Abstract
From a viewpoint of direct separation of trivalent minor actinides (MA: Am, Cm etc.) from fission products (FP) including rare earths (RE) in high level radioactive liquid waste, the authors have developed a simplified separation process using a single column packed with novel extraction adsorbents. Attention was paid to a new type of nitrogen-donor ligand, R-BTP (2,6-bis(5,6-dialkyl-1,2,4-triazin-3-yl)pyridine, R: alkyl group) as an extractant because it has higher extraction selectivity for Am(III) than RE(III). Since the R-BTP ligands show different properties such as adsorbability and stability when they have different alkyl groups, several R-BTP extraction adsorbents were prepared by impregnating the R-BTP ligands with different alkyl groups (isohexyl-, isoheptyl- and cyheptyl-BTP) into a porous silica/polymer composite support (SiO2-P particles). This work investigated: (1) fundamental properties of the synthesized R-BTP/SiO2-P adsorbents, (2) adsorption and desorption properties of Am and FP in nitric acid solution and water using the adsorbents in a batch experiment, (3) radiolytic and chemical stabilities of the adsorbents, and (4) the possibility for developing a simplified separation process of MA using the most promising adsorbent (isohexyl-BTP/SiO2-P) under temperature control between 25 and 50°C.
1. Introduction
For minimizing the long-term radiotoxicity and facilitating nuclear waste management and disposal, numerous efforts have been made for recovery of trivalent minor actinides (MA: 241,242m,243Am, 243,244Cm etc.) from fission products (FP) in high level radioactive liquid waste (HLLW). Various ligands have been actively developed for the extraction process to separate the MA from FP including trivalent rare earths (RE) [Citation1]. Due to the similarity in chemical properties between MA(III) and RE(III), the selective extraction of MA(III) over RE(III) is one of the challenging issues, especially in acidic media.
As a potential candidate to be used in ≥1 mol/dm3 M HNO3, new type nitrogen-donor ligands, R-BTP (BTP: 2,6-bis-(5,6-dialkyl-1,2,4-triazin-3-yl)-pyridine, R: alkyl group), which were first utilized to separate MA(III) from RE(III) as extractants by Kolarik et al. [Citation2,Citation3], have received wide interest due to their higher extraction selectivity for Am(III) over RE(III) (the separation factor (SF) between Am and Eu: 100–150). In addition, the ligands consist of the elements C, H, O, and N only (CHON principle), which means they can be incinerated when they are no longer usable. Recently, Ekberg et al. [Citation4] reviewed various nitrogen-donor ligands for extraction and separation of MA(III) from RE(III), and pointed out that the R-BTP solvent extraction system exhibited the above excellent property but had a slow kinetic rate and instability to radiolytic and chemical degradation.
In order to overcome the demerits of the solvent extraction method and to use the merits of R-BTP extractants, an alternative technology, extraction chromatography, which uses a minimal organic solvent (much less extractant and diluent) in compact equipment, has been proposed for separation of MA(III). Furthermore, several R-BTP extraction adsorbents with different alkyl groups has been synthesized by impregnating the R-BTP extractants into a macroporous silica/polymer composite support (SiO2-P particles), and their fundamental properties have been investigated [Citation5–Citation10]. It was found that the separation property and stability of the R-BTP/SiO2-P adsorbents depended on the structure of the alkyls, i.e., number of carbon atoms (length of chain) and branching of the chain. SFs between Am(III) and Eu(III) are relatively high (up to 100) in the case of normal R-BTP/SiO2-P adsorbents with straight 3–7 carbon atom chains [Citation6,Citation7]. On the other hand, a branched R-BTP/SiO2-P (for example, isobutyl-BTP/SiO2-P) adsorbent is more stable than normal R-BTP/SiO2-P adsorbents in ≤3 M HNO3 solution [Citation7,Citation10].
The objective of this work is to directly separate trivalent MA from FP including RE in HLLW by extraction chromatography using new type R-BTP/SiO2-P adsorbents and eluents composed of nitric acid and water in order to establish a single-column MA separation process. In the authors’ previous articles [Citation11,Citation12], isohexyl-BTP/SiO2-P adsorbent (R: 4-methylpentyl) was prepared as a novel R-BTP adsorbent. The adsorption property of RE(III) in HNO3 solution on the adsorbent and its desorption property with water were evaluated by batch method using Dy(III) as a substitute for Am(III). In addition, the chemical stability and thermal decomposition property of the R-BTP adsorbents were examined. Quite recently, researchers of Japan Atomic Energy Agency (JAEA) have also investigated separation of Am(III) and Cm(III) using isohexyl-BTP/SiO2-P adsorbent by batch and column methods and demonstrated selective recovery of MA(III) by adjusting the acidity of the feed solution to 1 M HNO3 and/or by utilizing a complexing agent such as diethylenetriaminepentaacetic acid (DTPA) as eluent [Citation13–Citation15].
In this article, not only isohexyl-BTP/SiO2-P but also isoheptyl-BTP/SiO2-P (R: 3-methylhexyl) and cyheptyl-BTP/SiO2-P (R: 2-cyclopentylethyl) adsorbents were prepared. Then, their fundamental and adsorption/desorption properties as well as radiolytic/chemical stabilities were investigated and compared. Furthermore, the single-column separation process of MA from FP including RE was demonstrated by evaluation of the column experiment using the most promising R-BTP/SiO2-P adsorbent under temperature control between 25 and 50°C.
2. Experimental
2.1. Synthesis of silica-based R-BTP adsorbents
Three types of R-BTP/SiO2-P adsorbents, (1) isohexyl-BTP/SiO2-P (2,6-bis(5,6-bis(4-methylpentyl)- 1,2,4-triazin-3-yl)pyridine), (2) isoheptyl-BTP/SiO2-P (2,6-bis(5,6-bis(5-methylhexyl)-1,2,4-triazin-3-yl)pyridine), and (3) cyheptyl-BTP/SiO2-P (2,6-bis(5,6-bis(2-cyclopentylethyl)-1,2,4-triazin-3-yl)pyridine) were prepared by impregnating the respective R-BTP ligands (or extractants, see ) into the macroporous silica/polymer composite support (SiO2-P particles) [Citation5–Citation9]. The support contained macroreticular styrene-divinylbenzene copolymer which was immobilized in porous silica particles (mean diameter: 60 μm).
Purity of the respective extractants was measured by high performance liquid chromatography. Adsorptive capacity was evaluated using the isotherm plotting between Dy content (mmol/g) in the adsorbent and the equilibrated concentration (M) in aqueous phase. The highest value of the metal content was selected as capacity of the adsorbent. Thermal decomposition properties of the R-BTP/SiO2-P adsorbents were examined by thermogravimetry-differential thermal analysis (TG-DTA) up to 600°C after the adsorbents were washed with H2O and dried at 35–40°C in vacuum overnight.
2.2. Evaluation method for adsorption/desorption and separation properties of Am(III) and main FP
Distribution ratio between aqueous and solid phases was defined as distribution coefficient, Kd , obtained by the batch method for evaluating adsorption property:
In the batch adsorption experiment, a weighed amount of the adsorbent (0.1–1 g) was combined in a glass vial with Teflon stopper with a measured volume of an aqueous solution (5–10 mL, 0.01–3 M HNO3) including simulated FP elements (0.1–1 mM). The resultant mixture was maintained at constant temperature (mainly 25 and 50°C) and shaken mechanically at 160 rpm. The solution was separated from the adsorbent by vacuum filtration and sampled for analysis.
Desorption behavior was examined in the batch desorption experiment by mixing the solid phase (R-BTP/SiO2-P adsorbent which had previously adsorbed metal ions in 3 M HNO3 solution) with H2O at 25 and 50°C. The desorption kinetic rate was evaluated by analyzing concentration of the metal ions in aqueous phase.
To evaluate the separation performance, the column experiment using a glass column (φ8 mm × h150 mm or φ10 mm × h120 mm) with a glass jacket to maintain constant temperature (25 and 50°C) was done by packing a weighed amount of the adsorbent (4–5 g) in the column. Elution curves were drawn using the weight of effluent versus the ratio of C (metal concentration in effluent) to C 0 (metal concentration in initial solution).
The concentrations of the simulated FP (Cs, Sr, Zr, Mo, Ru, Rh, Pd, etc.) including RE (Y, La, Ce, Nd, Eu, Gd, Dy, etc.) in solution were analyzed by inductively coupled plasma-optical emission spectrometry and atomic absorption spectrometry. The radioactivities of 241Am and 99Tc were determined by well type NaI(Tl) scintillation spectrometry and liquid scintillation counting, respectively.
The acidity of aqueous phase in the batch desorption experiment and effluent in the column experiment was ascertained with a pH meter.
2.3. Evaluation method for radiolytic and chemical stabilities of R-BTP/SiO2-P adsorbents
The weighted R-BTP/SiO2-P adsorbents (0.5–1 g) were contacted with 0.01–3 M HNO3 solution (10–20 mL). For evaluation of radiolytic stability, the adsorbents were irradiated with γ-rays up to about 120 d at room temperature by utilizing a 60Co source (dose rate: 35–40 Gy/h). The Kd values of RE(III) on the irradiated R-BTP/SiO2-P adsorbents were compared with those on the nonirradiated adsorbents under similar conditions as control samples. Leakage of the R-BTP extractants from the absorbents or degradation of the extractants over the irradiation period was obtained by measuring the concentration of total organic carbon (TOC) in aqueous phase by TOC analysis. For evaluation of the stability against HNO3 (chemical stability), the extractant leakage or the degradation was determined at different HNO3 concentrations (≤5 M) and temperatures (≤80°C). Concentrations of leaked R-BTP extractants were obtained by extracting them into 1,2-dichloroethane and detecting by UV/VIS (ultraviolet/visible) spectrophotometry.
3. Results and discussion
3.1. Fundamental properties of the synthesized R-BTP/SiO2-P adsorbents
Appearance, chemical structure and bonding state of the synthesized R-BTP/SiO2-P adsorbents were verified by analyzing the adsorbents as products, R-BTP ligands as extractants and SiO2-P particles as composite support by scanning electron microscopy, Fourier transform infrared spectrophotometry, and/or nuclear magnetic resonance spectroscopy [Citation11,Citation12].
summarizes purity of the respective R-BTP extractants and adsorption capacity of the R-BTP/SiO2-P adsorbents for Dy(III) in 3–4 M HNO3 solution. The extractants had almost the same purity (97–99%). With respect to capacity of Dy(III) adsorbed on the adsorbents, it was found that the isohexyl-BTP/SiO2-P absorbent had the highest capacity (0.12 mmol/g in 3 M HNO3) among them.
Table 1. Purity of the respective R-BTP extractants and adsorption capacity of the R-BTP/SiO2-P adsorbents for Dy(III).
Thermal decomposition properties of isoheptyl- and cyheptyl-BTP/SiO2-P adsorbents were almost the same as those of isohexyl-BTP/SiO2-P adsorbent [Citation11]. shows weight composition of the adsorbent obtained by TG-DTA. The weight percentage of all extractants was 33–34%, and such high content results in sufficient adsorption capacity for target ions.
Table 2. Weight composition of the R-BTP/SiO2-P adsorbents obtained by TG-DTA.
3.2. Adsorption and desorption properties of Am(III) and main FP
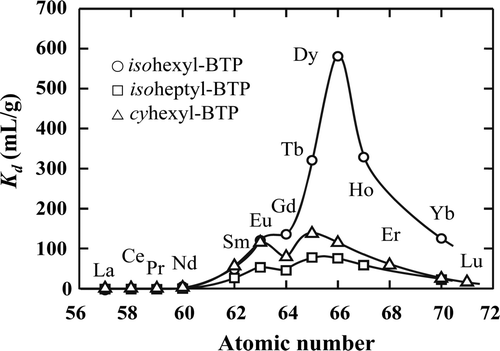
Previously, it was reported that trace amounts of Dy(III) had much higher Kd value on isohexyl-BTP/SiO2-P adsorbent than other RE(III) in 2–4 M HNO3 so that Dy(III) could be utilized as a substitute ion for Am(III) and Cm(III) from the viewpoint of separation [Citation11]. compares the Kd values of various RE(III) on isohexyl-, isoheptyl- and cyheptyl-BTP/SiO2-P adsorbents. The isoheptyl- and cyheptyl-BTP/SiO2-P adsorbents also showed the tendency that intermediate-to-heavier RE(III) had rather high Kd values, with isohexyl-BTP/SiO2-P adsorbent being the most conspicuous. This behavior suggests that selective separation of Dy(III) from the other RE(III) is possible.
Figure 2. Distribution coefficients (Kd ) of RE(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 solution (0.1 mM RE(III), phase ratio: 0.25 g/10 cm3, contact time: 72 h, temperature: 20°C).
The adsorption property of Am(III) was evaluated in 0.1–3 M HNO3 solution on the R-BTP/SiO2-P adsorbents at 25 and 50°C. In 2–3 M HNO3, the Kd values of Am(III) for a 3 h contact time were much higher than those of all the FP elements except Pd(II), which had the dominantly high value on the adsorbents. shows the Kd of Am(III) and SF of Am/Eu for the respective adsorbents. Adsorbability of Am(III) and the separation property between Am(III) and RE(III) seemed to be excellent for all the adsorbents, especially for isohexyl-BTP/SiO2-P adsorbent which exhibited the highest Kd value for Am(III). shows the Kd values of RE(III) for the R-BTP/SiO2-P adsorbents in 3 M HNO3 solution at 25 and 50°C. Generally the adsorption reaction is an exothermic reaction and higher temperature results in weaker adsorption, but the observed Kd values of RE(III) at 50°C were much increased, becoming several times more than those at 25°C due to the rapid adsorption kinetic rate within the contact time. Therefore, it is presumed that the adsorbability of Am(III) has the same tendency with temperature as that of RE(III).
Table 3. Distribution coefficients (Kd ) of Am(III) and separation factors (SF) between Am(III) and Eu(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 at 25°C.
Table 4. Distribution coefficients (Kd ) of RE(III) on R-BTP/SiO2-P adsorbents in 3 M HNO3 solution.
This unique adsorption behavior of Am(III) and RE(III) on the R-BTP/SiO2-P adsorbents at 25°C resembled that in the cyMe4-BTBP (6,60-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[Citation1,Citation2,Citation4]triazin-3-yl)-[Citation2,20]bipyridine) solvent extraction system reported by Geist et al [Citation16]. They observed such behavior in the extraction of Am(III), Cm(III) and RE(III) from 1 M HNO3 at (20 ± 0.5)°C using cyMe4-BTBP ligand and suggested that the size fit of the ligand cavity was preferable for the intermediate-to-heavier lanthanide cations.
On the other hand, it was found that the adsorption and desorption kinetic rate of RE(III) for isohexyl-BTP/SiO2-P adsorbent at 25°C was considerably slow [Citation11,Citation12]. Unfortunately, the rate for isoheptyl- and cyheptyl-BTP/SiO2-P adsorbents were much slower than that for isohexyl-BTP/SiO2-P adsorbent.
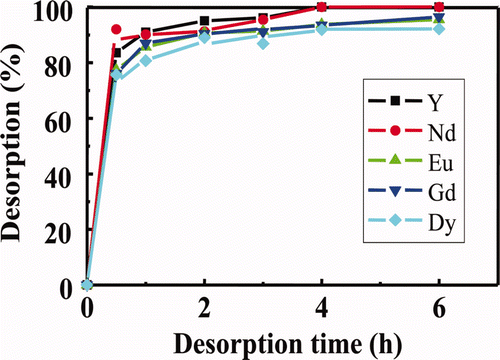
shows the effects of contact time on desorption of several RE(III) ions loaded in isohexyl-BTP/SiO2-P adsorbent with H2O at 50°C. Since the desorption kinetic rate of the ions at 50°C was more rapid than that at 25 C, more effective elution of the ions from the column packed with the adsorbent is expected at the elevated temperature.
3.3. Radiolytic and chemical stabilities of R-BTP/SiO2-P adsorbents
The radiolytic and chemical stabilities of R-BTP/SiO2-P adsorbents were evaluated by comparing the adsorbability of RE(III) and leaked amounts of the R-BTP extractants from the adsorbents between the irradiated and nonirradiated absorbents, on contact with HNO3 solution.
shows the effect of γ-ray irradiation on Kd of Dy(III) with isohexyl-BTP/SiO2-P adsorbent in 0.01 M HNO3 solution. The Kd values of Dy(III) increased with irradiation dose at the beginning and then declined with further increase in irradiation dose. It was found that the adsorbability of other ions such as Eu(III) and Gd(III) also showed similar changes with irradiation. However, except for the above results in dilute acid solution (0.01 M), all the other experimental results with irradiation at higher HNO3 concentrations (0.1M, 1M, and 3M) indicated that the Kd values for three type of the R-BTP/SiO2-P adsorbents decreased significantly with irradiation dose. It is difficult to explain the phenomena that the Kd values of RE(III) in 0.01 M HNO3 solution increased with γ-ray irradiation. For similar cases, it was reported that Kd values of Am(III) and RE(III) remain constant or increase in the solvent extraction system at low irradiation dose due to the formation of better extracting radiolysis products [Citation17,Citation18].
Figure 4. Effects of γ-ray irradiation on Kd of Dy(III) with isohexyl-BTP/SiO2-P adsorbent in 0.01 M HNO3 solution (dose rate: ≈40 Gy/h, room temperature).
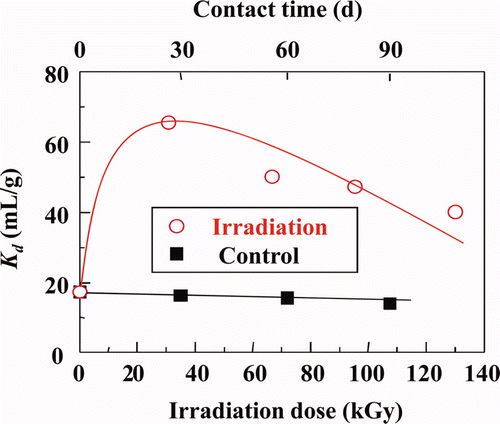
On the other hand, for non-irradiation isohexyl-BTP/SiO2-P adsorbent, the Kd values of RE(III) (Eu, Gd, and Dy) were steadily maintained at an equilibrium state even in 3 M HNO3 solution for 20 d [Citation11], so the adsorbent is considered to have relatively good resistance against HNO3 solution.
shows the effects of γ-ray irradiation on leakage of isohexyl-BTP extractant from isohexyl-BTP/SiO2-P adsorbent into 3M HNO3 solution as a typical example. In the case without irradiation, the leaked amount of isohexyl-BTP increased only slightly with contact time, indicating that the adsorbent was relatively stable in 3M HNO3. However, it increased drastically with irradiation dose (i.e. contact time), revealing that the decomposition or degradation of the adsorbent was considerably enhanced by γ-ray irradiation in 3M HNO3.
Figure 5. Effects of γ-ray irradiation on leakage of isohexyl-BTP extractant from isohexyl-BTP/SiO2-P adsorbent to 3M HNO3 solution (dose rate: ≈40 Gy/h, room temperature).
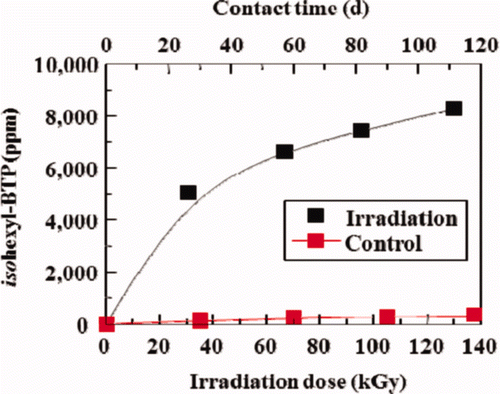
Leakage behavior of the isohexyl-BTP extractant from isohexyl-BTP/SiO2-P adsorbent containing 3 M HNO3 was previously checked [Citation11]. It was found that the leaked amount gradually increased with concentration of HNO3 at 50°C and the leakage fraction was <0.3% (20–50 ppm of the extractant), while the fraction was about 0.1% (10–25 ppm of the extractant) at 25°C. It has been confirmed that the branched isohexyl-BTP/SiO2-P adsorbent was more stable even in 2–4 M HNO3 [Citation7,Citation10], compared with normalhexyl-BTP/SiO2-P adsorbent at 25°C, from which the leakage was 1.8% at 3 M HNO3.
Concerning the chemical stability of the R-BTP/SiO2-P adsorbents against HNO3 solution, the leakage was examined for a broad range of conditions. shows leakage of the R-BTP extractants from the adsorbents in contact with 1 and 5 M HNO3 solution at room temperature, 50 and 80°C during 1 week and 1 month. The amounts of leaked R-BTP extractants increased with concentration of HNO3, temperature and contact time. Such degradation was also confirmed by observing UV-Vis spectra of the leaked R-BTP extractants and thermal decomposition properties of the degraded R-BTP/SiO2-P adsorbents. The leakage is considered to result from the solubility of the extractant in aqueous solution due to protonation and was also observed in solvent extraction [Citation3]. The amount of isohexyl-BTP extractant in 1M HNO3 during 1 week at 80°C was 66 times higher than that at room temperature and estimated to be <1% (0.6%) of the extractant impregnated into the isohexyl-BTP/SiO2-P adsorbent.
Table 5. Leakage of the R-BTP extractants from the R-BTP/SiO2-P adsorbents in contact with HNO3 solution.
3.4. Single-column separation behaviors of Am(III) and main FP
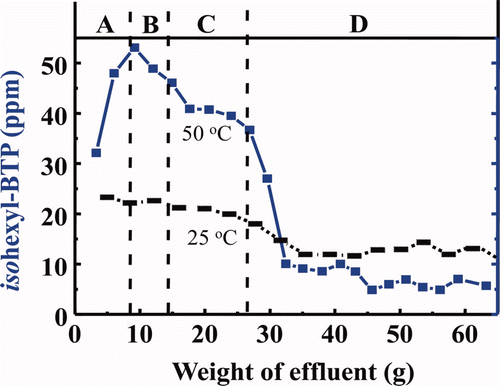
From the above results, the isohexyl-BTP/SiO2-P adsorbent was selected for single-column separation of MA(III) from FP because it had the most promising properties among the synthesized adsorbents. and are elution curves of various metal ions in simulated HLLW obtained in the column experiment using a column packed with isohexyl-BTP/SiO2-P adsorbent at 25 and 50°C, respectively.
Figure 6. Elution curves of various metal ions in simulated HLLW in a column experiment using isohexyl-BTP/SiO2-P adsorbent at 25°C (concentration of stable metal ions: 1 mM, 241Am and 99Tc: trace amounts, column size: φ8 mm × h150 mm, A: dead volume (3M HNO3), B: feed solution (3M HNO3), C: washing solution (3M HNO3), and D: eluting solution (H2O), flow rate: 0.25 cm3/min).
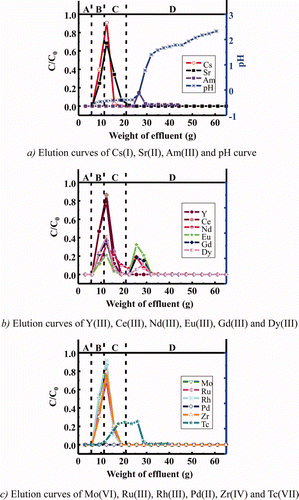
Figure 7. Elution curves of various metal ions in simulated HLLW in a column experiment using isohexyl-BTP/SiO2-P adsorbent at 50°C (concentration of stable metal ions: 1 mM, 241Am and 99Tc: trace amounts, column size: φ10 mm × h120 mm, A: dead volume (3M HNO3), B: feed solution (3M HNO3), C: washing solution (3M HNO3), and D: eluting solution (H2O), flow rate: 0.25 cm3/min).
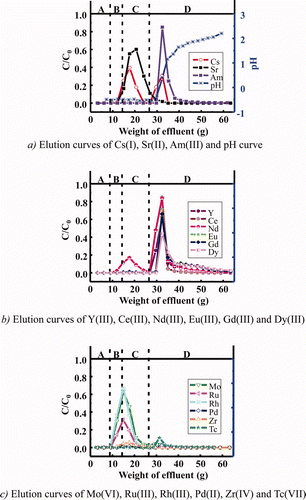
In the column experiment at 25°C, Am was separated from alkali (Cs(I)), alkali earth (Sr(II)) (see a), light RE(III) (seei b) and other FP (Zr(IV), Mo(VI), Ru(III), and Rh(III)) (seei c). However, separation of Am(III) from intermediate and heavy RE(III) was not enough. In addition, recovery yield of Am with H2O was extremely low (14%) probably due to the slow desorption kinetic rate. Since the pH values immediately increased at eluting Am(III) with H2O (see a), some hydrolysis of Am nitrate may be occurring at 25°C [Citation14]. On the other hand, in the column experiment at 50°C, Am(III) was not separated sufficiently from light RE(III) and even Cs(I) with 3 M HNO3 solution (see a–c), but the recovery yield of Am with H2O was much improved (≈70%) principally due to the rapid desorption kinetic rate.
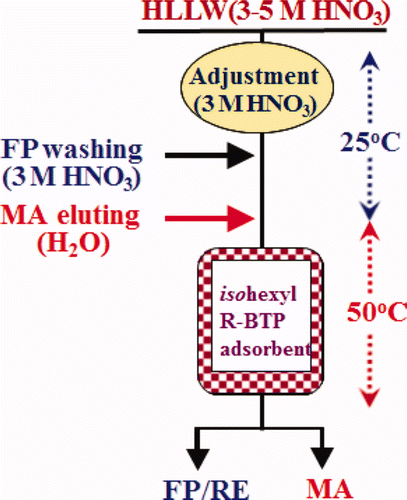
The above results (see a–c and 7a) show the potentiality for the successful separation of Am(III) from the main FP, which can be realized by charging feed solution and washing FP in 3 M HNO3 at 25°C and by eluting Am(III) with H2O at 50°C as shown in . From this figure, scheme for single-column separation of MA(III) under temperature control can be successfully proposed as “a simplified separation process of trivalent MA” as shown in . The use of 3 M HNO3 at 25°C for feed and washing are expected to diminish radiolytic and chemical damage to the adsorbent due to the elimination of 137Cs and 90Sr at an early separation stage.
Figure 8. Estimated elution curves of Am and some FP ions using isohexyl-BTP/SiO2-P adsorbent under temperature control (A: dead volume, B: feed soln., C: washing soln., D: eluting soln., flow rate: 0.25 mL/min, C0 : initial concentration, C: concentration in effluent).
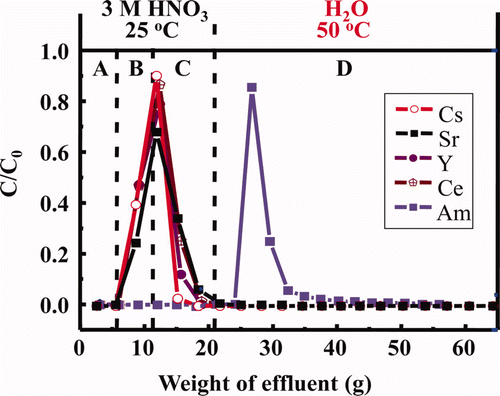
Figure 9. Single-column separation of MA(III) under temperature control (a simplified separation process of trivalent MA) (FP: Cs, Sr, RE, Zr, Mo, etc., RE: Y, La, Ce, Pr, Nd, Sm, Eu etc., MA: Am, Cm).
Under the elution conditions shown in , however, washing of intermediate and heavy RE(III) with 10 mL of 3 M HNO3 was insufficient as seen in b. For more effective separation of Am(III) from the RE(III), sufficient washing with HNO3 (larger quantity and/or lower concentration) is required. In addition, for more complete recovery of Am retained in the column, complexing agents such as 0.01–0.05 M DTPA, instead of H2O, may be necessary as an eluting solution [Citation14]. Since Pd(II) was strongly adsorbed in the column, it may be recovered finally with 0.1 M thiourea (CH4N2S) solution at 50°C [Citation12]. Furthermore, the recovery of 99Tc(VII) was extremely low (≤10%) in the column experiment at 25 and 50°C due to its complicated adsorption/desorption behaviors as ascertained in the batch experiment [Citation11].
compares the leakage behaviors of isohexyl-BTP extractant, which were simultaneously obtained in the column experiment at 25 and 50°C. The leakage at 50°C was about twice as high as that at 25°C. The total amount of the leaked extractant was <0.5%.
4. Conclusions
Three types of silica-based R-BTP adsorbents for extraction chromatography were synthesized with the objective of direct recovering MA(III) from FP in HLLW. Then, a simplified separation process of MA(III) by using the synthesized adsorbents was developed.
Adsorption/desorption properties of target metal ions and radiolytic/chemical stabilities of the novel R-BTP/SiO2-P adsorbents were examined by the batch method. The isohexyl-BTP/SiO2-P adsorbent was selected as the most promising adsorbent. Regarding isoheptyl- and cyheptyl-BTP/SiO2-P adsorbents, desired improvement was not obtained, especially for the slow adsorption/desorption kinetic rate at 25°C. Although all adsorbents were relatively stable against 3 M HNO3 solution even at elevated temperature, they were generally unstable against γ-ray irradiation except for the case of the isohexyl-BTP/SiO2-P adsorbent in dilute HNO3 (0.01 M).
In the column experiment using isohexyl-BTP/SiO2-P adsorbent at 25°C, Am(III) was selectively separated from the main FP including light RE(III) but the recovery yield of Am with H2O was extremely low probably due to the slow desorption kinetic rate and hydrolysis. At 50°C, the recovery yield of Am became much higher due to the rapid desorption kinetic rate. The detected leakage of isohexyl-BTP extractant from the adsorbent was small (≤0.5%) in the column experiment. Consequently, the simplified separation process of MA(III) from the main FP including RE(III) was successfully demonstrated by the evaluation of column experiment using isohexyl-BTP/SiO2-P adsorbent under temperature control between 25 and 50°C.
In actual HLLW, not only Pd(II) and 99Tc(VII) as the main FP but also small amounts of other actinides such as U(VI), Pu(IV) and Np(V) may exist and the optimum conditions for separating MA(III) from such ions should be considered. As future work, the process needs to be demonstrated for actual HLLW. In addition, development of more endurable adsorbents against severe radiolysis is necessary.
Acknowledgements
A part of this study is results of the project “Development of a Simplified MA Separation Process Using Novel R-BTP Adsorbents” carried out under the Strategic Promotion Program for Basic Nuclear Research by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Additional information
Notes on contributors
Yuezhou Wei
†Present address: School of Engineering, Tohoku University, Aoba 6-6-01-2, Aramaki, Aoba-ku, Sendai 980-8578, Japan. ††Present address: School of Nuclear Science and Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China.References
- Van Hecke , K. and Goethals , P. 2006 . Research on advanced aqueous reprocessing of spent nuclear fuel: literature study . SCK.CEN-BLD-1030 ,
- Kolarik , Z. , Mullich , U. and Gassner , F. 1999 . Selective extraction of Am(III) over Eu(III) by 2,6-ditriazoyl- and 2,6-ditriazinylpyridines . Solv. Extr. Ion Exch , 17 : 23 – 32 .
- Kolarik , Z. , Mullich , U. and Gassner , F. 1999 . Extraction of Am(III) and Eu(III) nitrates by 2,6-di-(5,6-dipropyl-1,2,4-triazine-3-yl-) pyridines . Solv. Extr. Ion Exch , 17 : 1155 – 1170 .
- Ekberg , C. , Fermvik , A. , Retegan , T. , Skarnemark , G. , Foreman , M. R.S. , Hudson , M. J. , Englund , S. and Nilsson , M. 2008 . An overview and historical look back at the solvent extraction using nitrogen donor ligands to extract and separate An(III) from Ln(III) . Radiochim. Acta , 96 : 225 – 233 .
- Wei , Y.-Z. , Hoshi , H. and Kumagai , M. A hot test on minor actinides/lanthanides separation from HLLW using an R-BTP extraction resin . Proceedings of 16th Pacific Basin Nuclear Conference (16PBNC) . Oct 13–18 . Paper ID P16P 1033
- Wei , Y.-Z. , Hoshi , H. , Kumagai , M. , Asakura , T. and Morita , Y. 2004 . Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinyl pyridine extraction chromatography for radioactive waste management . J. Alloys Comp , 374 : 447 – 450 .
- Wei , Y.-Z. , Hoshi , H. , Kumagai , M. , Asakura , T. and Uchiyama , G. 2002 . Preparation of novel silica-based R-BTP extraction-resins and their application to trivalent actinides and lanthanides separation . J. Nucl. Sci. Technol. Suppl , 3 : 761 – 764 .
- Wei , Y.-Z. , Sabharwal , K. N. , Kumagai , M. , Asakura , T. , Uchiyama , G. and Fujine , S. 2000 . Preparation of novel silica-based nitrogen donor extraction resins and their adsorption performance for trivalent americium and lanthanides . J. Nucl. Sci. Technol , 37 : 1108 – 1109 .
- Zhang , A. , Kuraoka , E. and Kumagai , M. 2007 . Preparation of a novel macroporous silica-based 2,6-bis(5,6-diisobutyl-1,2,4-triazine-3-yl)pyridine impregnated polymeric composite and its application in the adsorption for trivalent rare earths . J. Radioanal. Nucl. Chem , 274 : 455 – 464 .
- Hoshi , H. , Wei , Y.-Z. , Kumagai , M. , Asakura , T. and Morita , Y. 2006 . Separation of trivalent actinides from lanthanides by using R-BTP resins and stability of R-BTP resin . J. Alloys Comp , 408–412 : 1274 – 1277 .
- Usuda , S. , Liu , R. , Wei , Y.-Z. , Xu , Y. , Yamazaki , H. and Wakui , Y. 2010 . Evaluation study on properties of a novel R-BTP extraction resin−From a viewpoint of simple separation of minor actinides . J. Ion Exch , 21 : 35 – 40 .
- Liu , R. , Wei , Y.-Z. , Xu , Y. , Usuda , S. , Yamazaki , H. and Ishii , K. in press . Evaluation study on properties of isohexyl-BTP/SiO2-P resin for direct separation of trivalent minor actinides from HLLW . J. Radioanal. Nucl. Chem ,
- Surugaya , N. , Sano , Y. , Yamamoto , M. , Kurosawa , A. and Hiyama , T. Extraction chromatographic separation of trivalent minor actinides using Hex-BTP/SiO2-P resin . American Chemical Society, ACS Symposium Series 1046 (Nuclear Energy and the Environment) (ISBN: 978-0-8414-2585-5) . pp. 131 – 139 .
- Sano , Y. , Surugaya , N. and Yamamoto , M. 2010 . “ Selective recovery of minor trivalent actinides from high level liquid waste by R-BTP/SiO2-P adsorbents ” . In Actinides 2009 (IOP Conf. Series: Materials Science and Engineering 9, 012064) 1 – 8 .
- Matsumura , T. , Matsumura , K. , Morita , Y. , Koma , Y. , Sano , Y. and Nomura , K. 2011 . Separation of trivalent minor actinides from fission products using single R-BTP column extraction chromatography . J. Nucl. Sci. Technol , 48 : 855 – 858 .
- Geist , A. , Hill , C. , Modolo , G. , St J. Foreman , M. R. , Weigl , M. , Gompper , K. and Hudson , M. J. 2006 . 6,6’-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl) [2.,2’]bipyridine, an effective extracting agent for the separation of americium(III) and curium(III) from the lanthanides . Solv. Extr. Ion Exch , 24 : 463 – 482 .
- Retegan , T. , Ekberg , C. , Englund , S. , Fermvik , A. , Foreman , M. R.S. and Skarnemark , G. 2007 . The behaviour of organic solvents containing C5-BTBP and CyMe4-BTBP at low irradiation doses . Radiochim. Acta , 95 : 637 – 642 .
- Mariania , M. , Macerataa , E. , Gallettaa , M. , Buttafavab , A. , Casnatic , A. , Ungaroc , R. , Faucitanob , A. and Giola , M. 2007 . Partitioning of minor actinides: effects of gamma irradiation on the extracting capabilities of a selected calixarene-based picolinamide ligand . Radiat. Phys. Chem , 76 : 1285 – 1289 .