving spiders have been relatively well studied in regards to their parasites and natural predators (eg Gillung and Borkent Citation2017; Durkin et al. Citation2021), reports of non-agonistic interactions are scarce and lack thorough research. This scarcity may be partially attributed to the predominantly generalist and predatory nature of spiders (Elgar Citation1994), as well as the difficulty of observing the natural behaviour of nocturnal animals without affecting them. Regarding tarantulas (Mygalomorphae: Theraphosidae), in particular, the vast majority of reports on potentially mutualistic interactions describe an association with frogs, with observations dating back to as early as 1936. Information on interactions with other animal taxa are very rare in the literature, and in almost all cases they are only briefly mentioned as a small part of taxonomic publications (Lapinski Citation2019).
In this paper, our aim is to (1) review published cases of mutualistic and similar ecological associations between tarantulas and other animals, (2) report on a substantial number of new such cases, (3) propose and discuss a new hypothesis regarding the evolution of hirsuteness in tarantulas as a response to evolutionary pressure from army ants, (4) document an escape strategy in New World arboreal tarantulas against ants, and (5) briefly discuss the possibility of a chemical defence strategy in the form of specialised epidermal glands in tarantulas.
Material and methods
An ecological interaction involving tarantulas is herein considered a potential mutualistic association if there is the possibility for long-term or continuous encounters between the two species without any antagonistic reactions from either of them. This broad definition also encompasses simple tolerated cohabitations.
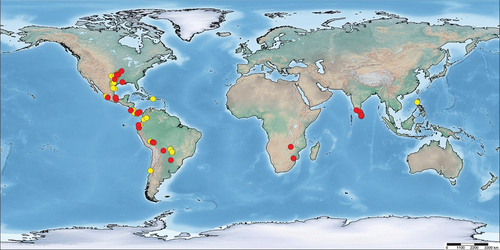
Published reports were collected through an extensive bibliographic search using the Thomson-Reuters database, Scopus databases, Google Scholar, and ProQuest Dissertations and Theses. The search was conducted using the terms ‘tarantula’ and ‘Theraphosidae’, combined with at least one of the following keywords: ‘Anura’, ‘ants’, ‘commensalism’, ‘ecological interaction’, ‘frogs’, ‘Microhylidae’, ‘mutualism’, ‘symbiosis’, ‘termitaria’, and ‘termites’. References cited in each publication were carefully screened for additional publications in other languages. The associations newly recorded in this paper were either documented by the authors or obtained from social media platforms, particularly iNaturalist, Facebook, and Flickr, or as a result of circulating requests to individuals or within social media groups. Theraphosid species identifications, particularly concerning the genus Aphonopelma Pocock, 1901, have predominantly been made not by the authors but rather by the photographer and members of the public on iNaturalist. These identifications are based on species familiarity and their zoogeographical ranges, rather than through the examination of diagnostic characters. To ensure ongoing accessibility in the future, these observations have been and will continue to be added to a dedicated ‘project’ focused on these associations (https://www.inaturalist.org/projects/mutualistic-ecological-associations-involving-tarantulas). The nomenclature for theraphosids, anurans, reptiles and ants follows the World Spider Catalog (WSC Citation2024), Frost (Citation2024), Uetz et al. (Citation2023) and AntWiki (Citation2024), respectively. The map () was prepared using SimpleMappr (Shorthouse Citation2010).
Figure 1. Geographic distribution of reported cases of associations between tarantulas and anurans. Red dots: literature records; yellow dots: new records.
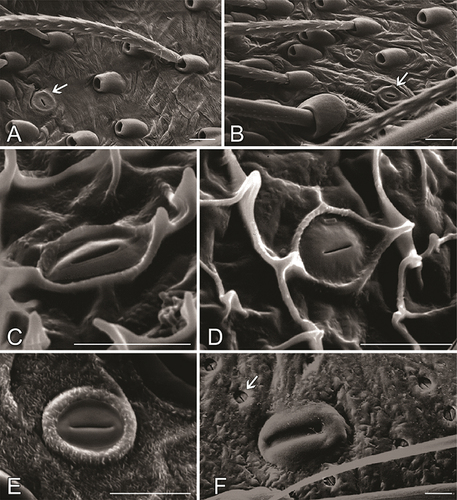
Scanning electron micrographs () were made using a Zeiss DSM-950 at the Neue Kantonsschule Aarau (Switzerland) by R. Foelix. Exuviae were sputtered with gold in a Polaron Sputter Coater SC 7610 before viewing them in the microscope. Pictures were taken at 15 kV with a Pentax digital SLR K20D camera and images were adjusted for brightness and contrast in Adobe Photoshop CS6.
Cases of associations between tarantulas and vertebrates
In the literature, all reported cases of non-agonistic interactions between tarantulas and vertebrates involve frogs and toads. These are all reviewed here, and additional new cases are also reported. Such interactions are currently known from a total of 17 countries (). Additionally, we report herein, for the first time, three associations possibly of a mutualistic nature between tarantulas and snakes.
Frogs and toads (Anura)
So far, 29 cases of associations between theraphosids and anurans from 12 countries have been reported in the literature (), and we report on 63 new cases from 10 countries () (). The majority of these cases (83: 22 in the literature, 61 new) involve the subfamily Theraphosinae (, , , ), followed by Poecilotheriinae (4 in the literature; ), Harpactirinae (2 in the literature; ), and Psalmopoeinae (1 new), Selenocosmiinae (1 new; ) and Thrigmopoeinae (1 in the literature). Theraphosid–anuran associations are herein reported for the first time from Brazil, Chile, the Dominican Republic and the Philippines ().
Figure 2. Associations between tarantulas and anurans. A. Aphonopelma cf. armada and Gastrophryne sp., Burleson, Texas, USA. B. Aphonopelma seemanni and Engystomops pustulosus, Tamarindo, Guanacaste, Costa Rica. C. Nhandu carapoensis and Chiasmocleis albopunctata, Balneario Pinamar, Paraguarí, Paraguay. D. Aphonopelma hentzi and Gastrophryne olivacea, Double Helix Ranch, Pontotoc, Texas, USA. E. Aphonopelma anax and Gastrophryne sp., Brownsville, Cameron County, Texas, USA. F. Pamphobeteus sp., female with late instars, and Chiasmocleis ventrimaculata, Tambopata Reserve, Madre de Dios Region, Peru. B reproduced from Hooijer (Citation2005), C reproduced from Bascoulés and Smith (Citation2021). Photo credits: Kassy Myers (A), Alex Hooijer (B), Sébastien Bascoulès (C), David Hillis (D), John Edward (E), and Reginald Cocroft (F).

Figure 3. Associations between tarantulas and anurans, continued. A. Aphonopelma armada and Gastrophryne olivacea, Austin, Texas, USA. B, C. Aphonopelma hentzi and Gastrophryne olivacea (marked with an arrow), Bandera County, Texas, USA. D. Aphonopelma hentzi and Gastrophryne olivacea. Bexar County, Texas. Photo credits: Kenneth Bader (A), Alec Gaudiesus (B), Alejandro Santillana (C), and Josh Benavidez (D).

Figure 4. Associations between tarantulas and anurans, continued. A. Sericopelma sp. and Engystomops pustulosus, nr. La Soledad, Veraguas Province, Panama. B. Pterinochilus sp. and Sclerophrys sp., Mana Pools NP, Zimbabwe. C. Ceratogyrus darlingi and Sclerophrys sp., South Africa. D. Orphnaecus sp., juvenile (marked with an arrow, the adult female not in the frame), and Rhinella marina, Sison, Pangasinan, Philippines. E. Poecilotheria fasciata and Uperodon taprobanica, Eluwankulama, Puttalam, Sri Lanka. E reproduced from Karunarathna et al. (Citation2012). Photo credits: Martin Hüsser (A), Delwin Eggers (B, C), Darrell Camacho (D), and Suranjan Karunarathna (E).

Figure 5. Associations between tarantulas and anurans, continued. A. Pamphobeteus sp., juveniles eating a tree-frog at maternal burrow entrance with Chiasmocleis royi untouched, Los Amigos Biological Station, Madre de Dios, Peru. B. Pamphobeteus sp., late instars living with Chiasmocleis ventrimaculata in maternal burrow entrance, Tambopata Reserve, Madre de Dios, Peru. C. Acanthoscurria sp. and Chiasmocleis albopunctata (marked with an arrow), Taunay, Aquidauana, Mato Grosso do Sul, Brazil. D. Pamphobeteus sp. and Chiasmocleis royi, Los Amigos Biological Station, Madre de Dios, Peru. Photo credits: Francesco Tomasinelli and Emanuele Biggi (A, D), Reginald Cocroft (B), and Platon Yushchenko (C).

Table 1. A list of mutualistic associations between theraphosids and anurans reported in the literature.
Table 2. A list of mutualistic associations observed between theraphosids and anurans reported herein. [Web sources all accessed 13 May 2024.]
The new data represent the first record of such association for the following 13 theraphosid taxa: Selenocosmiinae, Aphonopelma anax (Chamberlin, 1940), Aphonopelma armada (Chamberlin, 1940), Brachypelma klaasi (Schmidt and Krause, 1994), Cymbiapophysa Gabriel and Sherwood, Citation2020, Orphnaecus Simon, 1892, Phormictopus Pocock, 1901 (and P. atrichomatus Schmidt, 1991), Psalmopoeinae, Psalmopoeus Pocock, 1895 (and P. reduncus [Karsch, 1880]), and Xenesthis Simon, 1891 (and X. intermedia Schiapelli and Gerschman, 1945), and the following 20 anuran taxa: Anaxyrus Tschudi, 1845 (and A. debilis [Girard, 1854]), Dendrobatidae, Eleutherodactylidae, Eleutherodactylus Duméril and Bibron, 1841 (and E. inoptatus [Barbour, 1914]), Hylidae, Hypopachus Keferstein, 1867, Incilius Cope, 1863 (and I. nebulifer [Girard, 1854]), Oophaga Bauer, 1994 (and O. pumilio [Schmidt, 1857]), Pleurodema Tschudi, 1838 (and P. thaul [Schneider, 1799]), Pristimantis Jiménez de la Espada, 1870, Rhinella horribilis (Wiegmann, 1833), Rhinella marina (Linnaeus, 1758), Strabomantidae, and Trachycephalus Tschudi, 1838 (and T. cf. typhonius [Linnaeus, 1758]).
Theraphosinae
In the literature, there are 22 reports of 10–15 species in seven genera of Theraphosinae associated with anurans. The anurans reported belong to 10 species in seven genera, in the families Bufonidae, Craugastoridae, Leptodactylidae, and Microhylidae. The highest number of records (7) involve Aphonopelma hentzi (Girard, 1852), associated with the microhylids Gastrophryne olivacea (Hallowell, 1856) (6) and G. carolinensis (Holbrook, 1835) (1).
Amongst the associations newly reported here, 61 involve 17–26 species in at least 10 genera of Theraphosinae (). The associated anurans belong to 14–16 species in 12 genera, in the families Bufonidae, Eleutherodactylidae, Hylidae, Leptodactylidae, Microhylidae, and Strabomantidae. The highest number of new observations (21) involve A. hentzi, associated with 1–2 species of Gastrophryne Fitzinger, 1843 ().
Most of these observations describe the presence of more than one anuran individual either at the entrance or within an occupied terrestrial burrow of a tarantula (). For instance, Blair (Citation1936) and Dundee (Citation1999) reported the presence of up to 9 and 22 individuals of G. olivacea within occupied tarantula burrows, respectively. However, some observations reported only a single individual per burrow (eg Samadi Citation2014). In many cases, the anurans at the entrance were reported to quickly shelter in deeper parts of the burrow upon disturbance, without eliciting any reaction from the tarantula.
Hunt (Citation1980) conducted an experiment to test the tolerance of G. olivacea by A. hentzi in a terrarium. The spider did not attack the frogs, as expected. In the same environment, when a snake – a natural predator of G. olivacea – was introduced, the tarantula swiftly attacked the snake after the frogs sought shelter in its burrow. Interestingly, the frogs completely ignored the spiderlings but actively fed on introduced termites. Gastrophryne readily feed on small ants in their natural environment. Hunt observed the captive toads eating small ants in the tarantula’s burrow attracted to organic remains of the tarantula’s prey, that could otherwise attack the tarantula’s eggs and small spiderlings. Another experiment was conducted by Cocroft and Hambler (Citation1989) on an unidentified species of Pamphobeteus Pocock, 1901 (sub Xenesthis immanis). They found that the tarantula ignored individuals of the microhylid frog Chiasmocleis ventrimaculata (Andersson, 1945), while readily attacking other bufonid, hylid, and leptodactylid anurans.
Interestingly, Schalk and Sezano (Citation2014) did not observe frogs and tarantulas using the same burrow simultaneously. They also documented a predation attempt by the tarantula on a female Leptodactylus bufonius Boulenger, 1894. Consequently, it is possible that the relationship described in their work is not mutualistic but more likely to be commensalistic. Additionally, they noted that the burrows housing anurans had a larger diameter and were in closer proximity to water sources compared to those occupied solely by tarantulas.
Poecilotheriinae
There have been four documented cases of species of Poecilotheria Simon, 1885 sharing a tree cavity with one or multiple frogs (). These interactions involve four species of Poecilotheria and two species of the microhylid genus Uperodon Duméril and Bibron, 1841 ().
Karunarathna and Amarasinghe (Citation2009) reported observing juvenile stages of both organisms cohabiting the same retreat, in 12 cavities out of the 17 that they found housing both species. They further reported that the tarantulas readily attacked Sri Lanka leaf-nosed geckos, Hemidactylus depressus Gray, Citation1842 (Gekkonidae), that were trying to feed on the eggs of U. nagaoi (Manamendra-Arachchi and Pethiyagoda, 2001). Additionally, the frogs were observed feeding on ants that were attacking the spider eggs. Interestingly, they also noted that the remnants of the spider’s prey falling into a small water hole in the cavity potentially provided nutrients for the microhylid tadpoles.
Harpactirinae
There are two documented cases of associations between species of Harpactirinae and anurans (). These interactions involve a single species of each Ceratogyrus Pocock, 1897 and Pterinochilus Pocock, 1897, and an unidentified species of the bufonid genus Sclerophrys Tschudi, 1838 (). The report for the unidentified species of Pterinochilus described two toads occupying the retreat of the tarantula in a tree, which was monitored over the course of three days. The observation for Ceratogyrus darlingi Pocock, 1897 described a large toad within the ground burrow entrance of the tarantula, with the tarantula itself waiting nearby for passing prey (‘Spider keeping frogs as pets’ Citation2017).
Psalmopoeinae
Herein we report the first case of an association between a species of this subfamily and anurans, on the basis of a photo by the late Gregory G. Dimijian (). The interaction involves the strawberry poison frog, Oophaga pumilio, and the tarantula Psalmopoeus reduncus.
Selenocosmiinae
Herein we report the first case of an association between a species of this subfamily and anurans. It involves a cane toad, Rhinella cf. marina, sharing the burrow of an unidentified species of Orphnaecus (), a very small tarantula.
Thrigmopoeinae
There is only one known case of association between species of this subfamily and anurans, briefly described by Siliwal and Ravichandran (Citation2008). They reported Sanjay Molur (Executive Director, Zoo Outreach Organization, Coimbatore, India) observing a frog of an unidentified species of Microhyla Tschudi, 1838 within the burrow of Haploclastus kayi Gravely, 1915.
Snakes (Squamata: Serpentes)
Herein, we report three potential cases of mutualistic interactions involving tarantulas sharing their retreats with snakes (). The first case was observed in Guerrero State of Mexico by the second author. While turning over large angular rocks lying on the ground in search of theraphosids, one rock was turned to expose the burrow of a large female Brachypelma boehmei Schmidt and Klaas, 1993 (Theraphosinae) standing mere centimetres away from a coiled Michoacán ground snake, Sonora michoacanensis (Duges, 1884) (Colubridae). No predation attempt was made by the tarantula on the snake. While preparing the camera to record this event, the exposed snake, approximately 30 cm in length, started to burrow into the soft soil surrounding the spider’s burrow ().
Figure 6. Associations between tarantulas and whip spiders, a harvestman and a snake. A. Sericopelma sp. sharing its retreat with an unidentified whip spider (marked with an arrow), La Chorrera, Panama. B. Megaphobema velvetosoma sharing its retreat with an unidentified whip spider (marked with an arrow), Yasuní National Park, Ecuador. C. Sericopelma sp. and Paraphrynus laevifrons, Santa María de Dota, San José Province, Costa Rica. D. Phormictopus cautus sharing its burrow with an unidentified whip spider, Vinales, Pinar del Río Province, Cuba. E. Sericopelma sp. sharing its retreat with an unidentified harvestman (marked with an arrow), Upala, Alajuela Province, Costa Rica. F. Brachypelma boehmei sharing its retreat with Sonora michoacanensis (marked with an arrow), Guerrero State, Mexico. Photo credits: John G. Phillips (A), Aidan Craner (B), Johnson Jou (C), José Garrido (D), Dan MacNeal (E), and Rick C. West (F).

Table 3. A list of potentially mutualistic associations between theraphosids and non-anurans. [Web sources all accessed 13 May 2024.]
Figure 7. Interactions between tarantulas and ants. A. Avicularia purpurea (marked with an arrow) in its arboreal retreat, living with a colony of an unidentified species of Camponotus, Tena, Napo, Ecuador. B. Tapinauchenius cupreus living with Camponotus femoratus in a tree cavity, Río Momón, Loreto, Peru. C. Stichoplastoris cf. obelix, spiderlings, and Labidus coecus ants not being interested in the spiders, Reserva Biológica Tirimbina, Heredia Province, Costa Rica. D. Same, spiderlings gathered at the maternal burrow entrance, with ants moving close to the entrance. E. Avicularia hirschii displaying an escape strategy against Labidus ants, Madre de Dios, Peru. F. Tapinauchenius plumipes caught, killed and carved up by the army ants Eciton burchellii, Montsinéry-Tonnegrande Commune, French Guiana. D reproduced from Lapinski (Citation2019). Photo credits: Rick C. West (A, B, F), Witold Lapinski (C, D), and Emanuele Biggi (E).

Figure 8. Tarantulas living in termitaria. A. Avicularia juruensis, nr. Iquitos, Loreto, Peru. B. Vitalius dubius, Dona Amélia Farm, Santo Antônio de Possee, São Paulo, Brazil. C. Nhandu coloratovillosus, São Geraldo do Araguaia, Pará, Brazil. D. Nhandu coloratovillosus, Peixe, Tocantins, Brazil. E. Psalmopoeus cambridgei, Tamana Hill, Sangre Grande, Trinidad Island, West Indies. F. Brachionopus sp. with Trinervitermes sp., Ezemvelo Nature Reserve, Tshwane, Gauteng Province, South Africa. Photo credits: Alexey Yakovlev (A), Ivan Sazima (B), Fernando J.M. Rojas-Runjaic (C), Danté Fenolio (D), Sarah Crews (E), and Luke Goddard (F).

Figure 9. Nesiergus insulanus living in drywood termite frass (Kalotermitidae) on Frégate Island, Seychelles. A. Decaying palm tree with a retreat (marked by an arrow). B. Same, detailed view of the retreat. C, E. Fallen decayed palm tree with retreats (marked by arrows). D, F. Same, detailed views of the retreats. A and F reproduced from Canning et al. (Citation2014). Photo credits: Greg Canning.

The second and third observations are sourced from iNaturalist. One of them details an unidentified species of Phoneyusa Karsch, 1884 (Eumenophorinae) cohabiting with a ‘small blue snake’ in Banalia, Democratic Republic of the Congo. The other, from Texas, USA, describes Aphonopelma armada (Theraphosinae) cohabiting with a Texas blind snake, Rena dulcis Baird and Girard, 1853 (Leptotyphlopidae). Further observations and research are needed to better understand the dynamics and frequency of these cohabitations.
Additionally, we report observations of tarantulas actively removing snakes from the vicinity of their burrows. In November 1993, RCW and WWL attempted to document a predation interaction between a large female Megaphobema velvetosoma and a Catesby’s snail-eater snake, Dipsas catesbyi (Sentzen, 1796) (Colubridae), for a wildlife photographer near the village of Nueve de Octubre on the Río Marañón, Loreto, Peru. The snake was released and approached the tarantula, which was lying in wait at the entrance of its ground retreat. The tarantula grabbed the snake with its chelicerae, causing the snake to expel an odorous liquid musk from its anal glands. The tarantula transported the uninjured snake approximately 50 cm away from the retreat entrance and then released it before returning to its retreat. This behaviour was repeated, with the tarantula again capturing, carrying, and releasing the snake, which appeared unharmed. To determine whether this behaviour was specific to the individual tarantula, a similar predation attempt was conducted with a different adult female M. velvetosoma, located earlier by a guide. The observed behaviour was consistent with the previous event, with the tarantula immediately grasping the snake, carrying it to the edge of the clearing in front of its ground retreat, and releasing it unharmed. We hypothesise that the odorous liquid expelled by the snake may render it unpalatable to these tarantulas, thereby providing a protective mechanism for the snake. Alternatively, the snake may simply not have represented suitable prey and the spider’s behaviour therefore involved mere removal from its burrow.
Cases of associations between tarantulas and invertebrates
Regarding invertebrates, records of non-agonistic associations with tarantulas are exceedingly rare in the literature and limited to ants and termites. In this review, we examine these cases and present information on new observations of associations with species from these groups, along with new potential associations with species of Amblypygi and Opiliones ().
Whip spiders (Amblypygi) and harvestmen (Opiliones)
Six observations are documented here wherein theraphosine tarantulas share their retreats or microhabitats with unidentified species of Amblypygi in Panama (), Ecuador (), Costa Rica (), Belize, and Cuba (), and eight wherein they are associated with unidentified species of Opiliones in Costa Rica (), French Guiana, Mexico, and Peru ().
Although all Opiliones possess repugnatorial glands (ozadenes), it is unknown whether the secretions from these glands affect the much larger spiders that tolerate sharing their burrows. Despite the absence of agonistic reactions from the tarantulas in these associations, the frequency and dynamics of these interactions require further research.
Ants (Hymenoptera: Formicidae)
Given that both ants and spiders are amongst the most abundant arthropods, especially in tropical regions, interactions between various species of the two groups are common (Lapinski Citation2019; ). However, reports of such interactions in the literature are very rare, with almost all of them describing a predatory behaviour by the ants (). Three exceptions to this are mentioned here. Lapinski (Citation2019) documented in detail an interaction in Costa Rica between the army ants Labidus coecus (Latreille, 1802) and a female Stichoplastoris cf. obelix (Valerio, 1980) (Theraphosinae) and her juveniles (). No aggressive behaviour was observed in this association. Instead, the ants were seen entering the tarantulas’ burrow to collect and remove organic material. This caused the spiders to temporarily vacate the burrow, but they returned once the ants had left the area. In another note, Hirschi (Citation1991) reports having observed a small species of Tapinauchenius Ausserer, 1871 (Psalmopoeinae) in Ecuador, exclusively in association with an unidentified, aggressive species of ant that inhabits abandoned termitaria. The author notes that despite provocation, the tarantula remained unharmed by the ants, which otherwise attacked the author himself. Finally, Chomphuphuang et al. (Citation2017) briefly noted that some specimens of Phlogiellus longipalpus Chomphuphuang et al., Citation2017 (Selenocosmiinae) described in their paper were collected from retreats built in ant colonies.
Here, we report on a few cases of non-agonistic interactions between tarantulas and ants, mostly based on observations of RCW and WWL in South America. The first cases involve Avicularia avicularia (Linnaeus, 1758) (Aviculariinae) and Tapinauchenius plumipes (C.L. Koch, 1842). In Venezuela, juveniles of the two aforementioned theraphosid species were observed cohabiting with the arboreal ant Camponotus femoratus (Fabricius, 1804) in ‘ant gardens’. Ant gardens are an association between ants and plants, consisting of soil and decayed organic matter, with living epiphytic plants growing out of it and the ant colony itself living inside (Orivel and Leroy Citation2011). When the ant garden with a juvenile tarantula living on it was bumped, the spider retreated to the protection of its self-made silken retreat while the ants readily swarmed over the garden’s exterior, attacking in defence by biting and spraying formic acid. In about 30 minutes, the ant swarming subsided and the tarantula emerged and resumed its hunting position. By day, the spiders were secure in their individual retreats which, in part, bore into the ant garden. The foraging ants avoided the spider’s silken retreat, the entrance of which was not always silked over to prevent an ant from entering. At night, worker ants were more visibly active and going about their cultivating and food gathering activities on the exterior of the garden and surrounding vegetation. At the same time, the juvenile theraphosid would be out of its silken retreat and stretched out on the side of the ant garden waiting for passing prey. The ants were observed scurrying around the theraphosid, but never interacting with it nor trying to enter its retreat. Additionally, the juvenile theraphosid was never observed trying to predate a passing ant. The ant gardens observed were not large enough or located in such a place that they could support the needs of a subadult or adult-sized arboreal theraphosid of the aforementioned species. As observed in the past, as arboreal theraphosids and their prey demands and sizes grow, they relocate higher towards the canopy of the same tree or on an adjacent tree.
In Ecuador and Peru, small arboreal termite mounds were observed constructed on the sides of spiny palm trees and in the fork of living trees that had been taken over by colonies of either C. femoratus or stinging ants, possibly of the genus Pseudomyrmex Lund, 1831. Juveniles of both Avicularia purpurea Kirk, 1990 () and Tapinauchenius cupreus Schmidt and Bauer, 1996 () were observed in their self-made silken retreats in cracks or crevices of the abandoned termite mound. By day, each juvenile theraphosid remained concealed in its retreat. Small numbers of worker ants foraged on the living tree. At night, the tarantulas would emerge from their retreat and position themselves on the support tree or palm to wait for passing prey. As noted above, ant activity on the side of the support tree or palm was busier at night with passing ants ignoring the juvenile tarantula and vice versa.
On another occasion, WWL and RCW noticed a large active mound of the leafcutter ant Atta sexdens (Linnaeus, 1758), approximately 6 m across and 1 m in height. There were multiple entry holes into the mound made by the ants, some actively being used by the ants while some of the earlier-made holes in the mound were no longer being used by the ants and were now being utilised as retreats by an unidentified species of Pamphobeteus. On this particular leafcutter ant mound, 12 individuals of Pamphobeteus of varying sizes were found individually occupying unused entry holes into the mound as their retreat.
One another occasion, while photographing an adult female Megaphobema velvetosoma Schmidt, 1995 (Theraphosinae) eating a bush cricket at its fossorial burrow entrance in rainforest near the Río Nanay, Loreto, Peru, long columns of the army ants Eciton burchellii Westwood, 1842 were observed swarming over the ground and low vegetation hunting for invertebrates and small vertebrates. Sensing the foraging army ants’ approach, the tarantula retreated to the rear of its short ground burrow while still grasping the bush cricket in its chelicerae. By flashlight, the spider was observed pressed against the rear of its blind end retreat; it had dropped its partially consumed prey and drew its legs up and remained still at the back of its retreat. The army ants entered the burrow and swarmed over the dead bush cricket and tarantula. The ants quickly carved up and carried off pieces of the bush cricket from the retreat. Several ants tried to bite the tarantula but were unsuccessful due to the hirsuteness of the tarsal scopula, palps, legs and abdomen. The majority of ants that entered the burrow, however, appeared disinterested in the large tarantula that would, potentially, have been a major food source for them. The ants eventually gave up trying to subdue the tarantula and left the retreat while the spider remained motionless for about another 20 minutes, before returning to a hunting stance at the entrance of its retreat.
During recent fieldwork in the Pará State of Brazil, R. Bertani documented an instance of cohabitation involving a subadult Acanthoscurria geniculata (C.L. Koch, 1841) residing within a subterranean ant colony, likely of the genus Solenopsis Westwood, 1840. Upon disturbing the colony, the ants aggressively swarmed the observer’s legs, preventing him from capturing photographs (Bertani, pers. comm.). Typically, Solenopsis ants exhibit defensive behaviours and will attack and sting any intruding animal to protect their colony. However, in this particular observation, the theraphosid spider was not attacked by the ants, suggesting a possible association or tolerance between the species.
Finally, we also found four photographs on iNaturalist illustrating a potential association between theraphosids and ants in India and Thailand (), which warrant further investigation.
Termites (Blattodea: Isoptera)
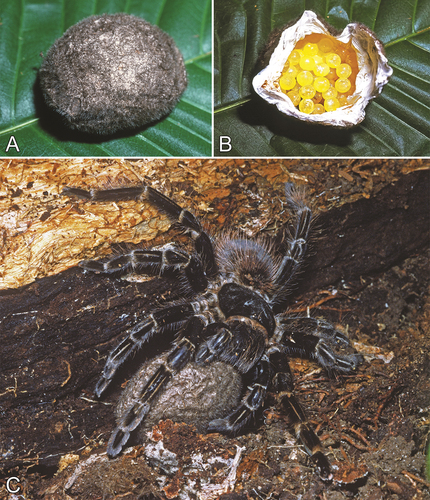
There are a few records of associations between tarantulas and termites in the literature. Lourenço (Citation1978) reported that Acanthoscurria paulensis Mello-Leitão, 1923 (sub A. atrox Vellard, 1924) inhabits the mounds of an unidentified species of Armitermes Wasmann, 1897 (Nasutitermitidae), while its diet primarily consists of orthopterans and small spiders and vertebrates. Canning et al. (Citation2014) found that some individuals of the Seychelles endemic Nesiergus insulanus Simon, 1903 (Ischnocolinae) construct their retreats within arboreal and terrestrial termitaria () and feed on termites as a part of their diet. Chomphuphuang et al. (Citation2017) reported that some individuals of Phlogiellus longipalpus Chomphuphuang et al., Citation2017 were found in their retreats built in colonies of ants and termites, which were preyed upon by the tarantulas. The holotype and the only known specimen of Umbyquyra gurleyi Sherwood and Gabriel, Citation2020 (Theraphosinae) was collected from termite mounds (Sherwood and Gabriel Citation2020), and Cifuentes and Bertani (Citation2022) listed a female specimen of Psalmopoeus pulcher Petrunkevitch, 1925 collected off a termitarium in San José Island, Panama. Inhabitation of termite mounds has been further reported for Acanthoscurria gomesiana Mello-Leitão, 1923 and an unidentified species of Acanthoscurria Ausserer, 1871 in Brazil (Costa et al. Citation2009; Macedo et al. Citation2021), as well as an unidentified species of Selenocosmiinae in Indonesia (Hood et al. Citation2020).
Herein, we also report on a few observations of theraphosids living in association with termites (). These include one observation of Avicularia juruensis Mello-Leitão, 1923 from Peru (), one observation of Vitalius dubius (Mello-Leitão, 1923) and two observations of Nhandu coloratovillosus (Schmidt, 1998) (both Theraphosinae) from Brazil (), one observation of Psalmopoeus cambridgei Pocock, 1895 from the West Indies (), one observation of Psalmopoeus pulcher from Panama, and one observation of an unidentified species of Brachionopus Pocock, 1897 and one observation of Harpactira marksi Purcell, 1902 (both Harpactirinae) from South Africa ().
Most, if not all, of these associations are likely not mutualistic, given that termite alates and workers are typically preyed upon by the spider and its young. Indeed, the use of termite mounds as retreats is common for a large number of vertebrate and invertebrate species (eg Haddad and Dippenaar-Schoeman Citation2002; Costa and Vanin Citation2010; Duleba and Ferreira Citation2014). Nonetheless, we opted to include them in the manuscript owing to their prevalence and the poorly understood dynamics of these interactions. Termitaria may be chosen by certain theraphosids as an optimal retreat due to prey availability for the young and the stability of the burrow itself.
Discussion
Theraphosidae is the largest family of mygalomorph spiders, currently comprising over 1100 species in 167 genera worldwide (WSC Citation2024). Most tarantula systematists recognise 12–13 subfamilies within this family, albeit with controversial phylogenetic relationships (Lüddecke et al. Citation2018; Foley et al. Citation2019; Pérez-Miles Citation2020). Mutualistic and similar associations have been documented in the following nine subfamilies.
Theraphosinae is the largest theraphosid subfamily, comprising more than 500 species in 76 genera (Pérez-Miles and Perafán Citation2020), which account for almost half of the known global diversity of the family. This subfamily is endemic to the Americas, distributed from southern North America to temperate zones of South America, and inhabiting a wide variety of terrestrial habitats, from sea level up to an altitude of 4500 m (Pérez-Miles and Perafán Citation2020). Most cases of theraphosid associations involve species of this subfamily (110 in total), with interactions documented with species of anurans, snakes, whip spiders, harvestmen, termites, and ants ().
Aviculariinae and Psalmopoeinae are both primarily arboreal subfamilies endemic to South America, comprising seven and five genera, respectively, and around 30 species each (WSC Citation2024). Three associations are known for Aviculariinae, and seven are documented for Psalmopoeinae, involving interactions with termites, ants, and a frog ().
Poecilotheriinae consists solely of the genus Poecilotheria, which currently includes 15 species found in India and Sri Lanka (WSC Citation2024). These arboreal tarantulas typically inhabit large cavities within trees, particularly in dry deciduous forests. Five associations involving this subfamily are currently known, involving interactions with anurans and ants ().
Selenocosmiinae comprises approximately 130 species in 11 genera, all distributed in South-East and East Asia and Oceania. They are typically terrestrial tarantulas living in silk-lined burrows at the bottom of trees or on the ground, within deciduous and humid forests. Five associations, with ants, termites, and a toad, are currently known for this subfamily ().
Ischnocolinae is a para- or polyphyletic subfamily currently comprising 17 genera and more than 100 species from Central and South America, Africa, the Mediterranean Basin, the Middle East, and India (WSC Citation2024). Four associations, with harvestmen, ants, and termites, are currently known for this subfamily ().
Harpactirinae comprises approximately 60 species in 10 genera, all of which are distributed exclusively in eastern to southern Africa. These tarantulas primarily inhabit dry grasslands and savannah woodlands, often constructing their burrows under large rocks or logs, or within tree roots (Smith Citation1990). Four associations involving Harpactirinae are currently known, with anurans and termites ().
Eumenophorinae comprises 13 genera and more than 50 species distributed in Africa and the Arabian Peninsula. Only a single association, with an unidentified snake, is currently known for this subfamily ().
Thrigmopoeinae is the smallest theraphosid subfamily. It is endemic to the Western Ghats of India and comprises only nine species, within the genera Haploclastus Simon, 1892 (seven species) and Thrigmopoeus Pocock, 1899 (two species). They are generally burrowing spiders excavating their burrows in muddy substrates or forest floor (Sankaran and Sebastian Citation2018). Only a single association, with a microhylid frog, is currently known for this subfamily ().
While some of the interactions presented and reviewed in this paper appear to be merely tolerated cohabitations (such as those with whip spiders, harvestmen, termites, and snakes), those involving anurans and ants seem to be more complex and prevalent, clearly offering benefits for both organisms. Therefore, these associations are discussed in more detail in the following section.
Interactions with anurans
It has been suggested that anurans living within the retreats of tarantulas benefit from shelter and protection against predators while feeding on insects attracted to the remnants of the spider’s prey in the burrow, which could otherwise be harmful to the spider, its eggs and its young (Hunt Citation1980; Mulvany Citation1983). In particular, anurans offer protection from parasitoid flies (Acroceridae, Phoridae) and predatory ants, which may be especially harmful to the tarantula’s eggs and spiderlings. It is noteworthy that species of Gastrophryne, the anurans most commonly cited for their association with theraphosids, are ant specialists (Deyrup et al. Citation2013) and inefficient burrowers that usually seek shelter in the burrows of other animals (Dundee et al. Citation2012). Given the spider’s selective tolerance of only a specific species of anuran in its retreat, while repelling or attacking others that are of similar size to their associated species (Dundee et al. Citation2012; Nyffeler and Altig Citation2020), the relationship appears to be mutualistic rather than commensalistic (Hénaut and Machkour-M’Rabet Citation2020), as suggested by some authors (eg Miller Citation2003). All known cases of tarantula–anuran mutualistic associations are facultative, as each involved organism can survive without the other. In very rare cases, the spider may prey upon its associated frog species. Miller (Citation2003) reported a mutualistic association between a species of Pamphobeteus and Hamptophryne boliviana (Parker, 1927) (Microhylidae), while von May et al. (Citation2019) reported predation of a species of Pamphobeteus on the same frog species. It is possible, however, that the theraphosids observed in the two reports belong to different species, with only one tolerating the presence of H. boliviana.
In a few cases, some anuran species not known to produce toxic skin secretions were observed cohabiting with theraphosid species. It can only be surmised that some tarantulas have a higher tolerance of sharing their retreat with a variety of non-toxic vertebrates and invertebrates than others (). A study of the skin chemistry of Gastrophryne carolinensis demonstrated that they produce toxic skin secretions, making them unpalatable to spiders and thus allowing them to coexist with tarantulas within their burrows (Garton and Mushinsky Citation1979). Szelistowski (Citation1985) found that the neotropical nocturnal-hunting spider Cupiennius coccineus F.O. Pickard-Cambridge, 1901 (Trechaleidae) readily accepted non-toxic frogs as prey but avoided predating dendrobatid frogs which possess toxic skin alkaloids. This would suggest that chemoreception by spiders may play a role in prey selection by larger hunting spiders, especially with nocturnal hunters. Inasmuch as little is known about toxicity or repugnant qualities of anuran skin secretions, at least in terms of the intended recipient, we tentatively make this inference. Moreover, anuran species known to be highly toxic or repugnant to many organisms still have their predators. Among spiders, species from at least five families are known to feed on these frogs (Nyffeler and Altig Citation2020). Specifically regarding theraphosids, Gray et al. (Citation2010) reported that although Sericopelma rubronitens Ausserer, 1875 (Theraphosinae) attacks the highly toxic Dendrobates auratus (Girard, 1855), the tarantula rejects or does not fully consume these frogs significantly more often than it does sympatric non-toxic species. In another observation, Costa et al. (Citation2006) reported Nhandu cerradensis Bertani, 2001 feeding on Ameerega flavopicta (Lutz, 1925), seemingly unaffected by the frog’s toxic alkaloids.
Interactions with ants and their potential role in the evolution of hirsuteness in tarantulas
Because of their large size and nutritional content, 63% protein and 9.8% fat by body weight (Taylor Citation1975), tarantulas are particularly vulnerable to a wide array of both invertebrate and vertebrate predators, and also serve as hosts for several groups of parasites and parasitoids (Hénaut and Machkour-M’Rabet Citation2020). Tarantulas employ various antipredator defence strategies, such as hiding in well-concealed retreats and burrows, displaying defensive postures, biting and delivering venom, autotomy, stridulating, defaecation, and feigning death. Most New World tarantulas (all Theraphosinae and Aviculariinae, and Ephebopus Simon, 1892 of Psalmopoeinae) use a unique defence mechanism in the form of releasing modified barbed setae called urticating setae, which not only deters most predators but may also kill certain small intruders (eg rodents) by entering their respiratory organs (Cooke et al. Citation1972). Some tarantulas also release their urticating setae on their egg sacs or moulting mats (Kaderka Citation2020). Seven types of these setae are recognised, which occur either on the dorsal abdominal surface (Theraphosinae, Aviculariinae) or on the prolateral side of the palpal femora (Ephebopus). In Theraphosinae, the spider releases the setae by rubbing them off with its hind legs, while in the case of the palpal setae in Ephebopus, they are released by moving the palps against the chelicerae (Bertani and Guadanucci Citation2013). The mechanism for releasing abdominal urticating setae in Aviculariinae differs from that in Theraphosinae and is primarily done via direct contact (Bertani and Marques Citation1996; Cifuentes and Perafán Citation2020).
One of the more puzzling interspecific interactions involving fossorial tarantulas is their relationship with predacious ants. Many species of spiders are known to live in close proximity to or within ant colonies (i.e. myrmecophiles), and some have evolved various degrees of morphological, behavioural, and chemical mimetic resemblance to ants (i.e. myrmecomorphs). Such adaptations enable certain species (more than 100 documented) to feed on ants, either as euryphagous or stenophagous predators (Cushing Citation2012).
Previous authors have suggested that the interactions between tarantulas and ants may represent a case of facultative mutualism, as the ants gather food for themselves and, in the process, clean the tarantula’s burrow. However, this interaction could potentially have a negative effect on the tarantula’s fitness, as the spider becomes fully exposed to potential predators and parasites during the ants’ visit to its burrow (Lapinski Citation2019). During their raids, army ants are known to attack most arthropods, but based on the limited observations reported here and by Lapinski (Citation2019), they seem to ignore fossorial tarantulas and even their spiderlings. Lapinski (Citation2019) hypothesised that these tarantulas may emit a rather neutral odour, resulting in being ignored by the ants, although this appears to be unlikely, as discussed below.
Herein, we propose an alternative hypothesis supported by observations and field experiments: the hirsuteness of theraphosids, particularly those of Theraphosinae, could have evolved partially as a defensive strategy against army ants. In April 1996, these findings were shared with Edward O. Wilson, the late world-renowned ant specialist from Harvard University, who agreed with the hypothesis. Indeed, many invertebrates have evolved characteristic hairs, spines or similar structures on their bodies or appendages in response to evolutionary pressures from their predators. A well-known example is the dense hairs and conspicuous spines of many caterpillars, which are thought to function as a physical defensive barrier against predators and parasitoids (Sugiura and Yamazaki Citation2014; Kageyama and Sugiura Citation2016). Even the scales of winged adults are believed to have evolved partially as an escape strategy from spider webs (Köchling et al. Citation2020).
Herein, we describe a field experiment conducted in Peru in 1994 in support of this hypothesis. Two female Acanthoscurria cf. theraphosoides (Doleschall, 1871) were collected from ground burrows, each with a body length of about 35 mm. Army ants of the species E. burchellii were found foraging on the forest floor, some observed carrying parts of beetles, larvae, other types of small spiders (possibly ctenids), and parts of a small frog. The two collected theraphosids were placed on the ground in front of the advancing column of army ants. As with the previous observation of M. velvetosoma, the two Acanthoscurria tarantulas seemed to sense the advancing ants, drew their legs up against their body and remained motionless, while the ants swarmed over them, trying to secure a hold with their mandibles but to no avail due to the hirsuteness of the spider. The ants quickly became disinterested and the column of foraging ants moved on. Once the ants had left, again, the theraphosids remained motionless for more than 10 minutes before relaxing and walking off.
However, there have been observations of army ants feeding on theraphosids, particularly arboreal species, though such incidents appear to be quite rare. For instance, in their study on the prey spectrum of army ants Eciton burchellii and Labidus praedator (Smith, Citation1858) in the Brazilian Amazon, Vieira and Hoefer (Citation1994) found that among the 312 spiders predated by E. burchellii, only two were (unidentified) theraphosids, while finding no theraphosids in a sample of 173 spiders predated by L. praedator.
Here we describe a field observation of army ants feeding on a theraphosid: in 1999, while in the rainforest near Montsinéry-Tonnegrande, French Guiana, army ants (E. burchellii) were observed foraging over the forest floor, on low vegetation and up the sides of larger trees in search of prey. A subadult Tapinauchenius plumipes was observed falling to the ground from under a piece of tree bark, possibly trying to escape the advancing ants, whereupon it was swarmed by army ants. In a matter of seconds, the theraphosid was barely visible under the number of army ants that began to bite, sting, and carve it up (). It can be speculated that Eciton burchellii are perhaps triggered to attack moving prey by grabbing with their mandibles and stinging. Tapinauchenius plumipes is not as hirsute as many other arboreal and ground-dwelling theraphosid species, thus allowing the army ants to hold it with their mandibles while stinging to subdue the spider. This is in line with the fact that medium- to large-sized theraphosines also tend to be more hirsute than small- to medium-sized ischnocolines, which are more likely to be attacked and killed by ants (eg Montemor et al. Citation2020). Indeed, it is likely that this hirsuteness could be an evolving character unique to Theraphosinae. This is supported by the fact that species in the genus Catumiri Guadanucci, Citation2004 (Ischnocolinae), the basal group of tarantulas (Foley et al. Citation2019), are not very hirsute. Ischnocolines typically produce more silk in their retreat entrances and burrows, creating more of a physical barrier and entanglement for other injurious invertebrates, such as ants.
Bertani and Guadanucci (Citation2013) suggested that incorporating urticating setae (especially those of type I) on egg sacs and moulting mats could serve as a barrier against predatory ants and effectively hinder their movement. During moulting, the spider is vulnerable to attacks from ants and phorid flies; therefore, urticating setae may help protect the spider against ants. The presence of urticating setae on egg sacs, which sometimes makes them hirsute (), further supports our hypothesis regarding the evolutionary force behind the overall hirsuteness of theraphosines. Therefore, both the hirsuteness and the urticating setae of theraphosids seem to have a defensive function against ants.
Figure 10. Egg sacs of theraphosids, heavily covered in protective urticating setae. A, B. Intact and opened egg sac of Megaphobema velvetosoma, Loreto, Peru. C. Female Tekoapora wacketi (Mello-Leitão, 1923) with her egg sac, Ubatuba, São Paulo, Brazil. Photo credits: Rick C. West (A, B) and Ivan Sazima (C).
At least some arboreal theraphosids appear to use a unique defensive strategy against predatory ants. In a field observation by E. Biggi in Madre de Dios, Peru, army ants of an unidentified species of Labidus Jurine, 1807 were observed foraging over low vegetation in search of live prey. A female Avicularia hirschii Bullmer, Thierer-Lutz and Schmidt, 2006 was observed exiting its thin silken retreat, sensing the approach of the army ants and hanging by two tarsi from the edge of the retreat leaf (). It can only be speculated that this is an escape strategy from predacious ants. This behaviour has been observed in other arboreal Neotropical theraphosids, whereupon the theraphosid either suspends itself from vegetation by a tarsus or immerses itself underwater inside the hollow of a tree for a long period of time, reportedly 15–20 minutes. The dense pile and guard setae effectively trap and envelop air around the tarantula’s body, enabling it to remain submerged for an extended period.
In all instances where predatory ants were observed interacting with tarantulas, the only vulnerable site on the spider that would be prone to injury from the ant’s mandibles is the ventral connective membrane of the appendage joints, particularly between the femur and patella and the tibia and metatarsus (). When theraphosids were observed drawing their legs up to their body, not only did the connective membrane between the opposing appendage segments become reduced, but a fringe of stiff guard setae (and, in some species, an intermix of spines) surrounded the membrane on all the lateral and ventral surfaces. When the appendage segments are drawn up, and the exposure to the membrane reduced, the opposing stiff setae crisscross, further making it difficult for an ant to secure a hold or cause injury to this softer vulnerable region with their mandibles.
Figure 11. Leg IV (prolateral view) of a female Nhandu tripepii (Dresco, 1984) from Belém, Pará, Brazil. Photo credit: Rick C. West.

Finally, theraphosids possess slit-like epidermal gland openings (Foelix Citation2011, fig. 2.33b; ) of unknown function which may serve as an additional defensive mechanism. Such structures are found also in Liphistiidae of Mesothelae (). When sniffing a live pet tarantula, both cats and dogs readily wince and move away despite the spider not releasing any urticating setae or making a threat posture that would cause such a reaction (West, pers. obs.). Considering the highly developed olfactory senses of these mammals, these reactions may be attributed to repugnatorial glands in the theraphosid cuticle, although further investigation is needed to substantiate this hypothesis, which at this stage is very speculative.
Figure 12. Slit-like cuticular pores representing epidermal gland openings. A, B. Ephebopus cyanognathus West and Marshall, 2000, palpal femur, with urticating setae and one gland opening (marked with an arrow). C, D. Exuvia of juvenile Ephebopus cyanognathus, abdomen. E. Psalmopoeus sp., dorsal side of metatarsus. F. Liphistius sp., a slit sensillum (large) and several gland openings (small, one marked with an arrow). Photo credits: Rainer Foelix. Scale bars: 0.01 mm.
Acknowledgements
Rick C. West wishes to thank the late Edward O. Wilson for discussion on the evolution of the hirsuteness in theraphosids, and Rainer Foelix for discussion and providing scanning electron micrographs. The following people are graciously thanked for sharing their field observation information, species identification, videos, and photographs for this study: Darrell Acuña, Wes Adams, Robin Agarwal, Kenneth Bader, Sebastien Bascoulès, Josh Benavidez, Rogerio Bertani, Emanuele Biggi, Greg Canning, Judith Cerepera M., John T. Cherry, Rex Cocroft, Gregory G. Dimijian, John Edwards, Delwin Eggers, Danté Fenolio, Alejandro Santillana Fernandez, Harper Forbes, Tiffany M. Garcia, José Garrido, Alec Gaudiesus, Luke Goddard, Chris Hamilton, Brent Hendrixson, Eddy Hijmensen, David Hillis, Alex Hooijer, Martin Hüsser, Witold Lapinski, Oly Lnskns, Is Ma, Dan MacNeal, Joseph Mappilacherry, Isiah McCracken, Mark Moffett, Blake Molone, Wes Molone, Kassy Myers, Guillermo Oramas, Nicolai Pedersen, Andrew Raciti, Sam Remillard, Stephen Ridgway, Gerardo Cobian Romo, Fernando J.M. Rojas-Runjaic, Jan-Philipp Samadi, Ivan Sazima, Travis Taggart, Paul Tardie, Rodrigo Orozco Torres, Antonio Tosto, Nathan Villareal, Volker von Wirth, Dirk Weinmann, Alexey Yakovlev, and Platon Yushchenko. Finally, we thank the editor for diligently handling this manuscript, and the three anonymous reviewers for their constructive feedback and valuable suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AntWiki. 2024. AntWiki; [ accessed 2024 May 13]. https://www.antwiki.org.
- Bascoulés S, Smith P. 2021. Mutualism between frogs (Chiasmocleis albopunctata, Microhylidae) and spiders (Eupalaestrus campestratus, Theraphosidae): a new example from Paraguay. Alytes. 38:58–63.
- Bertani R, Guadanucci JPL. 2013. Morphology, evolution and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia. 30:403–418. doi:10.1590/S1984-46702013000400006.
- Bertani R, Marques OAV. 1996. Defensive behaviors in mygalomorph spiders: release of urticating hairs by some Aviculariinae (Araneae, Theraphosidae). Zool Anz. 234:161–165.
- Blair WF. 1936. Ecological notes on Microhyla olivacea (Hallowell). Copeia. 1936:115. doi:10.2307/1436661.
- Bokma J. 2006. Rain frogs and a tarantula. [ accessed 2024 Mar 7]. http://www.johnbokma.com/mexit/2006/12/17/rain-frogs-and-a-tarantula.html.
- Canning G, Reilly BK, Dippenaar-Schoeman AS. 2014. Burrow structure and microhabitat characteristics of Nesiergus insulanus (Araneae: Theraphosidae) from Frégate Island, Seychelles. J Arachnol. 42(3):293–298. doi: 10.1636/J13-59.1.
- Chomphuphuang N, Smith D, Wongvilas S, Sivayyapram V, Songsangchote C, Warrit N. 2017. New species of Southeast Asian dwarf tarantula from Thailand: Phlogiellus Pocock, 1897 (Theraphosidae, Selenocosmiinae). ZooKeys. 684:57–73. doi: 10.3897/zookeys.684.12558.
- Cifuentes Y, Bertani R. 2022. Taxonomic revision and cladistic analysis of the tarantula genera Tapinauchenius Ausserer, 1871, Psalmopoeus Pocock, 1985, and Amazonius n. gen. (Theraphosidae, Psalmopoeinae). Zootaxa. 5101(1):1–123. doi: 10.11646/zootaxa.5101.1.1.
- Cifuentes Y, Perafán C. 2020. Arboreal tarantulas and their allies: Aviculariinae and Psalmopoeinae. In: Pérez-Miles F, editor. New world tarantulas. Zoological monographs. Vol. 6. Berlin/Heidelberg: Springer; p. 93–119.
- Cocroft RB, Hambler K. 1989. Observations on a commensal relationship of the microhylid frog Chiasmocleis ventrimaculata and the burrow theraphosid spider Xenesthis immanis in southeastern Peru. Biotropica. 21(1):2–8. doi: 10.2307/2388434.
- Cooke JAL, Roth VA, Miller FH. 1972. The urticating hairs of theraphosid spiders. Am Mus Novit. 2498:1–43.
- Costa C, Vanin SA. 2010. Coleoptera larval fauna associated with termite nests (Isoptera) with emphasis on the “bioluminescent termite nests” from Central Brazil. Psyche. 2010:723947. doi: 10.1155/2010/723947.
- Costa D, Carvalho RA, de Lima Filho GF, Brandão D. 2009. Inquilines and invertebrate fauna associated with termite nests of Cornitermes cumulans (Isoptera, Termitidae) in the Emas National Park, Mineiros, Goiás, Brazil. Sociobiology. 53(2B):443–453.
- Costa MC, Fenolio DB, Tonial IJ, Vieira V Jr, Silva HLR, Silva NJ Jr. 2006. Hyla albopunctata (spotted tree frog), H. multifasciata (many-banded tree frog), Barycholos ternetzi (Savage’s Goiás frog), Epipedobates flavopictus (Cerrado poison frog). Predation. Herpetol Rev. 37(3):337–338.
- Csakany JJ. 2003. Some aspects of the relationship between the Peruvian microhylid frog, Chiasmocleis ventrimaculata, and a theraphosid spider, Pamphobeteus sp [ Doctoral dissertation]. State University of New York College of Environmental Science and Forestry.
- Cushing PE. 2012. Spider–ant associations: an updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche. 23:151989. doi: 10.1155/2012/151989.
- Deyrup M, Deyrup L, Carrel J. 2013. Ant species in the diet of a Florida population of Eastern Narrowmouthed Toads, Gastrophryne carolinensis. Southeast Nat. 12:367–378. doi: 10.1656/058.012.0210.
- Dimijian GG. 2000. Evolving together: the biology of symbiosis, part 1. Bayl Univ Med Cent Proc. 13:217–226. doi:10.1080/08998280.2000.11927677.
- Duleba S, Ferreira VL. 2014. Herpetofauna associated with termite mounds in a pasture, Mato Grosso do Sul State, Brazil. Herpetol Bull. 127:10–16.
- Dundee HA. 1999. Gastrophryne olivacea (Great Plains Narrowmouth Toad). Aggregation with tarantula. Herpetol Rev. 30:91–92.
- Dundee HA, Shillington C, Yeary CM. 2012. Interactions between tarantulas (Aphonopelma hentzi) and narrow-mouthed toads (Gastrophryne olivacea): support for a symbiotic relationship. Tulane Stud Zool Bot. 32(1):31–38.
- Durkin ES, Cassidy ST, Gilbert R, Richardson EA, Roth AM, Shablin S, Keiser CN. 2021. Parasites of spiders: their impacts on host behavior and ecology. J Arachnol. 49(3):281–298. doi: 10.1636/JoA-S-20-087.
- Elgar MA. 1994. Experimental evidence of a mutualistic association between two web-building spiders. J Anim Ecol. 63:880–886. doi:10.2307/5265.
- Foelix R. 2011. Biology of spiders. New York: Oxford University Press.
- Foley S, Lüddecke T, Cheng D-Q, Krehenwinkel H, Künzel S, Longhorn SJ, Wendt I, von Wirth V, Tänzler R, Vences M, et al. 2019. Tarantula phylogenomics: a robust phylogeny of deep theraphosid clades inferred from transcriptome data sheds light on the prickly issue of urticating setae evolution. Mol Phylogenet Evol. 140:106573. doi: 10.1016/j.ympev.2019.106573.
- Frost DR. 2024. Amphibian species of the World: an online reference. Version 6.2. New York (USA): American Museum of Natural History; [ accessed 2024 May 13]. https://amphibiansoftheworld.amnh.org/index.php.
- Garton JD, Mushinsky HR. 1979. Integumentary toxicity and unpalatability as an antipredator mechanism in the narrow mouthed toad, Gastrophryne carolinensis. Can J Zool. 57(10):1965–1973. doi: 10.1139/z79-260.
- Gillung JP, Borkent CJ. 2017. Death comes on two wings: a review of dipteran natural enemies of arachnids. J Arachnol. 45(1):1–19. doi: 10.1636/JoA-S-16-085.1.
- Gray HM, Kaiser H, Green DM. 2010. Does alkaloid sequestration protect the green poison frog, Dendrobates auratus, from predator attack? Salamandra. 46:235–238.
- Haddad CR, Dippenaar-Schoeman AS. 2002. The influence of mound structure on the diversity of spiders (Araneae) inhabiting the abandoned mounds of the snouted harvester termite Trinervitermes trinervoides. J Arachnol. 30(2):403–408. doi: 10.13156/arac.2006.17.1.28.
- Hénaut Y, Machkour-M’Rabet S. 2020. Predation and other interactions. In: Pérez-Miles F, editor. New World tarantulas. Zoological monographs. Vol. 6. Berlin/Heidelberg: Springer; p. 237–270.
- Hirschi H. 1991. Biotopstudien, Beobachtungen und Überlegungen zur Lebensweise verschiedener Vogelspinnen-Arten in Costa Rica und Ecuador, Teil II. Arachnol Anz. 20:14–17.
- Hood ASC, Pashkevich MD, Dahlsjö CAL, Advento AD, Agung Ketut Aryawan A, Caliman JP, Naim M, Head JJ, Turner EC. 2020. Termite mounds house a diversity of taxa in oil palm plantations irrespective of understory management. Biotropica. 52(2):345–350. doi: 10.1111/btp.12754.
- Hooijer A. 2005. Interesting encounter in Tamarindo, Costa Rica. Br Tarantula Soc J. 20(4):120–123.
- Hunt RH. 1980. Toad sanctuary in a tarantula burrow. Nat Hist. 89:49–53.
- Kaderka R. 2020. Enemies and defences: urticating setae of Theraphosidae. In: Pérez-Miles F, editor. New World tarantulas. Zoological monographs. Vol. 6. Berlin/Heidelberg: Springer; p. 271–296.
- Kageyama A, Sugiura S. 2016. Caterpillar hairs as an anti-parasitoid defence. Sci Nat. 103:86. doi: 10.1007/s00114-016-1411-y.
- Karunarathna S, Kumarasinghe A, Perera N, Peabotuwage I, Abeywardene T, Pradeep G, Wickramaarachi S. 2012. Eine Symbiose zwischen einem Frosch (Microhylidae: Kaloula) und einer Spinne (Theraphosidae: Poecilotheria) in Sri Lanka. Sauria. 34(4):39–45.
- Karunarathna SDMS, Amarasinghe AT. 2009. Mutualism in Ramanella nagaoi Manamendra-Arachchi & Pethiyagoda, 2001 (Amphibia: Microhylidae) and Poecilotheria species (Aracnida [sic]: Thereposidae [sic]) from Sri Lanka. Taprobanica. 1:16–18. doi: 10.4038/tapro.v1i1.2772.
- Köchling P, Niebel A, Hurka K, Vorholt F, Hölscher H. 2020. On the multifunctionality of butterfly scales: a scaling law for the ridges of cover scales. Faraday Discuss. 223:195–206. doi: 10.1039/D0FD00038H.
- Langerhans R. 2008. Coevolution, and Fath B, editor. Encyclopedia of ecology. Oxford, UK: Academic Press, Elsevier; p. 644–648. doi: 10.1016/B978-008045405-4.00471-7.
- Lapinski W. 2019. The army ants and the tarantula – a rare encounter in a neotropical rainforest (Hymenoptera: Formicidae; Araneae: Theraphosidae). Entomol Z. 129:51–56.
- Leung TLF, Poulin R. 2008. Parasitism, commensalism, and mutualism: exploring the many shades of symbioses. Vie Et Milieu-Life Env. 58:107–115.
- Lourenço WR. 1978. Notas sobre a biologia de Acanthoscurria atrox Vellard, 1924 (Araneae: theraphosidae). Rev Bras Biol. 38:161–164.
- Lüddecke T, Krehenwinkel H, Canning G, Glaw F, Longhorn SJ, Tänzler R, Wendt I, Vences M. 2018. Discovering the silk road: nuclear and mitochondrial sequence data resolve the phylogenetic relationships among theraphosid spider subfamilies. Mol Phylogenet Evol. 119:63–70. doi: 10.1016/j.ympev.2017.10.015.
- Macedo KWR, Costa LJ, Costa LJ, Souza JO, Vasconcelos IA, Castro JS, Santana CJ, Magalhães AC, Castro MD, Pires OR. 2021. Brazilian Theraphosidae: a toxicological point of view. J Venom Anim Toxins Incl Trop Dis. 27:e20210004. doi: 10.1590/1678-9199-jvatitd-2021-0004.
- Margulis L, Fester R. 1991. Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Massachusetts: MIT Press.
- Miller G. 2003. Observations of commensalism between burrowing theraphosid spiders and the microhylid frog species, Hamptophrynes boliviana. CNR Student Research Symposium, Stevens Point, Wisconsin, College of Natural Resources, University of Wisconsin - Stevens Point: 24.
- Montemor VM, West RC, Zamani A, Moradmand M, von Wirth V, Wendt I, Huber S, Guadanucci JPL. 2020. Taxonomy of the genus Ischnocolus in the Middle East, with description of a new species from Oman and Iran (Araneae: Theraphosidae). Zool Middle East. 66(1):76–90. doi: 10.1080/09397140.2020.1675994.
- Mulvany PS. 1983. Tarantula preys on a western ribbon snake with notes on the relationship between narrow-mouth toads and tarantulas. Bull Oklahoma Herpetol Soc. 8:91–99.
- Nguyen PL, van Baalen M. 2020. On the difficult evolutionary transition from the free-living lifestyle to obligate symbiosis. PLOS ONE. 15(7):e0235811. doi: 10.1371/journal.pone.0235811.
- Nyffeler M, Altig R. 2020. Spiders as frog-eaters: a global perspective. J Arachnol. 48(1):26–42. doi: 10.1636/0161-8202-48.1.26.
- Orivel J, Leroy C. 2011. The diversity and ecology of ant gardens (Hymenoptera: formicidae; Spermatophyta: angiospermae). Myrmecol News. 14:73–85.
- Pérez-Miles F, Perafán C. 2020. Theraphosinae. In: Pérez-Miles F, editor. New World tarantulas. Zoological monographs. Vol. 6. Berlin/Heidelberg: Springer; p. 121–151.
- Pérez-Miles F. 2020. Introduction to the Theraphosidae. In: Pérez-Miles F, editor. New World tarantulas. Zoological monographs. Vol. 6. Berlin/Heidelberg: Springer; p. 1–23.
- Powell R, Little LW, Smith DD. 1984. Eine Wohngemeinschaft von Physalaemus pustulosus (Cope, 1864) (Salientia: leptodactylidae) mit einer bodenbewohnenden Vogelspinne. Salamandra. 20:273–274.
- Samadi J-P. 2009. Auf Spinnensuche in Ecuador - Teil 2. Arachne. 14(3):12–33.
- Samadi J-P. 2014. Von spinnen und fröschen: beobachtungen in Panama. Arachne. 19(2):4–14.
- Sankaran PM, Sebastian PA. 2018. A new synonym in the subfamily Thrigmopoeinae Pocock, 1900 (Araneae, Theraphosidae). ZooKeys. 749:81–86. doi: 10.3897/zookeys.749.23414.
- Schalk CM, Sezano M. 2014. Observations on the use of tarantula burrows by the anurans Leptodactylus bufonius (Leptodactylidae) and Rhinella major (Bufonidae) in the Dry Chaco ecoregion of Bolivia. Acta Herpetol. 9(1):99–102. doi: 10.13128/Acta_Herpetol-13606.
- Sherwood D, Gabriel R. 2020. A new species of Umbyquyra Gargiulo, Brescovit & Lucas, 2018 from Goiás, Brazil (Araneae: Theraphosidae). Arachnology. 18(6):619–622. doi: 10.13156/arac.2020.18.6.619.
- Sherwood D, Gabriel R, Peñaherrera-R P, León-E RJ, Cisneros-Heredia DF, Brescovit AD, Lucas SM. 2023. Pamphobeteus Pocock, 1901: redescriptions, new species and records, description of a missing sex, with taxonomic notes and changes in Megaphobema Pocock, 1901 (Araneae: Theraphosidae). Arachnology. 19(6):894–930. doi: 10.13156/arac.2023.19.6.894.
- Shorthouse DP. 2010. SimpleMappr, an online tool to produce publication-quality point maps; [ accessed 2024 May 13]. http://www.simplemappr.net.
- Siliwal M, Ravichandran B. 2008. Commensalism in microhylid frogs and mygalomorph spiders. Zoos’ Print. 23(8):13.
- Smith AM. 1990. Baboon spiders: tarantulas of Africa and the Middle East. London: Fitzgerald Publishing.
- Spider keeping frogs as pets. 2017. SANSA News; [ accessed 2024 May 13]. https://www.arc.agric.za/arc-ppri/Newsletter%20Library/SANSA%20News,%20Issue%2029,%20May-Oct%202017.pdf
- Sugiura S, Yamazaki K. 2014. Caterpillar hair as a physical barrier against invertebrate predators. Behav Ecol. 25:975–983. doi: 10.1093/beheco/aru080.
- Szelistowski WA. 1985. Unpalatability of the poison arrow frog Dendrobates pumilio to the ctenid spider Cupiennius coccineus. Biotropica. 17(4):345–346. doi: 10.2307/2388601.
- Taylor RL. 1975. Butterflies in my stomach or: insects as human nutrition. California: Woodbridge Press Publishing Company.
- Uetz P, Freed P, Aguilar R, Hošek J, editors. 2023. The reptile database. [accessed 2024 May 19]. http://www.reptile-database.org.
- Vieira RS, Höfer H. 1994. Prey spectrum of two army ant species in central Amazonia, with special attention on their effect on spider populations. Andrias. 13:189–198.
- von May R, Biggi E, Cárdenas H, Isabel Diaz M, Alarcón C, Herrera V, Santa-Cruz R, Tomasinelli F, Westeen EP, Sánchez-Paredes CM, et al. 2019. Ecological interactions between arthropods and small vertebrates in a lowland Amazon rainforest. Amphib Reptile Conserv. 13(1):65–77.
- West RC. 2005. The Brachypelma of Mexico. Br Tarantula Soc J. 20(4):108–119.
- WSC. 2024. World spider catalog. Version 25.0. Bern: Natural History Museum; [ accessed 2024 Mar 7]. http://wsc.nmbe.ch.
- Yamamura N, Higashi M, Behera N, Wakano JY. 2004. Evolution of mutualism through spatial effects. J Theor Biol. 226(4):421–428. doi: 10.1016/j.jtbi.2003.09.016.
- Yeary CM. 1979. Support for a mutualistic relationship between the Western narrow-mouthed frog, Gastrophryne olivacea, and the tarantula, Dugesiella hentzii [ Master’s thesis]. Oklahoma: University of Tulsa.
