Abstract
Hydrogen production by γ-radiolysis of the mixture of mordenite, a zeolite mineral, and seawater was studied in order to provide basic points of view for the influences of zeolite minerals, of the salts in seawater, and of rise in temperature on the hydrogen production by the radiolysis of water. These influences are required to be considered in the evaluation of the hydrogen production from residual water in the waste zeolite adsorbents generated in Fukushima Dai-ichi Nuclear Power Station. As the influence of the mordenite, an additional production of hydrogen besides the hydrogen production by the radiolysis of water was observed. The additional hydrogen can be interpreted as the hydrogen production induced by the absorbed energy of the mordenite at the yield of 2.3×10−8 mol/J. The influence of the salts was observed as increase of the hydrogen production. The influence of the salts can be attributed to the reactions of bromide and chloride ions inhibiting the reaction of hydrogen with hydroxyl radical. The influence of the rise in temperature was not significantly observed up to 60°C in the mixture with seawater. The results show that the additional production of hydrogen due to the mordenite had little temperature dependence.
1. Introduction
Production of molecular hydrogen (H2) by the radiolysis of water is one of the important radiation effects for aqueous processes under ionizing radiation environment. For the safe operation of the processes, the H2 production is required to be adequately evaluated and handled. At Fukushima Dai-ichi Nuclear Power Station, a water treatment system was installed for the decontamination of radioactive water generated in the accident. The radioactive water was also contaminated by the salts from seawater since seawater was injected to cool the reactors and a large amount of seawater was left behind by the tsunami [Citation1]. The water treatment system employed ion-exchange processes using zeolite adsorbents to remove radioactive cesium (Cs) from the water. Since radioactive Cs accumulates in the zeolite adsorbents, water remaining in the spent zeolite adsorbents is assumed to be exposed to intensive radiations from the adsorbed Cs. Therefore, a particular attention should be given to the H2 production by the radiolysis of the residual water in the waste zeolite adsorbents during the storage period of them.
To evaluate the H2 production from the waste zeolite adsorbents, the influence of the zeolite adsorbents on the radiolysis of water should be taken into consideration. The experience of the accident at Three Mile Island suggests that the presence of the zeolite adsorbents increases the H2 production by the radiolysis of the residual water [Citation2,Citation3]. At Three Mile Island, a similar ion-exchanging process using zeolite adsorbents was carried out to decontaminate radioactive water generated in the accident. The waste zeolite adsorbents generated in Three Mile Island produced H2 and molecular oxygen (O2). The report on the H2 control of the waste in Three Mile Island notes that the production rates of the gaseous products were higher than those expected from the radiolysis of the residual water contained in the waste zeolite adsorbents [Citation3]. Moreover, some basic studies on the radiolysis of adsorbed water on zeolites also reported relatively high productions of H2 from the adsorbed water compared to that expected from the same amount of water in the absence of the zeolites [Citation4–Citation6]. In these studies, the high productions of H2 were interpreted as indicating that the energies originally absorbed by the zeolites transferred to the surfaces of the zeolites and decomposed the adsorbed water. In addition, the energy transfer process has been proposed as a possible mechanism of the H2 production in a lot of studies on the influences of fine particles or porous materials of insoluble ceramics [Citation7–16].
The salts from seawater in the contaminated water at Fukushima Dai-ichi are the major difference between the water treatments at Fukushima Dai-ichi and at Three Mile Island. Seawater contains many ions such as chloride ion (Cl−), bromide ion (Br−), and bicarbonate ion (HCO3 −) [Citation17]. These ions are involved in the radiation-induced reactions in bulk seawater, and thereby possibly in the reactions subsequent to the radiolysis of adsorbed water on the zeolite adsorbents. The radiation-induced phenomena at the interfaces are expected to have key roles in the H2 production from the waste zeolite adsorbents. However, the reaction scheme of the H2 production in the presence of zeolites or solid oxides is still not established. Therefore, further studies are required to predict the influence of the zeolite adsorbents on the radiolysis of water containing the salts.
In addition, the temperature effect on the H2 production should also be evaluated because the decay heat from the radioactive Cs would raise the temperature of the waste zeolite adsorbents. The temperature dependence of the H2 production by radiolysis of water has been studied, since the radiolysis of water at elevated temperatures is important for the control of water quality in light water nuclear reactors [Citation18]. However, little has been studied about the radiolysis of water at elevated temperatures in the presence of zeolites or other solid oxides. Therefore, the influence of the rise in temperature on the H2 production in the presence of zeolites is needed to be investigated.
Hence, the H2 production by the γ-radiolysis of the mixture of zeolites and seawater was studied. We have already reported the H2 production from the mixture of one of the zeolite adsorbents actually used at Fukushima Dai-ichi with seawater on some essential conditions [Citation19]. The purpose of this study is to provide basic points of view to take account of the influences of the zeolite adsorbents, of the salts in seawater, and of the rise in temperature in the evaluation of the H2 production from the waste zeolite adsorbents. In this study, the H2 production from the mixture of natural mordenite, a type of zeolite minerals, with seawater or with pure water at temperatures from room temperature to 60°C was investigated. Actual measured data on the H2 production from the mixture of the zeolite adsorbents and seawater are indispensable to evaluate the H2 production in the water processing at Fukushima Dai-ichi, while concurrently studies on the H2 production in various conditions will deepen our understanding on the radiation chemistry in heterogeneous systems of aqueous solutions and solid oxides.
2. Experimental
2.1. Sample preparation
The samples were the mixtures of mordenite with seawater or the mixtures of mordenite with pure water. The mordenite used in this study was natural mordenite (TOP ZEOLITE M) from Ayashi area in Miyagi prefecture supplied by SHIN TOHOKU Chemical Industry. Seawater was sampled at Oarai seafront in Ibaraki prefecture. Seawater was used after filtration with a filter paper of grade No. 5C purchased from ADVANTEC. The concentrations of anions in seawater were measured on an ion chromatography instrument, ICS-1000, DIONEX. Water used for sample preparation was purified by distillation and ion exchanging with a pure water system, SA-2100E1, EYELA. The electric conductivity of the pure water was less than 0.07 μS.
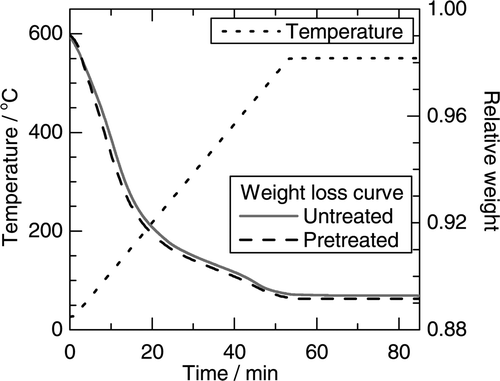
Mordenite contains a considerable amount of adsorbed water under moist atmosphere since mordenite has hygroscopic property. The water contents of the mordenite used in this study were determined by the weight losses at 550°C under nitrogen (N2) atmosphere by thermogravimetric analysis. The thermogravimetric analysis was carried out on a TGA-50, SHIMADZU. shows the thermal weight loss curves of the mordenite conditioned in a humidity controlled desiccator at 80% relative humidity (%RH) and at 22 ± 3°C. The water content of the mordenite was measured to be 0.108 ± 0.005 when the mordenite was conditioned in the humidity-controlled desiccator. The humidity in the desiccator was controlled by the saturated salt solution method using ammonium chloride. The variation in weight of the mordenite due to adsorption or desorption of water became negligible within 1 week of storage in the desiccator.
The mordenite used in the preparation of the mixtures with seawater was preliminarily treated with seawater in order to reduce the variations in salt concentrations in the samples due to the adsorbed water of the mordenite mixing into the added seawater. A given amount of seawater was diluted 10-fold with pure water and then the diluted seawater was added to the mordenite, so that the salts in seawater could be homogeneously distributed. The added amount of seawater was 12% of the dry weight of the mordenite, which corresponded to the amount of the adsorbed water at 80%RH and at 22 ± 3°C. The excess water in the mixture of the mordenite and the diluted seawater was evaporated at 110°C. This preliminary treatment had no significant influence on the water content of the mordenite as shown in .
The samples were prepared by adding seawater or pure water to the weighed mordenite in glass vials. The weights of the mordenite in the samples were measured after the variations in weights due to the moisture adsorption became negligible at 80%RH and at 22 ± 3°C. After the weight measurement of the mordenite, seawater or pure water was added to the vials. Then the vials were sealed with septa in air. The samples of the lower water contents were prepared by drying the mordenite using a sample dehydrator after the weight measurement. The mordenite was dried at various temperatures up to 200°C. The weight losses by the dehydration were measured to calculate the water contents of these samples. For the irradiation at room temperature, the sample vials of 8.7 cm3 with 23 mm in inner diameter were used. For the measurement of the temperature dependence of the H2 production, the sample vials of 5.15 cm3 with 12 mm in inner diameter were used.
The weights of seawater or pure water in the mixtures were calculated as the sum of the weights of the adsorbed water (m aq(ads)) and the added weights of seawater or pure water (m aq(ext)). The weight fractions of seawater or pure water in the samples (w aq) were calculated as below.
2.2. Irradiation and H2 measurement
The samples were irradiated by γ-rays from a 60Co radiation source up to 10 kGy. The irradiation experiments were carried out at Takasaki Advanced Radiation Research Institute. The absorbed doses of the samples were adjusted by the irradiation time. The dose rates were about 4 kGy/h. During the irradiation, the samples were not agitated. When the samples contained enough water, the mordenite sunk to the bottoms of the vials and the samples were separated to the layers of the sedimented moldenite below and of the supernatants above. The atmospheres of the samples in the vials were air.
For the measurement of the temperature dependence, the temperatures of the samples were controlled using dry thermo units with aluminum block baths, DYU-1C, TAITEC. The leaks of H2 from the vials at elevated temperatures were tested prior to the irradiation experiments. The vials were filled with Ar-balanced 1% H2 gas by displacement of water and then kept at elevated temperatures for 4 h. The keeping time corresponds to the period of the irradiation experiments including the preheating time of the samples. The concentrations of H2 in the vials did not significantly vary when the temperature was below 60°C. The concentrations of H2 in the several of the vials kept at 80°C decreased, and considerable concentrations of O2 and N2 were detected. Therefore, the measurement of the temperature dependence was performed at temperatures up to 60°C.
The H2 produced by the irradiation was measured by gas chromatography. Prior to the measurement, the samples were agitated by vortex mixer for 2 min to promote the transition of H2 from the mixtures to the gas phases and to homogenize the H2 concentrations in the gas phases. Further agitation did not affect the measurement. The H2 concentrations in the gases in the vials were measured using a gas chromatograph, GC-14A, SHIMADZU, equipped with a thermal conductivity detector and a molecular sieve 5A column. The carrier gas was Ar. When the empty vials were irradiated up to 10 kGy, H2 was not detected.
The gas phase volumes in the sample vials were determined by the water immersion method to calculate the quantities of produced H2 from the measured concentrations. After the gas analysis, the vials were opened and filled with pure water. The gas phase volumes were determined by the increases in the weights by the addition of pure water. The gas phase volume was determined individually for each sample.
As a reference to validate the measurement, 1 mM aqueous potassium bromide solution was irradiated and the production of H2 was measured in addition to the samples. The solution is known to produce H2 at the primary yield, 4.7 × 10−8 mol/J for the γ-radiolysis of water. The measured yield was (4.7 ± 0.2) × 10−8 mol/J. The error of the measurement was evaluated to be 5%.
The adsorption of H2 on the mordenite had negligible influence on the measurement of the quantities of H2. The influence of the adsorption of H2 on the mordenite was tested using the mixtures of the mordenite with pure water of w aq = 0.01, 0.1, and 0.48. A known amount of standard H2 gas was injected with a gas tight syringe to the sealed vials containing the mixtures. Then the quantities of H2 in the vials were measured 2, 4, or 6 h after the injection. No significant decrease in the quantities of H2 indicating the adsorption of H2 was observed.
2.3. Dosimetry
The absorbed doses were measured by the dichromate dosimeter [Citation20]. The respective doses of seawater, pure water, and the mordenite were evaluated because the samples were the heterogeneous mixtures. These doses for the materials were calculated by correcting the measured dose by the mass energy-absorption coefficients for 1.25 MeV photons [Citation21]. The coefficients were evaluated to be 2.95 × 10−2, 2.97 × 10−2, and 2.69 × 10−2 cm2/g for seawater, pure water, and the mordenite, respectively. The coefficient for seawater was calculated based on the reported composition [Citation17]. The coefficient for the mordenite was calculated from the chemical formula of the type material, Na8(H2O)24Al8Si40O96.
3. Results and discussion
3.1. H2 production from seawater
The H2 productions from the mixtures of the mordenite with seawater at various w aq were measured in order to elucidate the influence of the mordenite on the H2 production. shows the H2 productions per unit weight of the mixtures with seawater at room temperature. The horizontal axis of is the absorbed dose of the mixtures, which is calculated as the average of the absorbed doses of the mordenite and of seawater weighted by the weight fractions. The H2 production decreased with decreasing w aq. Therefore, the results suggest that H2 was produced primarily by the radiolysis of seawater in the mixture.
Figure 2. H2 productions per unit weight of the mixtures of the mordenite and seawater at room temperature as a function of the absorbed dose.
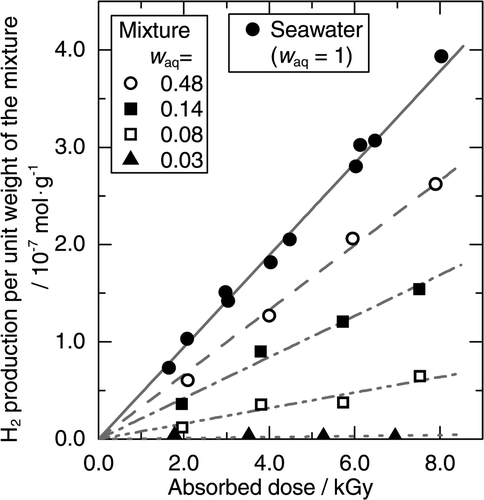
The radiolysis of seawater in the absence of the mordenite produced H2 at the yield of (4.8 ± 0.2) × 10−8 mol/J. The results are also shown in . The measured H2 production from seawater in the absence of the mordenite agrees with the numerical calculation by Bjergbakke et al. [Citation22,Citation23]. In their calculation, the yield of H2 from seawater was predicted to be equivalent to the primary yield, 4.7 × 10−8 mol/J for the γ-radiolysis of water. The H2 production at the primary yield means that the oxidation of H2 by hydroxyl radical (•OH) was inhibited in seawater. Otherwise, the H2 yield would become lower than the primary yield.
Table 1. Concentrations of anions in seawater used in this study/M.
3.2. Influence of the mordenite
In order to discuss the influence of the mordenite, the H2 productions per unit weight of seawater contained in the mixtures at different w aq were compared. For the comparison, the radiation-chemical yield of H2, G aq(H2), was evaluated using the following equations.
Figure 3. Yields of H2 from the mixtures of the mordenite and seawater, G aq(H2) calculated by Equation (2) shown in (a) and G total(H2) by Equation (4) shown in (b); the yields evaluated from the experimental results (□), expected supposing the mordenite had no influence (dashed line), and given by Equation (7) (solid line).
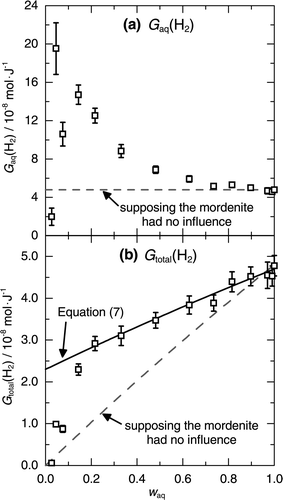
The increase of G aq(H2) clearly shows that the presence of the mordenite caused an additional production of H2. Supposing the mordenite had no influence on the H2 production, G aq(H2) was expected to be the same as that for seawater in the absence of the mordenite and to be constant at 4.8 × 10−8 mol/J as shown by the dashed line in (a). The additional production of H2 suggests that the energy of γ-rays originally absorbed by the mordenite was involved in the H2 production, as proposed in the studies on the radiolysis of heterogeneous systems of aqueous solutions and solid oxides [Citation4–Citation16]. However, G aq(H2) is inadequate for quantitative discussions on the additional production of H2 because the absorbed energy of the mordenite is neglected in the calculation of G aq(H2). When the energy absorbed by the mordenite is speculated to be involved in the H2 production, it is reasonable to include the energy absorbed by the mordenite in the calculation of the H2 yield.
For discussions on the involvement of the energy absorbed by the mordenite in the H2 production, the H2 yield was revaluated. A simple way to take account of the involvement of the energy absorbed by the mordenite is to calculate the H2 yield by dividing n(H2) by the sum of the absorbed energies of seawater and of the mordenite. Therefore, the revaluated H2 yield, G total(H2), was obtained from the experimental results using the following equations.
G total(H2) decreased monotonically with decreasing w aq, in contrast to G aq(H2) in (a). The dashed line in (b) shows the H2 yield calculated by Equation (4) supposing the mordenite had no influence, where n(H2) is given by multiplying E aq by the H2 yield from seawater, 4.8 × 10−8 mol/J. The additional production of H2 due to the mordenite can be distinguished as the difference between the experimental results shown by the symbols and the dashed line in (b). Therefore, the decrease of G total(H2) means the decrease of the efficiency of the H2 production toward the total energy absorption by the mixture, even though the mordenite caused the additional production of H2.
3.3. Empirical expression of the H2 production
A quite simple but quantitative interpretation of the H2 production from the mixture of the mordenite with seawater becomes possible when the H2 production induced by E MOR is supposed based on the discussion on the influence of the mordenite in the previous section. The H2 production from the mixture can be interpreted as a linear summation of the H2 production by the radiolysis of water and the additional production of H2 induced by E MOR. Namely, the following expression explains the experimental results for w aq > 0.2.
The value of g MOR(H2), 2.3 × 10−8 mol/J, was about half of the primary yield of H2 in the γ-radiolysis of water. Therefore, the results show that the additional production of H2 due to the mordenite was less efficient than the H2 production by the radiolysis of water. It seems reasonable that the energy originally absorbed by the mordenite is less efficient to produce H2 than the energy directly absorbed by water, since H2 is produced by the decomposition of water.
Comparison of Equation (7) with the experimental results suggests that the radiation-induced reactions in the pores of the mordenite or in the vicinity of the mordenite had an important role in the additional production of H2. The H2 production calculated by Equation (7) agrees with the experiment for w aq > 0.2. Since the second term in Equation (7) is proportional to E MOR with constant g MOR(H2), the agreement of Equation (7) with the experiment indicates that the efficiency of the additional production of H2 toward E MOR was independent of the excess amount of seawater for w aq > 0.2. For the lower w aq, the calculation by Equation (7) became higher than the experimental values. The experimental values approached to 0 with decreasing w aq. The discrepancy between Equation (7) and the experimental results suggests that the efficiency of the additional production of H2 due to the mordenite was no longer constant and was decreased by the loss of water molecules for w aq < 0.2. Therefore, the influence of the mordenite is considered to be spatially limited only in a region which was saturated by water molecules at w aq of around 0.2. Conversely, the mordenite is expected to have little influence on the radiolysis of seawater external to the region.
Although Equation (7) provides the simple interpretation of the H2 production as described above, the influence of the mordenite on the radiolysis of water might be more complicated. The energy absorption of the mordenite probably produces radicals, ions, and molecules other than H2 by the decomposition of water. Moreover, the presence of the interface or the pore structure of the mordenite constrains the diffusion of the products, and thereby might affect the reactions within a short time scale subsequent to the decomposition of water. Microscopic and kinetic understanding on the radiation-induced reactions in the presence of zeolites requires further studies.
3.4. Influence of the salts
In order to understand the influence of the salts in seawater on the H2 production from the mixture, the H2 productions from the mixtures of the mordenite with pure water were also measured and compared with those from the mixtures of the mordenite with seawater. shows G total(H2) from the mixtures with pure water at room temperature. In the calculation of G total(H2) from the mixture with pure water, the values of D aq and E aq for pure water were used. For comparison, contains G total(H2) from the mixture with seawater. The difference between the mixtures with pure water and with seawater indicates the influence of the salts.
Figure 4. Comparison of G total(H2) between the mixture with pure water and the mixture with seawater.
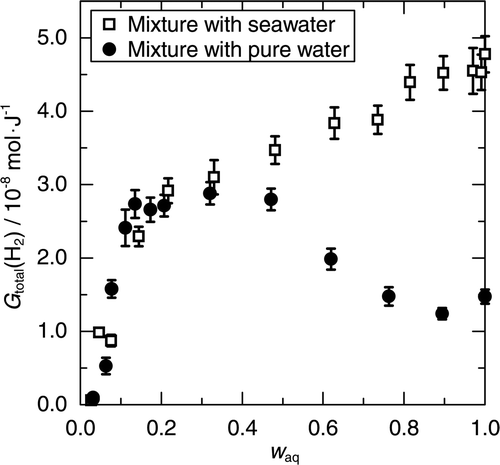
The influence of the salts was significant in the absence of the mordenite. The H2 production from pure water was remarkably lower than that from seawater. The low production of H2 from pure water means that the oxidation of H2 by •OH, reaction (R1) suppressed the H2 production. Meanwhile, reaction (R1) was inhibited in seawater due to the reactions of Br− and Cl−. The primary production of H2 by the radiolysis of water in seawater can be assumed comparable in pure water, because the measured H2 yield from seawater in the absence of the mordenite was (4.8 ± 0.2) × 10−8 mol/J.
The influence of the salts, however, was diminished with decreasing w aq. The diminution of the influence of the salts suggests that the salts in seawater had little influence on the additional production of H2 due to the mordenite. The H2 production from the mixture with pure water became comparable with that from the mixture with seawater for w aq < 0.2. For w aq < 0.2, the additional production of H2 due to the mordenite is expected to account for a considerable fraction of the measured H2. Therefore, the comparable production of H2 indicates that the quantities of H2 additionally produced due to the mordenite were not significantly different between in seawater and in pure water. Moreover, the H2 production at w aq < 0.2 would reflect the mechanism of the additional production of H2, because the efficiency of the additional production of H2 toward E MOR was dependent on w aq in seawater for w aq < 0.2. Therefore, the reaction scheme of the additional production of H2 is expected essentially the same between in seawater and in pure water.
On the other hand, for w aq > 0.2, the H2 production from the mixture with pure water increased with decreasing w aq, whereas the H2 production from the mixture with seawater decreased. The increase of the H2 production in the mixture with pure water is unlikely to be attributed to the additional production of H2, since the additional production of H2 is expected comparable between in seawater and in pure water. Therefore, the increase of the H2 production can be interpreted as showing that the suppression of H2 by reaction (R1) became less effective in the mixture even in the absence of the salts. Inhibition of reaction (R1) in the mixture with pure water would increase the H2 production from the mixture with pure water and concurrently would diminish the influence of the reactions of Br− and Cl−, reactions (R2) and (R3).
At least two possibilities can be considered for the inhibition of reaction (R1) in the mixture with pure water. One is diffusion and escape of H2 to the gas phase. As w aq decreased, the supernatant of the mixture became thin, and then the mixture contained air voids at w aq < around 0.5 to enlarge the interface area of the mixture to the gas phase. The decrease in the depth of the supernatant and the increase in the interface area might promote the escape of H2 to the gas phase. Another possibility is the decrease in the concentration of hydrogen peroxide (H2O2) due to the presence of the mordenite. The reaction of H2O2 with e − aq or H• produces •OH.
3.5. Temperature dependence
In order to investigate the influence of temperature on the H2 production, the H2 productions from the mixtures with seawater or with pure water were measured at 40°C and at 60°C in addition to room temperature. For comparison, the temperature dependences of H2 production from seawater and pure water in the absence of the mordenite were also measured. The results are shown in . No significant temperature dependences were observed in the H2 production from seawater and from the mixtures with seawater. Also, the H2 production from the mixture with pure water of w aq = 0.1 was not significantly dependent on temperature. Slight temperature dependences were observed in pure water in the absence of the mordenite and in the mixture with pure water of w aq = 0.48. When the mixtures with seawater and with pure water are compared, the H2 production from the mixture with seawater was comparable with or higher than the mixture with pure water at the same w aq within this temperature range.
Figure 5. Temperature dependences of G total(H2) from the mixtures with seawater of w aq = 0.1 (▵) and 0.48 (□), from seawater in the absence of the mordenite (○), from the mixtures with pure water of w aq = 0.1 (▴) and 0.48 (▪), and from the pure water in the absence of the mordenite (•).
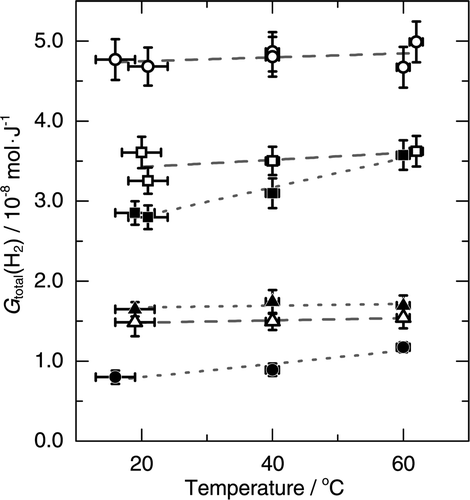
The temperature dependence of the primary yield of H2 in γ-radiolysis of water is not significant within this temperature range [Citation18]. Therefore, the oxidation of H2 remained to be inhibited in seawater up to 60°C since the H2 production from seawater was not significantly dependent on temperature. The results obtained from the mixtures with seawater show that the additional production of H2 due to the mordenite had little dependence on temperature, because the additional production of H2 is expected to account for a considerable fraction of the measured H2 especially at w aq = 0.1. Therefore, the temperature dependences observed in pure water and pure water mixture at w aq = 0.48 can be attributed to the temperature effect on the reaction processes in the aqueous phases before the transfer of H2 to the gas phases. The rise in temperature increased the rate of reaction (R1) [Citation18], while also accelerated the diffusion of H2. The increase of the H2 production with increasing temperature suggests that the rise in temperature accelerated the transfer of H2 to the gas phase more effectively than the oxidation of H2 by reaction (R1).
4. Conclusion
The H2 production from the γ-radiolysis of the mixture of the mordenite with seawater was studied in order to provide basic points of view for the influences of the zeolite adsorbents, of the salts from seawater, and of the rise in temperature on the H2 production by the radiolysis of water. These influences are required to be adequately included in the evaluation of the H2 production from the waste zeolite adsorbents generated in the water treatment at Fukushima Dai-ichi Nuclear Power Station.
The influence of the mordenite on the H2 production from the mixture with seawater was observed as an additional production of H2, although the H2 production from the mixture with seawater decreased with decreasing weight fraction of seawater. When the weight fraction of seawater was higher than 0.2, the additional production of H2 can be interpreted as the H2 production induced by the energy absorbed by the mordenite at the yield of 2.3 × 10−8 mol/J, which is about half of the primary yield of H2 by the γ-radiolysis of water. For the lower weight fraction of seawater, the H2 production from the mixture with seawater was significantly suppressed by further decrease of seawater. The significant decrease of the H2 production shows that the influence of the mordenite was diminished by the loss of water molecules.
The influence of the salts was discussed through the comparison of the H2 productions from the mixtures with seawater and with pure water. The H2 production from seawater was remarkably higher than that from pure water in the absence of the mordenite. The higher production of H2 in seawater can be explained by the reactions of Br− and Cl− inhibiting the oxidation of H2 by •OH. However, the influence of the salts in the mixture diminished with decreasing weight fraction of seawater, while the H2 production from the mixture with pure water increased with decreasing weight fraction of pure water. The diminution of the influence of the salts and the increase of the H2 production from the mixture with pure water suggests that the oxidation of H2 by •OH was inhibited in the mixture even in the absence of the salts in seawater. When the weight fraction of seawater was lower than 0.2, the H2 production from the mixture with seawater became comparable with that from the mixture with pure water containing the corresponding weight fraction of pure water. The comparable production of H2 suggests that the salts in seawater had a negligible influence on the additional production of H2 due to the mordenite.
The temperature dependence of the H2 production was not significantly observed in the mixture with seawater within the temperature range from room temperature up to 60°C. In this temperature range, the H2 production from the mixture with seawater was comparable with or higher than that from the mixture with pure water containing the corresponding weight fraction of pure water. The results show that the additional production of H2 due to the mordenite had little temperature dependence up to 60°C as well as the primary production of H2 by the radiolysis of water.
Acknowledgements
The authors gratefully acknowledge Dr. N. Nagasawa, Japan Atomic Energy Agency, for his help in the thermogravimetric analysis, Mr. K. Iwamatsu, The University of Tokyo, for his help in the experiments in Takasaki Advanced Radiation Research Institute, and Dr. R. Yamada, Japan Atomic Energy Agency, for discussions and his thought-provoking comments. This work was partially supported by JSPS KAKENHI Grant Number 24760721.
References
- 2011 . Situation of storing and treatment of accumulated water including highly concentrated radioactive materials at Fukushima Daiichi Nuclear Power Station (3rd Release), Press Release . Tokyo Electric Power Company , Jul : 13
- Quinn , GJ , Henrie , JO and Greenborg , J . 1984 . Submerged demineralizer system vessel shipment report , Idaho Falls, ID, USA : EG&G Idaho, Inc . (GEND-035)
- Henrie , JO , Flesher , DJ , Quinn , GJ and Greenborg , J . 1986 . Hydrogen control in the handling, shipping and storage of wet radioactive waste , Idaho Falls, ID, USA : EG&G Idaho, Inc . (GEND-052)
- Nakashima , M and Tachikawa , E . 1987 . Radiolytic gas production from titrated water adsorbed on molecular sieve 5A . J. Nucl. Sci. Technol , 24 : 41 – 46 .
- Nakashima , M and Aratono , Y . 1993 . Radiolytic hydrogen gas formation from water adsorbed on type A zeolites . Radiat. Phys. Chem , 41 : 461 – 465 .
- Nakashima , M and Masaki , NM . 1996 . Radiolytic hydrogen gas formation from water adsorbed on type Y zeolites . Radiat. Phys. Chem , 47 : 241 – 245 .
- Yamada , R , Nagaishi , R , Hatano , Y and Yoshida , Z . 2008 . Hydrogen production in the γ-radiolysis of aqueous sulfuric acid solutions containing Al2O3, SiO2, TiO2 or ZrO2 fine particles . Int. J. Hydrogen Energy , 33 : 929 – 936 .
- LaVerne , JA and Tandon , L . 2002 . H2 production in the radiolysis of water on CeO2 and ZrO2 . J. Phys. Chem. B , 106 : 380 – 386 .
- LaVerne , JA and Tonnies , SE . 2003 . H2 production in the radiolysis of aqueous SiO2 suspensions and slurries . J. Phys. Chem. B , 107 : 7277 – 7280 .
- Rotureau , P , Renault , JP , Lebeau , B , Patarin , J and Mialocq , JC . 2005 . Radiolysis of confined water: molecular hydrogen formation . Chem. Phys. Chem , 6 : 1316 – 1323 .
- Le Caër , S , Rotureau , P , Brunet , F , Charpentier , T , Blain , G , Renault , JP and Mialocq , JC . 2005 . Radiolysis of confined water: hydrogen production at a high dose rate . Chem. Phys. Chem , 6 : 2585 – 2596 .
- Petrik , NG , Alexandrov , AB and Vall , AI . 2001 . Interfacial energy transfer during gamma radiolysis of water on the surface of ZrO2 and some other oxides . J. Phys. Chem. B , 105 : 5935 – 5944 .
- Seino , S , Yamamoto , TA , Fujimoto , R , Hashimoto , K , Katsura , M , Okuda , S and Okitsu , K . 2001 . Enhancement of hydrogen evolution yield from water dispersing nanoparticles irradiated with gamma-ray . J. Nucl. Sci. Technol , 38 : 633 – 636 .
- Le Caër , S , Renault , JP and Mialocq , JC . 2007 . Radiolysis of confined water: hydrogen production at a high dose rate . Chem. Phys. Lett , 450 : 91 – 95 .
- Dimitrijevic , NM , Henglein , A and Meisel , D . 1999 . Charge separation across the silica nanoparticle/water interface . J. Phys. Chem. B , 103 : 7073 – 7076 .
- Schatz , T , Cook , AR and Meisel , D . 1999 . Capture of charge carriers at the silica nanoparticle-water interface . J. Phys. Chem. B , 103 : 10209 – 10213 .
- Bruland , KW and Lohan , MC . 2003 . Controls of trace metals in seawater . Treatise Geochem , 6 : 23 – 47 .
- Elliot , AJ and Bartels , DM . 2009 . The reaction set, rate constants and g-values for the simulation of the radiolysis of light water over the range 20° to 350°C based on information available in 2008. Chalk River , Ontario , , Canada : Atomic Energy of Canada Limited; . (Report AECL No. 153-127160-450-001)
- Kumagai , Y , Nagaishi , R , Kimura , A , Taguchi , M , Nishihara , K , Yamagishi , I and Ogawa , T . 2011 . Measurement and evaluation of hydrogen production from mixtures of seawater and zeolite in decomtamination of radioactive water . Trans. At. Energy Soc. Jpn , 10 : 235 – 239 . [in Japanese]
- Mai , HH , Tachibana , H and Kojima , T . 1998 . Effect of temperature during irradiation and spectrophotometry analysis on the dose response of aqueous dichromate dosimeters . Radiat. Phys. Chem , 53 : 85 – 91 .
- Hubbell , JH and Seltzer , SM . 1995 . Tables of X-ray mass attenuation coefficients and mass energy-absorption coefficients from 1 keV to 20 MeV for elements Z=1 to 92 and 48 additional substances of dosimetric interest , Gaithersburg, MD, USA : National Institute of Standards and Technology; . (NISTIR 5632)
- Bjergbakke , E , Draganić , ZD , Sehested , K and Draganić , IGD . 1989 . Radiolytic products in waters part I: computer simulation of some radiolytic processes in the laboratory . Radiochim. Acta , 48 : 65 – 71 .
- Bjergbakke , E , Draganić , ZD , Sehested , K and Draganić , IGD . 1989 . Radiolytic products in waters part II: Computer simulation of some radiolytic processes in nature . Radiochim. Acta , 48 : 73 – 77 .
- Kelm , M , Metz , V , Bohnert , E , Janata , E and Bube , C . 2011 . Interaction of hydrogen with radiolysis products in NaCl solution – comparing pulse radiolysis experiments with simulations . Radiat. Phys. Chem , 80 : 426 – 434 .
- LaVerne , JA , Ryan , MR and Mu , T . 2009 . Hydrogen production in the radiolysis of bromide solutions . Radiat. Phys. Chem , 78 : 1148 – 1152 .
- Roth , O , Hiroki , A and LaVerne , JA . 2011 . Effect of Al2O3 nanoparticles on rdiolytic H2O2 production in water . J. Phys. Chem. C , 115 : 8144 – 8149 .
- Yamada , R , Kumagai , Y and Nagaishi , R . 2011 . Effect of alumina on the enhancement of hydrogen production and the reduction of hydrogen peroxide in the g-radiolysis of pure water and 0.4 M H2SO4 aqueous solution . Int. J. Hydrogen Energy , 36 : 11646 – 11653 .