Abstract
In spent fuel pools at the Fukushima Daiichi nuclear power plant, hydrazine was added to salt-containing water in order to reduce dissolved oxygen. Hydrazine is known to reduce dissolved oxygen in high-temperature pure water, but its deoxygenation behavior in salt-containing water at ambient temperature in the presence of radiation is unknown. Deoxygenation using hydrazine in salt-containing water was thus investigated using a 60Co gamma-ray source and artificial seawater at room temperature. Water samples containing a small amount of hydrazine were irradiated at dose rates of 100–10,000 Gy/h. The concentration of dissolved oxygen in the water samples was measured before and after irradiation. Notably, a decrease in the dissolved oxygen was only observed after irradiation, and the dissolved oxygen concentration decreased with increasing dose rate and irradiation time. The rate of decrease in the amount of dissolved oxygen using hydrazine was slow in the presence of salts. Kinetic considerations suggested that the deoxygenation of the salt-containing water exposed to gamma-ray irradiation using hydrazine was suppressed by chloride ions.
1. Introduction
After the 2011 Tohoku earthquake and Tsunami, the cooling systems at the Fukushima Daiichi nuclear power plant failed. As an emergency measure, seawater was poured into the spent fuel pools (SFPs) of units 2, 3 and 4 in order to cool the spent fuels. This action led to high concentrations of chloride ions in the SFP water. In the current cooling system for the SFPs, oxygen from the air dissolves in the water. To reduce the amount of dissolved oxygen (DO), and inhibit the corrosion of the materials comprising the SFPs, after May 2011, Tokyo Electric Power Co. added hydrazine (N2H4) to the SFPs.
Prior to installation of a cooling system using fresh water, an analysis of the SFP water revealed that the chloride ion concentration was approximately 2500 ppm in the unit 4 SFP water in May 2011Citation[1]. Subsequent to the installation of a cooling system using fresh water, the analysis data regarding the N2H4 concentration were released in September 2011[Citation2]. As shown in , the concentration of chloride ion decreased to 997 ppm and the N2H4 concentration was 59 ppm. The DO concentration was not reported.
Table 1 Sampling result for water from the spent fuel pool at Fukushima Daiichi nuclear plant unit 4 [Citation2]
In thermal power plants, N2H4 is used as a deoxidant for high-temperature water, but it is known that the deoxygenation effect is weak for room temperature water Citation[3]. In the SFPs of nuclear power plants, the stored highly radioactive spent fuels emit alpha, beta and gamma rays, with the main radiation component being gamma rays. It has been reported that the gamma radiolysis products of N2H4 react with the oxygen molecules, and the DO concentration in a sample of irradiated water is reduced as a result of N2H4 addition [Citation4–Citation8]. However, these data were not obtained for salt-containing water, but for distilled water. The deoxygenation behavior of N2H4 on DO in salt-containing water is not known.
Therefore, in this study the reaction of DO and N2H4 was studied in salt-containing water exposed to gamma-ray irradiation. A small amount of N2H4 was added to salt-containing water, and the water was irradiated at room temperature. After irradiation, the reaction of DO and N2H4 was evaluated via quantitative analysis of both DO and N2H4. Herein is presented a discussion of the effect of gamma-ray irradiation on the reaction of DO and N2H4 in salt-containing water as a function of the concentration of N2H4 and the dose rates.
2. Experimental
2.1. Test solutions
Distilled water and artificial seawater (ASW) were used to prepare the test solutions. The ASW was prepared using Aquamarin (Yashima Pure Chemical Co. Ltd.), and the chemical composition of the ASW is listed in . The chloride ion concentration of the ASW was approximately 19,000 ppm. A sample of the ASW, diluted with distilled water at a ratio of 1:7, had a chloride ion concentration of approximately 2500 ppm. Hereafter, the diluted ASW is abbreviated as 1/8 ASW. Analytical grade hydrazine mono-hydrate (98.0 wt% purity) was also used. The test solutions containing 32 ppm N2H4 had pH of approximately 9.3 at 298 K, while the solutions without N2H4 were adjusted to pH 9.3 at 298 K using NaOH solution. All of the test solutions were aerated. Before gamma-ray irradiation, a test solution (25 cm3) was sealed in a test tube without any head space. It was found that the concentration of DO in the distilled water was greater than that in the ASW and that the presence of salts influenced the amount of DO. The quantity of DO in distilled water and the ASW at 293K under atmospheric pressure were reported to be 9.1 ppm and 7.2 ppm, respectively [Citation9].
Table 2 Chemical composition of artificial seawater (ppm)
2.2. Gamma-ray irradiation testing
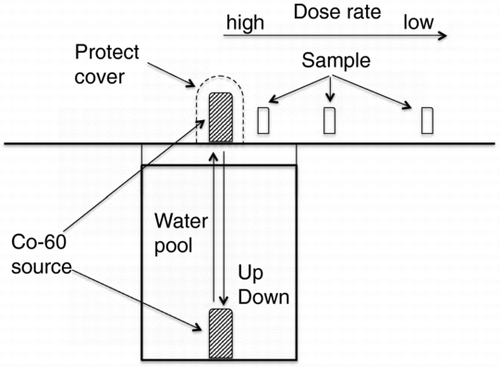
Gamma-ray irradiation testing was conducted at the 60Co gamma-ray irradiation facility of Japan Atomic Energy Agency. shows the layout of the 60Co gamma-ray source and samples. Gamma-ray irradiation was performed using an elevator system in which one irradiation cycle involved lifting the 60Co gamma-ray source from the bottom of a water pool to the top, followed by lowering it back to the bottom. The dose rate was controlled by changing the distance between the sample and the source. The total dose was controlled by changing the irradiation time. The test solutions were irradiated at room temperature at dose rates of 100–10,000 Gy/h. The dose rate at each irradiation position was determined using an alanine dosimeter (Aminogray made by Hitachi Cable, Ltd.). The temperature of the test solutions was not controlled and ranged from 298 to 303 K before and after irradiation.
2.3. Quantitative analysis of the test solutions
The DO and N2H4 concentrations were measured before and after irradiation. The amount of DO was quantified via the indigo carmine method, and the accuracy of the quantification was evaluated using a fluorescence-type DO meter. The amount of N2H4 was quantified via the para-dimethylaminobenzaldehyde method, and the samples were diluted with distilled water before each measurement. The amount of H2O2, which is one of the water radiolysis products that affects corrosion [Citation10], was quantified using the tetra-aminoantipyrine method.
3. Results
3.1. Effect of gamma-ray irradiation on deoxygenation by N2H4
The test solutions (distilled water, 1/8 ASW and ASW with 32 ppm N2H4) were irradiated at dose rates of 0.1–10 kGy/h for 1 h. The concentration of DO before and after irradiation is shown in . For comparison, the concentration of DO in samples, allowed to sit for 1 h in the absence of gamma radiation (0 Gy/h), is also plotted. As can be seen in the figure, the concentration of DO in the nonirradiated samples was nearly the same as that in the samples before the tests. In contrast, the concentration of DO in the irradiated samples decreased markedly with increasing dose rate and was less than 1 ppm at dose rates above 1 kGy/h. Notably, at a dose rate of 0.1 kGy/h, the decrease in the DO concentration in the ASW was less than that in the distilled water and 1/8 ASW.
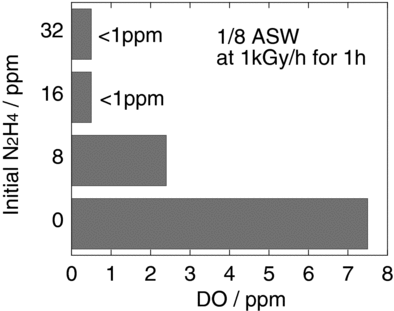
3.2. Effect of N2H4 concentration on deoxygenation under gamma-ray irradiation
The test solutions (1/8 ASW with N2H4 concentrations from 0 to 32 ppm) were irradiated at a dose rate of 1 kGy/h for 1 h, and the concentration of DO after irradiation is shown in . The initial concentration of DO in the test solutions was 8 ppm. In the solution with 8 ppm N2H4, the concentration of DO was reduced to approximately 2.3 ppm after irradiation, while in the solutions with more than 16 ppm N2H4, the amount of DO fell below 1 ppm. Therefore, the addition of N2H4 at more than double the concentration of DO enabled the removal of DO in dilute salt water exposed to gamma-ray irradiation.
3.3. Changes in the N2H4 and DO concentrations
Samples of distilled water, 1/8 ASW and ASW with 32 ppm N2H4 were irradiated at a dose rate of 1 kGy/h, and the change in the concentration of DO with time is plotted in . It can be seen in the figure that the DO concentration decreased with increasing irradiation time. It was reduced to less than 1 ppm, in 10 min for the distilled water and 1/8 ASW samples, and in 30 min for the ASW sample. Deoxygenation using N2H4 was clearly slower in the ASW. The change in the concentration of N2H4 with time was also investigated (). In this case, the tendency of the N2H4 concentration to decrease was similar for all test solutions – the concentration of N2H4 decreased with increasing irradiation time and fell from 32 ppm to approximately 8 ppm in 60 min.
3.4. Deoxygenation by gamma-ray irradiation without N2H4
Distilled water, 1/8 ASW and ASW without N2H4 (0 ppm N2H4) were irradiated at a dose rate of 1 kGy/h for 1 h. shows the change in the concentration of DO as a function of time. The quantity of DO in the distilled water decreased by approximately 2 ppm, while the change in the amount of the DO in the 1/8 ASW and ASW samples was small or not observed.
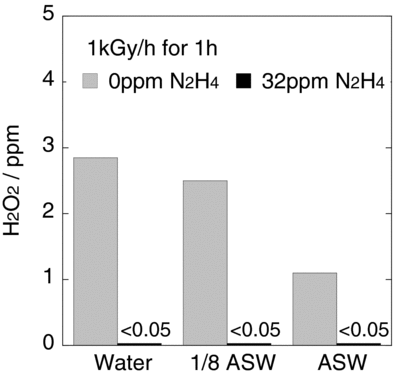
3.5. H2O2 production by gamma radiolysis, with and without N2H4
The concentration of H2O2 produced by gamma radiolysis, with and without N2H4, was measured. shows the H2O2 concentration in distilled water, 1/8 ASW and ASW, each without N2H4 and with 32 ppm N2H4 at 1 kGy/h for 1 h. Without N2H4 addition, the concentration of H2O2 decreased as the quantity of salts increased: 2.8 ppm in distilled water, 2.5 ppm in 1/8 ASW and 1.1 ppm in ASW. The concentration of H2O2 in the test solutions with 32 ppm N2H4 was less than 0.05 ppm.
4. Discussion
4.1. Decrease in N2H4 and dissolved oxygen during and after deoxygenation: G(-O2) and G(-N2H4)
As shown in Figures and , the concentration of DO and N2H4 decreased with increasing irradiation time. The values for G(-O2) and G(-N2H4) were calculated from the data in Figures and and are listed in Tables and , respectively. For the pH 9.3 distilled water, G(-O2) was 18.0 × 10−7 mol/J after exposure to 1 kGy/h radiation for 10 min (total dose - 167 Gy), while for the pH 9.3 ASW, G(-O2) was 11.0 × 10−7 mol/J after exposure to 1 kGy/h radiation for 10 min and 5.8 × 10−7 mol/J after 60 min (total dose - 500 Gy). G(-N2H4) in the pH 9.3 distilled water ranged from 7.6 × 10−7 to 21.5 × 10−7 mol/J, and that in the pH 9.3 ASW ranged from 8.3 × 10−7 to 24.9 × 10−7 mol/J.
Table 3 G(-O2) values in distilled water and artificial seawater with 32 ppm hydrazine at a dose rate of 1 kGy/h (10−7 mol/J)
Table 4 G(-N2H4) values in distilled water and artificial seawater with 32 ppm hydrazine at a dose rate of 1 kGy/h (10−7 mol/J)
The behavior of G(-N2H4) in distilled water has been previously studied by Lefort and Ershov. Lefort [Citation4] determined G(-N2H4) for pH 9.5 distilled water with 32 ppm N2H4, irradiated at a dose rate of 0.293 Gy/s (1 kGy/h), to be 13 × 10−7 mol/J at a dose of 500 Gy. Ershov [Citation5] also measured G(-N2H4) for pH 9.5 distilled water with 32 ppm N2H4 but at a dose rate of 0.113 Gy/s (∼ 400 Gy/h), and obtained the same result of 13 × 10−7 mol/J at a dose of 500 Gy. The value obtained for G(-N2H4) in this study for pH 9.3 distilled water with 32 ppm N2H4 was 10.3×10−7 mol/J at a dose of 500 Gy, which is quite similar to the previously reported results.
4.2. Deoxygenation mechanism in salt-containing water exposed to gamma-ray irradiation
4.2.1. Reaction among oxygen, water radiolysis products and hydrazine
The primary water radiolysis products were found to be e− aq, H·, OH·, H2 and H2O2 [Citation11]. The reaction between hydrated electrons (e− aq) and oxygen molecules (O2) [Equation (1)] was fast [Citation12], while the reaction between e− aq and N2H4 [Equation (2)] was slow [Citation13].
4.2.2. Deoxygenation by N2H4 in the presence of salts
As shown in Figure , the rate of decrease in the concentration of DO during gamma-ray irradiation was lower in the ASW than in the distilled water. This result suggests that the salts in the ASW affected the deoxygenation. The mechanism of deoxygenation by N2H4 in the ASW is considered to be as follows:
First, the oxygenation of water with N2H4 under gamma-ray irradiation can be expressed by the reactions in Equations (3), (4) and (5) [Citation6,Citation13]:
The hydroxyl radical (OH.) is therefore a key chemical species in the deoxygenation process. Since the ASW contained a large amount of chloride ions, the reaction expressed in Equation (6) should also occur:
This reaction can influence reaction (3) [Citation14]; for the ASW with 32 ppm N2H4, the value of k (3) was approximately five times greater than that of k (6), while the concentration of chloride ions was approximately 6000 times greater than that of N2H4. In another test using 3.5 wt% NaCl solution that had nearly the same chloride ion concentration (∼21,000 ppm) as that of the ASW, the rate of decrease of the DO concentration during 1 kGy/h irradiation was also slow. The concentration of DO in the ASW with 32 ppm N2H4 after 10 min was 3.8 ppm, and that in the 3.5 wt% NaCl solution was 2.4 ppm, whereas that in the distilled water was less than 1 ppm. The chloride ion was the main common anion in both ASW and 3.5 wt% NaCl solution. These results indicate that chloride ions affect the deoxygenation with N2H4 of salt-containing water that is exposed to gamma-ray irradiation. The small difference in the concentration of DO in the ASW and 3.5 wt% NaCl solution may be caused by the presence of additional anions (SO4 2−, HCO3 −, Br−, etc.) in the ASW.
4.2.3. Decrease of H2O2 in the presence of chloride ions or N2H4
Buxton [Citation8] reported on the deoxygenation of distilled water using N2H4. He investigated the reaction between oxygen and N2H4 molecules using a pulse radiolysis technique. The deoxygenation with N2H4 could be expressed as in Equations (3), (4) and (5). Water radiolysis gave H2O2 as follows [Citation15]:
In the absence of N2H4, as shown in Figure , the concentration of H2O2 decreased with increase in the quantity of salts. It suggests that the chloride ions scavenged preferentially the hydroxyl radical (·OH). The produced H2O2 can decompose according to Equation (8) [Citation16], and thus, it indicates that the rate of decomposition is very slow at room temperature.
The produced H2O2 can also react with ·OH according to Equation (9) [Citation17]. The value of k (7) was approximately 160 times greater than that of k (9). It indicates that the amount of produced H2O2 is much greater than that of decomposed H2O2.
In alkaline solutions,.OH reacts with OH− [Citation18]:
The pH of the test solutions was changed to a little lower direction (from 9.3 to 9.1) by gamma-ray irradiation; the change in the OH− concentration is approximately 7 × 10−6 mol · dm−3 (0.1 ppm). It suggests that hydroxyl radicals react preferentially with hydroxyl radicals and chloride ions. The value of k (6) was slightly less than that of k (7), and the concentration of zchloride ion was the greatest among that of anions in the test solutions. The chloride ion should affect the production process of H2O2. In the presence of N2H4, as shown in Figure , the concentration of H2O2 was less than 0.05 ppm owing to the reaction of N2H4 with OH·, as depicted in Equation (3); the value of k (3) was greater than that of k (7), and initial concentration of N2H4 (32 ppm) was greater than the maximum concentration of H2O2 (2.8 ppm), detected in the absence of N2H4.
5. Conclusion
Distilled water and salt-containing water samples with a small amount of N2H4 were irradiated with a 60Co gamma-ray source. After the gamma-ray irradiation, the concentrations of N2H4 and DO in the samples were measured. The same measurements were also conducted for nonirradiated samples. The following results were obtained:
| 1. | The rate of deoxygenation by N2H4 at room temperature was slow in the absence of gamma-ray radiation. In contrast, it was fast in the presence of gamma radiation. | ||||
| 2. | The deoxygenation occurred via the radiolysis of N2H4. | ||||
| 3. | The rate of deoxygenation by hydrazine under gamma-ray irradiation was slowed in the presence of salts. | ||||
| 4. | The deoxygenation of salt-containing water with hydrazine is suppressed by chloride ions. | ||||
Notes
a From reference [Citation4].
References
- METI Measures and Requests in response to the Great East Japan Earthquake at home page of MInistry of Economy, Trade and Industry http://www.meti.go.jp/earthquake/nuclear/pdf/120227_03aa.pdf
- Tokyo Electronic Power Company http://www.tepco.co.jp/en/nu/fukushima-np/images/handouts_110903_04-e.pdf, handouts at press conference Sep 03, 2011
- Tsubakizaki , S , Takada , M , Gotou , H , Mawatari , K , Ishihara , N and Kai , R. 2009 . Alternatives hydrazine in water treatment at thermal power plant . Mitsubishi Heavy Industries Tech Rev. , 46 ( 2 ) : 43
- Lefort , M and Hassinsky , M . 1956 . Decomposition radiochimique de l’hydrazine en solution aqueuse . J Chim Phys. , 53 : 527 – 535 .
- Ershov , B G , Mikhailova , T L and Emel’yanova , YuA . 1989 . Radiochemical oxidation of hydrazine in aqueous solutions containing oxygen . Russian Chem Bull. , 38 : 2249 – 2252 . Translated from Izv Akad Nauk SSSR, Ser Khim. 1988;11:2450–2458
- Ershov , B G , Mikhailova , T L and Emel’yanova , YuA . 1991 . A study by pulse radiolysis of the radiation-chemical transformation of aqueous solutions of hydrazine in the presence of oxygen . Russian Chem Bull. , 40 : 288 – 292 . Translated from Izv Akad Nauk SSSR, Ser Khim. 1991, 2:341–345
- Buxton , G V and Stuart , C R . 1997 . Radiation chemistry of aqueous solutions of hydrazine at elevated temperatures. Part 2 – Solutions containing oxygen . J Chem Soc Faraday Trans. , 93 ( 8 ) : 1535 – 1538 .
- Buxton , G V and Lynch , D A . 1999 . Radiation chemistry of aqueous solutions of hydrazine at elevated temperatures Part 3. The chain reaction in oxygenated solutions irradiated with 60Co γ-rays . Phys Chem Chem Phys. , 1 : 3293 – 3296 .
- Radtke , D B , White , A F , Davis , J V and Wilde , F D . 1998 . Field measurments techniques of water-resources investigations , U.S. geological survey . Section 6.2, Chapter 9, Books 9, Geological Survey. 34--35. Available from: http://pubs.water.usgs.gov/owq/FieldManual/Chapter6/6.2.v2.1.pdf
- Macdonald , D D . 1992 . Viability of hydrogen water chemistry for protecting in-vessel components of boiling water reactors . Corrosion. , 48 : 194 – 205 .
- Kiefer , Jurgen . 1989 . Biological radiation effects. , New York : Springer-Verlag .
- Elliot , A J . 1989 . A pulse radiolysis study of the temperature dependence of reactions involving H, OH and eaq − in aqueous solutions . Radiat Phys Chem. , 34 : 753 – 758 .
- Hayon , E and Simic , M . 1972 . Intermediates produced from the one-electron oxidation of hydrazine. Evidence for the formation and decay of tetrazane and triazene . J Am Chem Soc. , 94 : 42 – 47 .
- Grigor’ev , A E , Makarov , I E and Pikaev , A K . 1987 . Formation of Cl2 − in the bulk solution during the radiolysis of concentrated aqueous solutions of chlorides . High Energy Chem. , 21 : 99 – 102 .
- Elliot , A J , McCracken , D R , Buxton , G V and Wood , N D . 1990 . Estimation of rate constants for near-diffusion-controlled reactions in water at high temperatures . J Chem Soc Faraday Trans. , 86 : 1539 – 1547 .
- Hiroki , A and LaVerne , J A . 2005 . Decomposition of hydrogen peroxide at water-ceramic oxide interfaces . J Phys Chem B. , 109 : 3364 – 3370 .
- Buxton , G B , Greenstock , C L , Helman , W P and Ross , A B . 1988 . Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution . J Phys Chem Ref Data. , 17 : 513 – 886 .
- Buxton , G B . 1970 . Pulse radiolysis of aqueous solutions. Rate of reaction of OH with OH . Trans Faraday Soc. , 66 : 1656 – 1660 .
![Figure 2 Comparison of the dissolved oxygen concentrations without and with gamma-ray irradiation at different dose rates for 1 h. Water, distilled water; ASW, artificial seawater; 1/8 ASW, distilled water/ASW = 1:7. Initial [N2H4] = 32 ppm](/cms/asset/70cbc99c-d65b-455f-b349-57db51f5ad02/tnst_a_773159_o_f0002g.gif)
![Figure 4 Change in the dissolved oxygen concentration with time for distilled water, 1/8 ASW and ASW during 1 kGy/h irradiation. Water, distilled water; ASW, artificial seawater; 1/8 ASW, distilled water/ASW = 1:7. Initial [N2H4] = 32 ppm](/cms/asset/3b652709-c79a-4bdb-a2b1-96f2fa646c02/tnst_a_773159_o_f0004g.gif)
![Figure 5 Change in the hydrazine concentration with time for distilled water, 1/8 ASW, and ASW during 1 kGy/h irradiation. Water, distilled water; ASW, artificial seawater; 1/8 ASW, distilled water/ASW = 1:7. Initial [N2H4] = 32 ppm](/cms/asset/c9d1b5f5-e33b-4590-b58d-0d872cacb774/tnst_a_773159_o_f0005g.gif)
![Figure 6 Amount of dissolved oxygen in distilled water, 1/8 ASW and ASW without N2H4 for 0 and 1 h of 1 kGy/h irradiation at room temperature. Water, distilled water; ASW, artificial seawater; 1/8 ASW, distilled water/ASW = 1:7. Initial [N2H4] = 32 ppm](/cms/asset/4975f128-4547-49c1-954a-a60f2263f0b1/tnst_a_773159_o_f0006g.gif)