Abstract
This article describes the method for measuring the isotopic abundance of 10B in nuclear grade boron carbide using inductively coupled plasma-quadrupole mass spectrometry (ICP-QMS). The results of investigation revealed that both the integration time and the dwell time have a major influence on the reproducibility of ICP-QMS measurements. As a result of optimization of the measurement conditions, reproducibility below 0.2% relative standard deviation (RSD) (0.17% RSD maximum) was achieved. In addition, the measured value of the isotopic abundance of 10B for each sample well agreed with the values measured by the TIMS. Thus, the method described in the present investigation was very effective in the analysis of isotopic abundance of 10B in B4C or H3BO3. The results of this study suggest that ICP-QMS could be applied to the precise analysis of the isotopic abundance of 10B required in the field of nuclear applications.
1. Introduction
Boron has two stable isotopes 10B and 11B. The isotopic ratio of boron (10B/11B) in stones and the soil has often been used as a geochemical indicator of the origins and histories of the materials. A particular importance of the isotopic ratio of boron is in neutron-absorbing materials for nuclear reactors. In a boiling water reactor (BWR) and a fast breeder reactor (FBR), boron carbide (B4C) serves as the neutron-absorbing material for the control rods. Furthermore, in a pressurized water reactor (PWR), a solution of boric acid (H3BO3) is added to the primary cooling water to adjust the reactivity of the reactor core.
In the nuclear reactors, neutron absorption is mainly performed by 10B because 10B has a much greater neutron absorption cross-section than that of 11B. For example, the neutron absorption cross-section of 10B is 3840 barn and that of 11B is essentially zero (<0.01 barn) for thermal neutrons [Citation1,Citation2]. For fast neutrons with energy E ≺ 0.1 MeV, the neutron absorption cross-section of 10B becomes about 1 barn and that of 11B is negligibly small. Thus, the isotopic abundance of 10B is one of the most important factors that characterize the quality of B4C or H3BO3. In Japanese nuclear reactors, the accuracy of measurements specified for the isotopic abundance of 10B in B4C (natural or enriched) for BWR and FBR is 0.2% as the relative standard deviation (0.2% RSD). Therefore, it is important to measure the isotopic abundance of 10B with high reproducibility.
At present, the thermal ionization mass spectrometer (TIMS) and multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) are the two most popular tools for boron isotopic analysis because they enable high accuracy of measurement for the isotopic abundance of 10B. For example, the latest study of these instruments allows measurements of the isotopic ratio of boron to a reproducibility below 0.05% RSD [Citation3,Citation4]. However, it requires extremely high level of analytical skills and is time-consuming to obtain such a high accuracy of measurement. Usually, the accuracy of measurement becomes lower than that reported in the above literature. In the American Society for Testing and Materials (ASTM) C791-04, a method for measurement with TIMS is described for the isotopic abundance of 10B at RSD of 0.22% [Citation5]. Thus, achieving measurement with a RSD less than 0.2% for the isotopic abundance of 10B in nuclear grade B4C requires careful procedures. Furthermore, the TIMS and MC-ICP-MS instrumentation are very expensive. Since the accuracy of measurement required for 10B abundance might not be so high as could only be achieved by TIMS and MC-ICP-MS, it is desirable if we could make measurement with less expensive and simpler instrumentation than TIMS and MC-ICP-MS.
Inductively coupled plasma-quadrupole mass spectrometry (ICP-QMS) is an apparatus widely used for general and trace elemental analysis. It is generally recognized that the reproducibility of careful measurement of isotopic analysis by ICP-QMS is 0.1–1.5% RSD [Citation6–Citation9], which is inferior to those of TIMS and MC-ICP-MS. However, the simplified preparation and measurement processes as well as the low price of instruments would make the ICP-QMS more practical for use than TIMS and MC-ICP-MS if the accuracy of measurements could meet the required level for nuclear applications.
Kawamura et al. [Citation10] have made a round robin test using ICP-QMS for the analysis of boron isotopic ratio of H3BO3 solution used as chemical shim in PWR. The solutions of H3BO3 with 10.3–19.8 atom% of 10B isotopic abundances were prepared and measured in seven analytical laboratories in Japan. They showed that the RSD value in the determination of the isotopic abundance of 10B ranged from 0.11 to 0.81%, which does not meet the RSD value required for the quality assurance of B4C for BWR and FBR. It was recently reported that a measurement of 10B abundance with ICP-QMS, enabled a high reproducibility of less than 0.1% RSD by adopting the internal standard correction method using Li isotopes [Citation11]. Since the internal standard correction method requires skilful and elaborate procedures, it is not so favourable to apply for practical measurements. It was also recently reported that ICP-QMS could allow measurement of the isotope ratio of Ca elements with a reproducibility less than 0.1% RSD without the internal standard correction method, if measurement conditions, such as nebulizer gas flow rate, RF power, lens potential and dwell time were properly optimized [Citation12]. This method also requires a high level of analytical skills and is time-consuming, thus it is desirable if another simpler and effective measurement method is available in the ICP-QMS analysis.
This article describes the result of investigation of the method for measuring the isotopic abundance of 10B in the nuclear grade B4C using ICP-QMS, where a particular attention was paid to the instability of signal intensity associated with ICP-QMS. Studies were made on the effect of integration and dwell times in the data sampling on the reproducibility of the measurement, and the method for obtaining optimum conditions is presented.
2. Experimental
2.1. Sample
The samples used in the measurement were B4C powders of two different grades with different particle sizes. They are Grade #60–#80 (samples A and B) and Grade #200–#325 (samples C and D), each manufactured by Denki Kagaku Kogyo Kabushiki Kaisha. These samples were prepared using natural boron.
Each sample was ground to 100 μm or smaller. Then, 0.1 g of each sample was weighed in a platinum crucible for mixing with 1 g of sodium carbonate. The crucibles were placed in an electric furnace, and the samples were heated from 400 to 900°C (5°C per min, 100 min), kept at 900°C for 15 min and then allowed to melt. After cooling, ultrapure water produced by the ultra-high purity water generator Milli-Q Element (Millipore Corp.) was added to the products and each of the solutions obtained was passed through a column containing cation-exchange resin IR-120B (manufactured by Organo Corporation). Each eluate was concentrated on a sand bath and evaporated to dryness, yielding H3BO3 powder. In the process of evaporation to dryness, the temperature of the sand bath was carefully raised to 110°C and then kept for 18 h to remove water from the solution of H3BO3, thereby to avoid isotopic fractionation in the H3BO3.
The H3BO3 powder thus obtained was divided into two samples; one for the measurement by TIMS and the other for the measurement by ICP-QMS. For ICP-QMS measurement, the H3BO3 powder was mixed with ultrapure water, mannitol (special grade, Wako Pure Chemical Industries, Ltd.) and aqueous ammonia (special grade; Wako Pure Chemical Industries, Ltd.) to prepare a mixture of 0.25% mannitol and 0.1 M aqueous ammonia containing 200 ppb of boron [Citation13]. The mixtures thus prepared were subjected to measurements. The 0.25% mannitol–0.1 M ammonia solution was also used as the rinsing solution to reduce the memory effect of boron [Citation13]. We assume that the isotopic fractionation was negligible during the course of sample preparations.
Since the accurate isotopic abundance of 10B in the samples A–D was unknown, the isotopic abundance of 10B in each sample was first measured by TIMS in accordance with the ASTM-C791-04, where NIST SRM 951a (Boric Acid Isotope Standard, H3BO3, 10B = 19.827 atom%) was used as the standard sample. The H3BO3 powder was dissolved in water and loaded on the rhenium filament with NaOH aqueous to obtain the mass spectra of Na2BO2 +. The values thus obtained were used as the reference values for the isotopic abundance of 10B of each sample.
2.2. Equipment and instrument
It is well known that boron tends to remain in the analytical instrument, particularly when made of glass. The adherent and residual boron contamination degrades measurement accuracy, which is known as the memory effect [Citation11,Citation13]. Thus for the preparation of each sample solution, vessels made of polypropylene or polymethylpentene were used instead of glass vessels to prevent contamination from boron in glass. A spray chamber and nebulizer made of polyethylene were also used to reduce the memory effect.
The ICP-QMS used in this investigation was the ICP-MS Agilent 4500 (Yokokawa Analytical Systems Inc.). summarizes the measurement conditions. Plasma conditions were set so that the signal for the mass-to-charge ratio (m/z = 10) of 10B would be from 1 × 105 to 2 × 105 for the solution containing 200 ppb of boron. The torch position was adjusted for each measurement to enter the same mass-to-charge signal condition as described above. A peristaltic pump was used to introduce the samples.
Table 1. Measurement conditions of ICP-QMS (ICP-MS Agilent 4500).
The TIMS used in this investigation was the Varian MAT CH-5 mass spectrometer, where the measurement of isotopic abundance of 10B was carried out in accordance with the method described in the literature [Citation20]. summarizes the measurement conditions.
Table 2. Measurement conditions of TIMS (Varian MAT CH-5 mass spectrometer) [Citation20].
2.3. Method of measurement
Generally, in mass spectroscopy, the signal intensity ratio of each mass number does not necessarily agree with its isotopic ratio [Citation14]. This is because the loss of ion energy caused by collisions with gas molecules inside the mass spectrometer varies depending on the mass number of the ions. This phenomenon is known as the mass discrimination effect or mass bias effect [Citation15,Citation16]. In ICP-QMS using an argon plasma ionization source, the mass discrimination effect is particularly pronounced, and the variations in mass bias with time are a major factor in reducing the accuracy of the analysis values [Citation17].
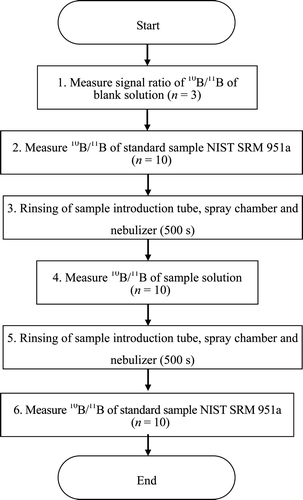
Therefore, to take into account the variations in mass bias with time, we employed the correction method using the NIST SRM 951a as standard sample [Citation18]. The signal intensity ratio of each target sample was calibrated using that of the standard sample. The correction factor or the mass bias coefficient was obtained from the signal intensity ratio of NIST SRM 951a by taking average of signal intensity ratio of NIST SRM 951a before and after the measurement of each sample. The signal intensity ratio of each sample was calibrated by multiplying the mass bias coefficient thus obtained. The actual procedure for measurement is shown in .
The isotopic abundance of 10B was calculated as shown in Equations (1)–(3). Here, the signal intensities of 10B and 11B in the sample solution is represented as 10Bobs and 11Bobs, the certified standard value of the 10B/11B ratio for NIST SRM 951a is represented as (10B/11B)cert, and the measured value of the isotopic ratio of boron for each sample as (10B/11B)meas. The signal intensities of 10B and 11B in the blank solution and NIST SRM 951a solution averaged over n measurements are represented as follows:
The mass bias coefficient f is calculated by Equation (1):
The isotopic ratio of boron (10B/11B)meas is calculated by Equation (2):
The abundance of 10B (10B atom%) is calculated by Equation (3):
The measurements were repeated 10 times for each sample and the average was set as the measured value.
2.4. Optimization of measuring conditions
It is generally accepted that ICP-QMS underperforms TIMS in terms of the reproducibility of isotopic ratio measurements. This is partially attributed to the instability of signal intensity of ICP-QMS [Citation17]. Accordingly, in this investigation, we tried to find the optimum condition to reduce the variations in signal instability by taking two types of variations into account, namely short-term variation and long-term variation.
The short-term variation was a fluctuation of the order of milliseconds to seconds caused by fluctuations in the argon plasma ionization source or variations in the volume of the sample introduced by the nebulizer or the pump [Citation9]. The long-term variation was a fluctuation of the order of minutes to hours caused by changes in plasma emission conditions associated with the elapsed time during measurements.
In ICP-QMS, which is unable to simultaneously determine more than one mass number, the short-term variations have different influences between signal intensity of 10B and that of 11B and thus provide a direct cause for the reduced reproducibility of the isotopic abundance of 10B. On the other hand, the long-term variations have different influences on the mass bias according to the ion mass number. Therefore, when the measurement time increases to several minutes to hours, then the isotopic ratio analysis value would vary and reproducibility would change for the worse. Thus, the reproducibility of measurements by ICP-QMS depends critically on the effects of integration time and dwell time [Citation19]. Here, the integration time is the product of dwell time and the number of data sampling. In this investigation, the dwell time and the integration time were changed to obtain the optimum condition for isotopic ratio measurements. The dwell time was changed from 0.5 to 10 ms and the integration time from 1 to 50 s. The measurement was repeated 10 times under the same conditions to calculate the RSD value for each sample.
3. Result and discussion
3.1. Effect of fluctuation of signal intensity
shows the relationship between integration time (1–50 s) and the RSD of the measurements using NIST SRM 951a, as a function of dwell time (0.5–10 ms), where the data fitting curves were obtained by the power approximation method. As shown in , the RSD value monotonically decreased with increasing integration time. This means that the longer the integration time, the better the reproducibility of the measurements. This is attributed to the fact that the increased integration time reduced the short-term variations in signal intensity. It also reveals that the change in RSD becomes small once the integration time reached or exceeded 30 s. Based on the relationship between the required measurement time and the reproducibility of measurements, the integration time was set to 30 s in the subsequent measurements.
Figure 2. Effect of integration time and dwell time on relative standard deviation (RSD) of isotopic abundance of 10B measured by ICP-QMS. Each RSD was obtained by 10 measurements.
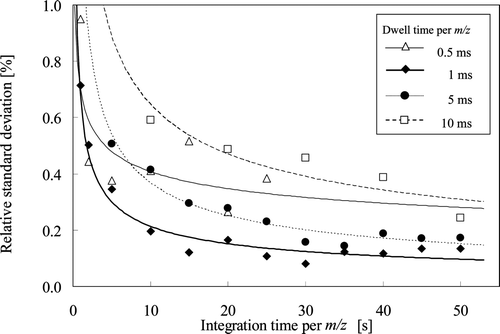
When we compared the reproducibility of measurements for the dwell times of 1, 5 and 10 ms, the smaller dwell time yielded better reproducibility. When the dwell time was 1 ms, the RSD at an integration time at 30s was 0.16%, whereas that for 10 ms was 0.43%, This was caused by the fact that a high-speed mass number scanning reduced the influence of short-term variations in signal intensity. On the other hand, when the dwell time was reduced to 0.5 ms, only a slight change in reproducibility was observed with relatively large RSD values as shown in . This is explained as follows.
When the dwell time is set as 0.5 ms, the effect of dwell time on the change in mass scanning speed becomes not so large because the time required for switching between the mass numbers was approximately 1 ms for the present ICP-QMS. Then the reduction in the short-term variations in signal intensity becomes relatively small. Furthermore, if the dwell time is as short as 0.5 ms, the number of switchings for mass number becomes large, provided the integration time is kept constant. Then, the total measurement time increases for the same integration time, thereby the susceptibility to long-term variations of signal intensity would become higher and result in poor RSD values as shown in .
Based on these results, the optimum dwell time was set to 1 ms at which the best reproducibility had been achieved as shown in . The reproducibility of measurements for NIST SRM 951a under the selected conditions, including the integration time of 30 s and the dwell time of 1 ms, was approximately 0.15% RSD, which compared with the reproducibility of TIMS measurements expressed as 0.22% RSD in ASTM C794-04.
3.2. Reproducibility of measurement
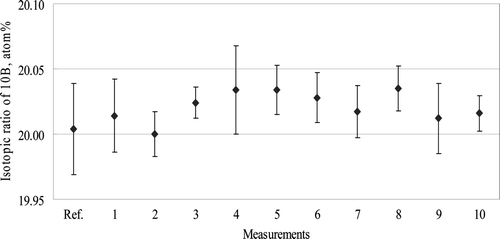
shows the isotopic abundance of 10B of the B4C sample A (Grade #60-#80) measured by TIMS and ICP-QMS. The measurement “Ref.” shows the data measured by TIMS. Each error bar represents the standard deviation (±1 σ), which was calculated from the data of 10 repeated measurements for ICP-QMS measurements and the value reported by Nomura [Citation20] for the TIMS measurement, respectively. The measurement by ICP-QMS was conducted 10 times (data indicated from No. 1 to 10 as shown in ) to confirm long-term reproducibility of measurement, where the measurement was done within a period ranging over six months.
As shown in , the value of ICM-QMS measurement fell within the range of error bar of the TIMS measurement and the isotopic abundance of 10B measured by ICP-QMS agreed well with that obtained by TIMS. Also, the reproducibility of all the measurements shown in fell below 0.2% RSD, indicating the long-term reproducibility of measurement.
summarizes the results of measurement of the isotopic abundance of 10B of B4C samples measured by TIMS and ICP-QMS. Here, the error in isotopic abundance of 10B measured by TIMS is calculated from the RSD value 0.2% reported for the measurements. As described above, the measurement of isotopic abundance by ICP-QMS was conducted 10 times for sample A, but 3 times for samples B–D. The values and errors (±1σ) of isotopic abundance shown in are the averages of these measurements. As shown in , the result of ICP-QMS measurement well agreed with those for the TIMS measurement. Furthermore, the reproducibility of ICP-QMS measurements fell below 0.2% RSD (0.05–0.17% RSD). This reproducibility was compared with the reproducibility of TIMS measurements of 0.22% RSD in ASTM C794-04.
Table 3. Isotopic abundance of 10B and the relative standard deviation (RSD) of B4C samples measured by TIMS and ICP-QMS.
From the results thus obtained, it was shown that the values of isotopic abundance of 10B measured by ICP-QMS could compare with those measured by TIMS in terms of the reproducibility if appropriate conditions are selected in the ICP-QMS measurement.
4. Conclusion
The results of this investigation revealed that both the integration time and the dwell time have a major influence on the reproducibility of ICP-QMS measurements. As a result of optimization of the measurement conditions, reproducibility below 0.2% RSD (0.17% RSD maximum) was achieved. In addition, the measured value of the isotopic abundance of 10B for each sample well agreed with the values measured by the TIMS. The method of measurement with ICP-QMS described in this investigation was very effective in the analysis of isotopic abundance of 10B in B4C or H3BO3. This study demonstrated that the examination and selection of appropriate conditions for isotopic measurements in ICP-QMS could achieve a reproducibility as high as that measured with TIMS shown in ASTM-C791-04. Consequently, it is suggested that ICP-QMS could be applied to the precise analysis of the isotopic abundance of 10B required in the field of nuclear applications.
Acknowledgements
The authors are grateful to Dr Masao Nomura of the Research Laboratory for Nuclear Reactors, Tokyo Institute of Technology for fruitful discussions and his help in the measurement of the isotopic abundance of 10B by TIMS.
References
- Shibata , K. , Kawano , T. Nakagawa , T. 2002 . Japanese evaluated nuclear data library version 3 revision-3: JENDL-3.3 . J. Nucl. Sci. Technol , 39 : 1125 – 1136 .
- Mahagin , D.E. and Dahl , R.E. 1977 . Boron and Refractory Boride , Berlin : Springer-Verlag .
- K. Aggarwal , S. , Wang , B.-S. , You , C.-F. and Ching , C.-H. 2009 . Fractionation correction methodology for precise and accurate isotopic analysis of boron by negative thermal ionization mass spectrometry based on BO2 − ions and using the 18O/16O Ratio from ReO4 − for internal normalization . Anal. Chem , 81 : 7420 – 7427 .
- Foster , G.L. 2008 . Seawater pH, pCO2 and [CO3 2−] variations in the Caribbean sea over the last 130 kyr: A boron isotope and B/Ca study of planktic foraminifera, Earth Planet . Sci. Lett , 271 : 254 – 266 .
- ASTM C791-04 . Standard Test Methods for Chemical, Mass Spectrometric, and Spectrochemical Analysis of Nuclear-Grade Boron Carbide, American Society for Testing and Materials 1975 ASTM, West Conshohocken , PA
- Beary , E.L. , Paulsen , P.J. and Fassett , J.D. 1994 . Sample preparation approaches for isotope dilution inductively coupled plasma mass spectrometric certification of reference materials . J. Anal. Atom. Spectrom , 9 : 1363 – 1369 .
- Gregoire , D.C. 1990 . Determination of boron in fresh and saline waters by inductively coupled plasma mass spectrometry . J. Anal. Atom. Spectrom , 5 : 623 – 626 .
- Smith , F.G. , Wiederin , D.R. , Houk , R.S. , Egan , C.B. and Surfass , R.E. 1991 . Measurement of boron concentration and isotope ratios in biological samples by inductively coupled plasma mass spectrometry with direct injection nebulization . Anal. Chim. Acta , 248 : 229 – 234 .
- Montaser , A. 1998 . Inductively Coupled Plasma Mass Spectrometry Wiley-VCH, New York
- Kawamura , H. , Sato , Y. Kino , K. 2010 . Interlaboratory comparison for boron isotope ratio measurement with inductively coupled plasma-quadrupole mass spectrometer . Bunseki Kagaku , 59 : 57 – 63 .
- Al-Ammar , A. , Reitznerova , E. and Barnes , R.M. 2000 . Improving boron isotope ratio measurement precision with quadrupole inductively coupled plasma-mass spectrometry . Spectrochim. Acta Part B , 55 : 1861 – 1867 .
- S. , F. Boulyga , Klotzil , U. , Stingeder , G. and Prohaska , T. 2007 . Optimization and application of ICPMS with dynamic reaction cell for precise determination of 44Ca/40Ca isotope ratios . Anal. Chem , 79 : 7753 – 7760 .
- Wright , C. , Fryer , F. and Woods , G. 2008 . Rinse Solution for Boron Analysis and Boron Isotope Ratios , Santa Clara : Agilent technologies . Agilent ICP-MS journal/5989-7727
- Hirata , T. 2002 . Introduction of isotopic ratio analysis – methodologies for isotopic ratio measurements [in Japanese] . Bunseki , 4 : 152 – 160 .
- Becker , J.S. 2002 . State-of-the-art and progress in precise and accurate isotope ratio measurements by ICP-MS and LA-ICP-MS . J. Anal. Atom. Spectrom , 17 : 1172 – 1185 .
- Beauchemin , D. 2004 . Inductively coupled plasma mass spectrometry . Anal. Chem , 76 : 3395 – 3415 .
- Hirata , T. 1993 . Precise determination of isotopic compositions of trace elements with ICP multiple-correction mass spectrometer [in Japanese] . Chishitsu News , 469 : 7 – 17 .
- JIS K 0133 . 2007 . General Rules for High Frequency Plasma Mass Spectrometry , Tokyo : Japanese Industrial Standards Committee .
- Katahira , K. 2005 . Examination of optimal conditions for analysis of lead isotope ratios in soil using ICP-MS . Seikatsu Eisei , 49 : 297 – 304 .
- Nomura , M. , Okamoto , M. and Kakihana , H. 1973 . Determination of boron isotope ratio by the surface ionization [in Japanese] . J. Mass Spectrom. Soc. Jpn , 21 : 277 – 281 .