Abstract
We studied the adsorption behavior of radioactive cesium (Cs) by the non-mica minerals kaolinite, halloysite, chlorite, montmorillonite, mordenite, MnO2, TiO2, Al2O3, and FeOOH to elucidate the environmental behavior of radioactive Cs fallout from the Fukushima Daiichi Nuclear Power Plant in the Tohoku region of Japan. The adsorption and desorption experiments of Cs on the minerals were carried out at the Cs concentrations 1 × 10−4, 1 × 10−5, and 2 × 10−9 mole L−1 at pH 5.5. The desorption of Cs from the minerals was examined using 0.1 mole L−1 LiCl, NaCl, KCl, RbCl, and CsCl solutions. The sequential desorption was examined using a 0.1 mole L−1 LiCl solution, a 1 mole L−1 KCl solution, and a 1 mole L−1 HCl solution. The distribution coefficient (K d) for the minerals at the Cs concentration 10−9 mole L−1 was in the order of mordenite > illite > montmorillonite, sericite, MnO2, kaolinite, and halloysite > chlorite, TiO2, Al2O3, and FeOOH, differing from the order observed at higher Cs concentrations. After the sequential desorption by the three reagent solutions, the residual fraction of Cs was higher at the Cs concentration 10−9 mole L−1 than at higher concentrations. Approximately 40%, 40%, 50%, and 25% of the adsorbed Cs were residual in montmorillonite, mordenite, MnO2, and kaolinite, respectively, after the sequential desorption. These results strongly suggest that (1) radioactive Cs at 10−9 mole L−1 is more strongly associated with the non-mica minerals than at higher concentrations of 1 × 10−4 and 1 × 10−5 mole L−1, and (2) the non-mica minerals montmorillonite, mordenite, kaolinite, and MnO2 contributed to the fixation of the radioactive Cs fallout on Fukushima soil.
1. Introduction
The nuclear accident at the Fukushima Daiichi Nuclear Power Plant (FDNPP) occurred as a consequence of the massive earthquake and associated tsunami that struck the Tohoku and north Kanto regions of Japan on 11 March 2011. A series of core melt [Citation1–Citation3] and hydrogen explosions occurred from 13 March to 15 March at FDNPP Units 1, 2, and 3. The rate of the release of radioactive cesium (134Cs and 137Cs) from FDNPP on 15 March was estimated to be between 1012 and 1015 Bq/hour [Citation4]. This fallout of radioactive Cs was dispersed from the FDNPP to the Pacific Ocean [Citation4–Citation10]. Some of the released radioactive Cs was deposited on the ground in the areas of Fukushima, Tochigi, Ibaraki, Chiba, and Tokyo. The spatial concentration, distribution, and depth profiles of radioactive Cs were measured [Citation9, Citation10] to estimate the dose rate and to estimate the fate of the Cs in the terrestrial environment. Those studies determined that the highest concentration of radioactive Cs in soil was approx. 105 Bq/kg [Citation10], being equivalent to approx. 2.3 × 10−10 mole/kg. Since the chemical concentration of Cs deposited on the soil is very low, the environmental behavior of Cs at very low concentrations should be clarified.
For the estimation of the environmental behavior of radioactive Cs, the sorption behavior of the radioactive Cs on soil should be clarified. Soil is composed of various components, including minerals, clay minerals, metal oxides/hydroxides, and organic materials. Cesium at a low concentration is strongly sorbed on mica-like minerals of sediments [Citation11–Citation13]. Kozai et al. have examined the Cs fallout on soils collected in Fukushima, Japan, and they found that not only mica-like minerals, but also minerals other than mica-like minerals (non-mica minerals) probably fixed the deposited radioactive Cs in the soil [Citation14].
Non-mica minerals include kaolinite, halloysite, chlorite, montmorillonite, mordenite, MnO2, TiO2, Al2O3, FeOOH, and others. The adsorption behavior of Cs on some of the non-mica minerals has been studied [Citation15–Citation20]. Most of these studies measured the distribution coefficient (K d) of Cs, and it was determined that 2:1 clay mineral of montmorillonite has a high K d and that 1:1 clay mineral of kaolinite has a low K d. However, the characteristics of the adsorption of Cs on non-mica minerals have not been fully understood.
In the present study, we conducted adsorption and desorption experiments for radioactive Cs using the non-mica minerals kaolinite, halloysite, chlorite, montmorillonite, mordenite, MnO2, TiO2, Al2O3, and FeOOH. We also carried out adsorption experiments of Cs on the mica-like minerals illite and sericite for a comparison of mica-like minerals and non-mica minerals. Based on the results, we discuss the association of radioactive Cs on non-mica minerals and mica-like minerals.
2. Experimental
2.1. Materials
The non-mica minerals used were the natural products mordenite, montmorillonite, todorokite (MnO2), kaolinite, halloysite, and chlorite, and synthetic TiO2 (rutile), Al2O3, and FeOOH. The mica-like minerals used were the natural products illite and sericite. The natural minerals kaolinite, sericite, halloysite, illite, MnO2, and chlorite were purchased from Nihon Chikagaku-sha Co. (Kyoto, Japan), and montmorillonite and mordenite from Kunimine Kogyo Co. (Tokyo, Japan). Synthetic TiO2, Al2O3, and FeOOH were used. The sizes of the minerals and metal oxides were less than 0.2 mm. X-ray diffraction (XRD) patterns showed that the minerals did not contain any other minerals. All minerals were washed with deionized water before being used for the adsorption experiments.
2.2. Sorption and desorption experiments
A Cs solution was prepared by diluting CsCl in a solution of CH3COONa (0.01 mole L−1). The solution was adjusted to pH 5.5 with the CH3COOH solution. The concentration of Cs in the 137Cs solution was 2 × 10−9 mole L−1. A stable Cs solution (1 × 10−4 or 1 × 10−5 mole L−1) was added to the 137Cs solution, and finally three different Cs concentrations of 1 × 10−4, 1 × 10−5, and 2 × 10−9 mole L−1 solution were prepared for the adsorption and desorption experiments. The adsorption of Cs on the minerals was changed with pH [Citation14]. Thus, CH3COO− was used for pH buffer.
The sorption and desorption experiments were carried out in polycarbonate tubes. Cesium sorption on the tube walls was less than 0.1% at pH 5.5 over a period of 4 days. Then, 0.5 g of each mineral (except for montmorillonite, mordenite, and illite) were contacted with 50 mL of the Cs solutions. For montmorillonite, mordenite, and illite, 0.1 g was used. Duplicate samples were equilibrated for 4 days at 20°C. The final pH values of the Cs solutions ranged from 5.4 to 5.7. Note that the time course of adsorption of Cs at the three concentrations showed that the adsorption attained equilibrium within 4 days.
After the adsorption experiments, each mixture of the adsorbents and the Cs solution was centrifuged for 1 hour at 10,000 rpm and then the supernatant of 10 mL was filtered through a hydrophilic PTFE filter (0.2 μm; Advantec, Tokyo, Japan) for measurements of the concentration of Cs and the pH of the solution. Subsequently, the distribution ratios of Cs between the minerals and the solutions were obtained. The distribution ratio (K d: mL g−1) was given by
All minerals were washed with deionized water after removing the Cs solutions and then were separately contacted with a 0.1 mole L−1 LiCl, NaCl, KCl, RbCl, or CsCl solution (pH 5.5) for 16 hours at 20°C. The solutions were separated from the minerals by centrifugation for 1 hour at 10,000 rpm. The radioactivity of the supernatant was measured after filtration through a hydrophilic 0.2-μm PTFE filter. The minerals treated with the 0.1 mole L−1 LiCl solution were then soaked with a 1 mole L−1 KCl solution (pH 7) for 16 hours at 20°C after being washed with deionized water. The minerals were then soaked with a 1 mole L−1 HCl solution for 16 hours at 20°C after being washed with deionized water. The KCl and HCl solutions were separated from the minerals by the centrifugation for 1 h at 10,000 rpm. The radioactivity of the supernatant was measured after filtration through a hydrophilic 0.2-μm PTFE filter. The residual minerals after the treatment were washed with deionized water and then used for the measurement of the Cs concentration. Note that the time course of desorption of Cs by all of the reagents solution showed that the desorption attained equilibrium within 16 hours.
The radioactivities of 137Cs in the minerals and in the solutions were measured by γ-spectrometer (Ortec, Oak Ridge, TN). An HM-30S pH meter (Toa, Kobe, Japan) with a combined electrode (GS-5015C; Toa) was used to measure the pH values of the solutions.
3. Results
3.1. Adsorption behavior of Cs at different concentrations
The distribution coefficients (K d) of Cs by minerals and clay minerals at different Cs concentrations (Figure ) showed that the K d for montmorillonite and mordenite was nearly constant at Cs concentrations between 2 × 10−9 and 10−4 mole L−1. At the 10−9 mole L−1 Cs concentration, the K d values for the minerals were in the order of: mordenite > illite > montmorillonite, sericite, MnO2, kaolinite, halloysite > chlorite, FeOOH, TiO2, Al2O3. The K d values for chlorite, FeOOH, TiO2, and Al2O3 were less than 10 mL g−1, indicating that Cs was hardly adsorbed by chlorite, FeOOH, TiO2, and Al2O3 at pH 5.5 solution. Thus, the K d values for chlorite, FeOOH, TiO2, and Al2O3 are not shown in Figure .
Figure 1 The distribution coefficients (K d) of Cs by minerals and clay minerals at different Cs concentrations. Deviation of duplicate measurement was within the symbols
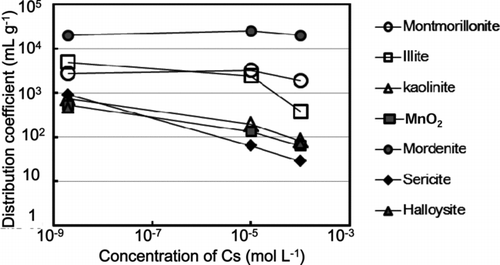
At the Cs concentrations 10−4 and 10−5 mole L−1, the K d values of Cs for the minerals were in the following order:
This order differs from that at the low Cs concentration, 10−9 mole L−1. These results strongly suggest that the sorption behavior of Cs depends on the concentration of Cs.
3.2. Desorption behavior of Cs at different concentrations
Fractions of Cs at 10−9 mole L−1 desorbed by different univalent cations at 25°C from montmorillonite, illite, kaolinite, MnO2, mordenite, halloysite, and sericite are shown in Figure . The desorption fraction of Cs from montmorillonite was approx. 2% by 0.1 M LiCl and NaCl solutions, and increased with the change in the reagent solution of a 0.1 M KCl solution to a a 0.1 M RbCl solution, and attained 40% by a 0.1 M CsCl solution. From mordenite, a monotonic increase in the desorbed fraction was achieved by changing from a 0.1 M LiCl solution to the NaCl, the KCl, and the RbCl solutions, and attained 80% by a 0.1 M CsCl solution. A monotonic increase was also observed for the desorption from halloysite, even though the desorbed fraction from halloysite attained 90% by the KCl solution. Approximately 8% of the adsorbed Cs was desorbed from kaolinite by the 0.1 M LiCl solution, and increased to about 40% by the 0.1 M KCl solution, and rose slightly to approx. 44% by the RbCl and Cs solutions. From the MnO2, about 4% of the adsorbed Cs was desorbed by the 0.1 M LiCl solution, and elevated to about 35% by the 0.1 M KCl solution, followed by a descent with the 0.1 M RbCl and CsCl solutions. Less than 1% of the adsorbed Cs was desorbed from illite by the 0.1 M LiCl solution and the 0.1 M NaCl solution, and increased to about 8% by 0.1 M KCl, the 0.1 M RbCl solution, and the 0.1 M CsCl solution. From sericite, less than 4% of the adsorbed Cs was desorbed by the 0.1 M LiCl solution, and the fraction increased to 10% by the 0.1 M NaCl solution, 40% by the 0.1 M KCl solution, 20% by the 0.1 M RbCl solution, and 17% by the 0.1 M CsCl solution.
Figure 2 Fractions of Cs at 2 × 10−9 mole L−1 desorbed by different univalent cations at 20°C from montmorillonite, illite, kaolinite, MnO2, mordenite, halloysite, and sericite. Deviation of duplicate measurement was within the symbols
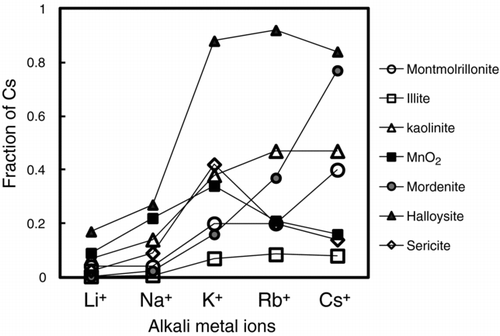
These results indicate that the desorption behavior of Cs at 10−9 mole L−1 from the minerals fit two categories: the first category contains montmorillonite and mordenite, with a monotonic increase in the desorbed fraction by Li to Cs. The second category includes illite, sericite, MnO2, and kaolinite, where the desorbed fraction increased between Li and K, followed by nearly the same fraction or the descent between K and Cs.
Figure a shows the sequential desorption of the adsorbed Cs at 10−9 mole L−1 by the 0.1 M LiCl solution, the 1 M KCl solution, the 1 M HCl solution, and the residual from montmorillonite, illite, kaolinite, MnO2, mordenite, sericite, and halloysite. The desorption fraction of Cs from montmorillonite was approx. 2% by the 0.1 M LiCl solution, about 45% by the 1 M KCl solution, 15% by the 1 M HCl solution, and approx. 40% was residual after the sequential desorption. Approximately 8% of the adsorbed Cs was desorbed from kaolinite by the 0.1 M LiCl solution, 60% by the 1 M KCl solution, and 8% by the 1 M HCl solution. More than 20% of the adsorbed Cs was residual in kaolinite after the treatment with the three reagent solutions. Approximately 4% of the adsorbed Cs was desorbed from MnO2 by the 0.1 M LiCl solution, 5% by the 1 M KCl solution, and 40% by the 1 M HCl solution. Approximately 50% of the adsorbed Cs was residual in MnO2 after the treatment with the three reagent solutions. Less than 1% of the adsorbed Cs was desorbed from mordenite by the 0.1 M LiCl solution, 55% by the 1 M KCl solution, and 5% by the 1 M HCl solution, and approx. 40% of the adsorbed Cs was residual after the treatment. Approximately 20% of the adsorbed Cs was desorbed from halloysite by the 0.1 M LiCl solution, 70% by the 1 M KCl solution, and 5% by the 1 M HCl solution, and approx. 5% of the adsorbed Cs was residual after the treatment. From illite, less than 20% of the adsorbed Cs was desorbed after the treatment with the 0.1 M LiCl solution, the 1 M KCl solution, and the 1 M HCl solution. Approximately 3% of the adsorbed Cs was desorbed from sericite by the 0.1 M LiCl solution, 25% by the 1 M KCl solution, and 20% by the 1 M HCl solution, and approx. 50% of the adsorbed Cs was residual after the treatment.
Figure 3 The sequential desorption of the adsorbed Cs at 10−9 mole L−1 (a), 10−5 mole L−1 (b), and 10−4 mole L−1 (c) by the 0.1 M LiCl solution, the 1 M KCl solution, the 1 M HCl solution, and the residual from montmorillonite, illite, kaolinite, MnO2, mordenite, sericite, and halloysite. Desorption data of Cs at 10−5 mole L−1 from halloysite was not measured because of the lack of the sample of halloysite
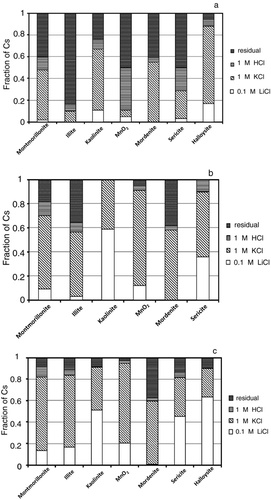
At the Cs concentration 10−5 mole L−1, the desorption behavior of Cs by the three different reagent solutions (Figure ) showed a manner that was slightly different from that at 10−9 mole L−1. The desorption fraction of Cs from montmorillonite was approx. 10% by the 0.1 M LiCl solution, about 60% by the 1 M KCl solution, and 10% by the 1 M HCl solution, and approx. 20% was residual after the sequential desorption. More than 50% of the adsorbed Cs was desorbed from kaolinite by the 0.1 M LiCl solution, and all of the adsorbed Cs was desorbed after the treatment with the 1 M KCl solution. Approximately 12% of the adsorbed Cs was desorbed from MnO2 by the 0.1 M LiCl solution, 80% by the 1 M KCl solution, and 4% by the 1 M HCl solution. Only 5% of the adsorbed Cs was residual in MnO2 after the treatment with the three reagent solutions. Less than 1% of the adsorbed Cs was desorbed from mordenite by the 0.1 M LiCl solution, 55% by the 1 M KCl solution, and 5% by the 1 M HCl solution, and approx. 40% of the adsorbed Cs was residual after the treatment. From illite, about 4% of the adsorbed Cs was desorbed by the 0.1 M LiCl solution, 50% by the 1 M KCl solution, and 10% by the 1 M HCl solution. Approximately 37% of the adsorbed Cs was desorbed from sericite by the 0.1 M LiCl solution and 50% by the 1 M KCl solution, and all of the adsorbed Cs was desorbed after the treatment with the 1 M HCl solution.
At the 10−4 mole L−1 Cs concentration, the desorption behavior of Cs by the three different reagent solutions (Figure ) showed that higher amounts of the adsorbed Cs was desorbed than that at 10−9 and 10−5 mole L−1. The desorption fraction of Cs from montmorillonite was approx. 15% by the 0.1 M LiCl solution, about 70% by the 1 M KCl solution, and 10% by the 1 M HCl solution, and approx. 10% was residual after the sequential desorption. Approximately 50% of the adsorbed Cs was desorbed from kaolinite by the 0.1 M LiCl solution and 40% by the 1 M KCl solution, and only 10% of the adsorbed Cs was residual after the treatment. Approximately 20% of the adsorbed Cs was desorbed from MnO2 by the 0.1 M LiCl solution, 75% by the 1 M KCl solution, and 3% by the 1 M HCl solution. Only 2% of the adsorbed Cs was residual in MnO2 after the treatment with the three reagent solutions. Less than 1% of the adsorbed Cs was desorbed from mordenite by the 0.1 M LiCl solution, 55% by the 1 M KCl solution, and 5% by the 1 M HCl solution, and approx. 40% of the adsorbed Cs was residual after the treatment. Approximately 60% of the adsorbed Cs was desorbed from halloysite by the 0.1 M LiCl solution, 30% by the 1 M KCl solution and 2% by the 1 M HCl solution, and approx. 7% of the adsorbed Cs was residual after the treatment. From illite, about 18% of the adsorbed Cs was desorbed by the 0.1 M LiCl solution, 65% by the 1 M KCl solution, and 10% by the 1 M HCl solution. Only 12% of the adsorbed Cs was residual in illite after the treatment. Approximately 45% of the adsorbed Cs was desorbed from sericite by the 0.1 M LiCl solution, 40% by the 1 M KCl solution, and 5% by the 1 M HCl solution.
4. Discussion
4.1. Adsorption of Cs by minerals
The major portion of Cs in aqueous solution in environments exists as a monovalent cation as Cs+ ions [Citation21]. This suggests that the adsorption of Cs+ ions by minerals is governed by the ion exchange reaction. The cation exchange capacities (CECs) of montmorillonite, illite, and mordenite are 100 (meq 100 g−1) [Citation22], 20 (meq 100 g−1) [Citation22], and 420 (meq 100 g−1) [Citation23], respectively. These CECs are higher than those of kaolinite (3.3 meq 100 g−1), halloysite (3.6 meq 100 g−1), chlorite (0.6 meq 100 g−1), sericite (1.9 meq 100 g−1), and MnO2 (8.5 meq 100 g−1) [Citation22]. The CECs of the minerals are in the order of:
At the Cs concentrations 10−4 and 10−5 mole L−1, the finding that the order of the K d values of Cs for the minerals is in accord with that of the CECs indicates that the adsorption of Cs by minerals is the same as that of the CEC at high Cs concentrations. The adsorption of Sr2+ by soils and minerals is governed by the ion exchange reaction, depending on the CEC of minerals [Citation22]. Thus, the adsorption of Cs at high concentrations (i.e., 10−4 and 10−5 mole L−1) is governed by the ion exchange reaction.
Regarding the desorption behaviors, the Cs adsorbed by the minerals was hardly desorbed by the 0.1 mole L−1 LiCl solution (Figure ). The desorbed fraction of Cs increased from Li+ ions to K+ ions because of the lower hydration energy of the desorption agents [Citation22]. The cation with low hydration energy is dehydrated and sorbed tightly to the interlayer of the 2:1 phyllosilicate and in the structure of zeolite [Citation24]. This reflects our present finding that the adsorbed Cs is desorbed by less than 5% from montmorillonite, illite, sericite, and mordenite by a 0.1 mole L−1 LiCl solution. About 5–15% Cs was desorbed from kaolinite, MnO2, and halloysite by the 0.1 mole L−1 LiCl solution, even though these minerals are not the 2:1 phyllosilicate.
The 2:1 phyllosilicate of montmorillonite and the zeolite structure of mordenite possess high CECs, showing high K d values of Cs at 10−4, 10−5, and 10−9 mole L−1. Since cations are loosely adsorbed in the expanded interlayer of montmorillonite and in the tetrahedral framework of mordenite, the adsorbed Cs was easily exchanged with the other cations [Citation23,Citation25]. Some of Cs can be tightly adsorbed at the “frayed edge” in the beidelite structure of montmorillonite [Citation18]. Even though the capacity of the “frayed edge” sites is low, approximately 40% of the adsorbed Cs at 10−9 mole L−1 was residual in montmorillonite after the treatment with the three different reagent solutions (Figure ). The selectivity of cations in zeolite depends on the Si/Al ratios [Citation23]. The Si/Al ratio of mordenite is about 4.0 to 5.0, nearly the same as that of clinoptiolite (Si/Al ratio: 4.3–5.3). The selectivity of monovalent cations by clinoptiolite is in the order of:
Illite and sericite have a mica structure. It is well known that the “frayed edge” sites of mica minerals are energetically more stable than the reversible sites of the montmorillonite and kaolinite structure [Citation26]. The capacity of the “frayed edge” sites of illite and sericite is sufficient for Cs at 10−9 mole L−1, reflecting our present sequential desorption behavior observation that most of the adsorbed Cs was residual after the desorption treatment. On the other hand, a pure mica structure reversibly adsorbs Cs [Citation27, Citation28]. In the present experiments, for Cs at 10−4 mole L−1, most of the Cs adsorbed by illite and sericite was desorbed by the three reagent solutions. These results indicate that Cs is adsorbed not only at the “frayed edge” site, but also at other sites where Cs is reversibly adsorbed.
Kaolinite and halloysite are 1:1 phyllosilicate minerals. Since the kaolinite structure does not expand [Citation29], kaolinite does not tightly adsorb Cs in its structure. Kaolinite is interstratified with vermiculite and micaceous layers [Citation29], suggesting the formation of a “frayed edge” site in the interstratified layers with vermiculite and micaceous layers being one of the possible sites for the residual fraction of Cs at 10−9 mole L−1 after the desorption treatment with the three different reagent solutions. An XRD analysis of kaolinite (data not shown) showed no impurity or interstratified minerals, suggesting that the “frayed edge” sites in kaolinite are very less. The “frayed edge” site of kaolinite was estimated by radiocesium interception potential (RIP) to approx. 1 × 10−8 mol g−1 [Citation30], being lower by 1000 than that of illite [Citation31]. Thus, approx. 30% Cs at 10−9 mole L−1 was residual after the desorption treatment, and almost all Cs was desorbed at 10−4 and 10−5 mole L−1. Halloysite contains additional one water molecule layer between 1:1 aluminosilicate layers, reflecting a layer repeat distance of 1.01 nm [Citation32]. This expandable layer structure does not enable a tight association with Cs+ ions. Since most of the adsorbed Cs was desorbed by the three different reagent solutions in the present study, the halloysite used was not interstratified with mica and/or smectite.
Manganese oxides of todorokite form a tunnel structure [Citation33]. The sorption of Cs on manganese oxides has been studied [Citation19, Citation20,Citation34, Citation35]. The adsorption of Cs was caused by a Freundlich-type isotherm at Cs concentrations between 10−6 and 10−3 mole L−1 [Citation20]. The adsorption of Cs was lowered at higher coexisting cations of K+, Mg2+, and Ca2+ [Citation19]. These results indicate that the adsorption of Cs at 10−5 and 10−4 mole L−1 by manganese oxides is due to an ion exchange-type reaction. On the other hand, todorokite has a higher affinity for 137Cs than for 60Co and 54Mn at low pH in a 0.1 mole L−1 HNO3 solution [Citation34]. XRD and TEM (transmission electron microscopy) analyses indicate that Cs is exchanged with interlayer Na in birnessite, which is layer-structured MnO2 [Citation35], suggesting that Cs is adsorbed in the tunnel structure of todorokite. These results strongly suggest that a small fraction of Cs is tightly adsorbed in the tunnel structure, and was residual after the desorption treatment with the three different desorption reagent solutions.
4.2. Implications of radioactive Cs in the soil of Fukushima
We analyzed the radioactive Cs levels in three different soils collected in Iitate-mura in Fukushima Prefecture [Citation14]. The results showed that more than 65% of the 137Cs resided in the soil samples after treatment with a 1 mole L−1 NH4Cl solution and a 1 mole L−1 CH3COOH solution, indicating that more than 60% of radioactive Cs was tightly associated with the soils. The mineral components of the treated soils analyzed by XRD after size fractionation showed the presence of mica-like minerals in the contaminated soils. The results of the present study indicate that the irreversible sorption by the contaminated soils is caused by fixation at the “frayed edge” of the mica-like minerals illite and sericite. However, some fractions in the size fractionation did not contain mica-like minerals yet they contained radioactive Cs. These results indicate that some fraction of 137Cs was irreversibly associated with the soils even though mica-like minerals were not present. The present study clarifies that the possible host minerals are montmorillonite, mordenite, kaolinite, and manganese oxides. Thus, the non-mica minerals, including montmorillonite, mordenite, kaolinite, and manganese oxides, in the soils probably associate tightly with some fractions of radioactive Cs.
5. Conclusion
We have elucidated the adsorption behavior of radioactive Cs by non-mica minerals (kaolinite, halloysite, chlorite, montmorillonite, mordenite, MnO2, TiO2, Al2O3, and FeOOH). At a concentration of 2 × 10−9 mole L−1, high distribution coefficients of Cs were obtained for kaolinite, halloysite, montmorillonite, mordenite, and MnO2, and very low distribution coefficients of Cs were obtained for chlorite, TiO2, Al2O3, and FeOOH. At a low concentration (10−9 mole L−1), the adsorption behavior of Cs was different from that at the high concentrations, 10−4 and 10−5 mole L−1. The desorption ratio between 0.1 mole L−1 K+ ions and 0.1 mole L−1 Cs+ ions for kaolinite and MnO2 was lower than that for montmorillonite and mordenite. These results indicate that the adsorption and desorption behaviors of kaolinite and MnO2 are different from those of montmorillonite and mordenite. Approximately 40%, 40%, 50%, and 25% of the adsorbed Cs were residual in montmorillonite, mordenite, MnO2 and kaolinite, respectively, after the desorption treatment using a 0.1 mole L−1 LiCl solution, 1 mole L−1 KCl solution, and 1 mole L−1 HCl solution. These results strongly suggest that the non-mica minerals montmorillonite, mordenite, kaolinite, and MnO2 contributed to the fixation of the radioactive Cs fallout on Fukushima soil.
References
- Tanabe , F. 2011 . Analysis of core melt accident in Fukushima Daiichi-Unit 1 Nuclear Reactor . J Nucl Sci Technol. , 48 : 1135 – 1139 .
- Liu , Q , Ishikawa , J , Maruyama , Y and Watanabe , N . 2012 . A simple method for estimating the structure temperatures and the cesium revaporization inside the reactor pressure vessel – I: Basic concepts and model descriptions for the Fukushima Daiichi Nuclear Power Plant . J Nucl Sci Technol. , 49 : 479 – 485 .
- Tanabe , F . 2012 . A scenario of large amount of radioactive materials discharge to the air from the Unit 2 reactor in the Fukushima Daiichi NPP accident . J Nucl Sci Technol. , 49 : 360 – 365 .
- Chino , M , Nakayama , H , Nagai , H , Terada , H , Katata , G and Yamazawa , H . 2011 . Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere . J Nucl Sci Technol. , 48 : 1129 – 1134 .
- Kawamura , H , Kobayashi , T , Furuno , A , In , T , Ishikawa , Y , Nanayama , T , Shima , S and Awaji , T . 2011 . Preliminary numerical experiments on oceanic dispersion of 131I and Cs discharged into the ocean because of the Fukushima Daiichi Nuclear Power Plant disaster . J Nucl Sci Technol. , 48 : 1349 – 1356 .
- Yasunari , T J , Stohl , A , Hayanoc , R S , Burkhart , J F , Eckhardt , S and Yasunari , T . 2011 . Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident . Prog Natl Acad Sci , 108 : 19530 – 19534 .
- Kinoshita , N , Suekia , K , Sasaa , K , Kitagawaa , J , Ikarashia , S , Nishimura , T , Wonga , Y S , Satoua , Y , Handa , K , Takahashi , T , Sato , M and Yamagata , T . 2011 . Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan . Prog Natl Acad Sci , 108 : 19526 – 19529 .
- Honda , M C , Aono , T , Aoyama , M , Hamajima , Y , Kawakami , H , Kitamura , M , Masumoto , Y , Miyazawa , Y , Takigawa , M and Saino , T . 2012 . Dispersion of artificial caesium-134 and -137 in the western North Pacific one month after the Fukushima accident . Geochem J. , 46 : e1 – e9 .
- Kato , H , Onda , Y and Teramage , M . 2012 . Depth distribution of 137Cs, 134Cs, and 131I in soil profile after Fukushima Daiichi Nuclear Power Plant Accident . J Environ Radioact , 111 : 59 – 64 .
- Tanaka , K , Takahashi , Y , Sakaguchi , A , Umeo , M , Hayakawa , S , Tanida , H , Saito , T and Kanai , Y . 2012 . Vertical profiles of Iodine-131 and cesium-137 in soils in Fukushima Prefecture related to the Fukushima Daiichi Nuclear Power Station accident . Geochem J. , 46 : 73 – 76 .
- Tamura , T and Jacobs , D G . 1989 . Structural implication in cesium sorption . Health Phys , 2 : 391 – 398 .
- Sawhney , B L . 1970 . Potassium and cesium ion selectivity in relation to clay mineral structure . Clay Clay Miner , 18 : 47 – 52 .
- Comans , R N , Haller , M and De Preter , P . 1991 . Sorption of cesium on illite: non-equilibrium behaviour and reversibility . Geochim Cosmochim Acta , 55 : 433 – 440 .
- Kozai , N , Ohnuki , T , Arisaka , M , Watanabe , M , Sakamoto , F , Yamasaki , S and Jiang , M. 2012 . Chemical states of fallout radioactive Cs in the soils deposited at Fukushima Daiichi Nuclear Power Plant accident . J Nucl Sci Technol. , 49 : 473 – 478 .
- Westrich , H R , Cygan , R T , Brady , P V , Nagy , K L , Anderson , H L , Kim , Y and Kirkpatrick , R J . 1995 . “ The sorption behavior of Cs and Cd onto oxide and clay surfaces ” . In Proceedings of the Conference on Waste Management ’95 (CONF-950216–75) , Washington , DC : US Department of Energy . 1995 26 Feb-2Mar; Tucson (AZ).
- Ejeckama , R B and Sherriff , B L . 2005 . 133Cs, 29Si, and 27Al MAS NMR spectroscopic study of Cs adsorption by clay minerals: implication for the disposal of nuclear wastes . Can Mineral , 43 : 1131 – 1140 .
- Saiers , J E and Hornberger , G M . 1999 . The influence of ionic strength on the facilitated transport of cesium by kaolinite colloids . Water Resour Res , 35 : 1713 – 1727 .
- Ohnuki , T and Kozai , N . 1994 . Sorption characteristics of radioactive cesium and strontium on smectite . Radiochim Acta , 66/67 : 327 – 331 .
- Singh , O V and Tandon , S N . 1977 . Studies on the adsorption of cesium and strontium radionuclides on hydrated manganese oxide . Intern J Appl Radiat Isot. , 28 : 701 – 704 .
- Hasany , S M and Chaudhary , M H . 2005 . Adsorption behaviour of microamounts of cesium on manganese dioxide . J Radioanal Nucl Chem , 84 : 247 – 256 .
- Pourbaix , M. 1966 . Atlas of electrochemical equilibria in aqueous solutions , New York : Permagon Press .
- Ohnuki , T . 1994 . Sorption characteristics of strontium on sandy soils and their components . Radiochim Acta , 64 : 237 – 245 .
- Ming , D W and Mumpton , F A . 1989 . “ Zeolites in soils ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 873 – 911 . Madison , WI : Soil Science Society of America .
- Douglas , L A . 1989 . “ Vermiculites ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 635 – 674 . Madison , WI : Soil Science Society of America .
- Borchardt , G. 1989 . “ Smectites ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 675 – 727 . Madison , WI : Soil Science Society of America .
- Goulding , K W . 1986 . “ Potassium-calcium exchange equilibria in aluminosilicate minerals and soils ” . In Geochemical processes at mineral surfaces , Edited by: Davis , J A and Hayes , K F . 327 – 340 . Washington , DC : American Chemical Society . ACS Symposium Series, Vol. 323
- Nakao , A , Thiry , Y , Funakawa , S and Kozaki , T . 2008 . Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand . Soil Sci Plant Nutr. , 54 : 479 – 489 .
- Fanning , D S , Keramidas , V Z and El-Desoky , M A . 1989 . “ Micas ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 551 – 634 . Madison , WI : Soil Science Society of America .
- Dixon , J B . 1989 . “ Kaolin and serpentine group minerals ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 467 – 525 . Madison , WI : Soil Science Society of America .
- Nakao , A , Thiry , Y , Funakawa , S and Kosaki , T . 2008 . Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand . Soil Sci Plant Nutr. , 54 : 479 – 489 .
- Delvaux , B , Kruyts , N , Maes , E and Smolders , E . 2001 . “ Fate of radiocesium in soil and rhizosphere ” . In Trace elements in the rhizosphere , Edited by: Gobran , G R , Wenzel , W W and Lombi , E . 61 – 91 . Boca Raton , FL : CRC Press .
- Brown , G , Newman , A CD , Rayner , J H and Weir , A H . 1978. p. 29–178 . “ The structures and chemistry of soil clay minerals ” . In The biochemistry of soil constituents , Edited by: Greenland , D J and Hayes , M HB . New York : John Wiley .
- Mc Kenzie , R M . 1989 . “ Manganese oxides and hydroxides ” . In Minerals in soil environments, SSSA book series 1 , Edited by: Dixon , J B and Weed , S B . 439 – 465 . Madison , WI : Soil Science Society of America .
- Dyer , A , Pillinger , M , Newton , J , Harjula , R , Moller , T and Amin , S . 2000 . Sorption behavior of radionuclides on crystalline synthetic tunnel manganese oxides . Chem Mater. , 12 : 3798 – 3804 .
- Lapano , C L , Heaney , P J and Post , J E . 2009 . Cs-exchange in birnessite: reaction mechanisms inferred from time-resolved X-ray diffraction and transmission electron microscopy . Am Mineral , 95 : 816 – 826 .