Abstract
Water chemistry control is one of the key technologies to establish safe and reliable operation of nuclear power plants. Continuous and collaborative efforts of plant manufacturers and plant operator utilities have been focused on optimal water chemistry control, for which, a trio of requirements for water chemistry should be simultaneously satisfied: (1) better reliability of reactor structures and fuel rods; (2) lower occupational exposure and (3) fewer radwaste sources. Various groups in academia have carried out basic research to support the technical bases of water chemistry in plants. The Research Committee on Water Chemistry of the Atomic Energy Society of Japan (AESJ), which has now been reorganized as the Division of Water Chemistry (DWC) of AESJ, has played important roles to promote improvements in water chemistry control, to share knowledge about and experiences with water chemistry control among plant operators and manufacturers and to establish common technological bases for plant water chemistry and then to transfer them to the next generation of plant workers engaged in water chemistry. Furthermore, the DWC has tried and succeeded arranging R&D proposals for further improvement in water chemistry control through roadmap planning. In the paper, major achievements in plant technologies and in basic research studies of water chemistry in Japan are reviewed. The contributions of the DWC to the long-term safe management of the damaged reactors at the Fukushima Daiichi Nuclear Power Plant until their decommissioning are introduced.
1. Introduction
In nuclear power plants (NPPs), water chemistry of cooling water is carefully monitored and controlled to keep integrity of structures, e.g. fuel cladding, pressure boundary structures and core internals, and to reduce occupational exposures of workers due to accumulated radioactive corrosion products. As demand increases for advanced applications of light water cooled reactors, e.g. application of upgraded fuels, safe and reliable operation of aging plants and future power rate up, water chemistry control is considered to play even more important roles in reliable plant operation [Citation1, Citation2]. Water chemistry affects all materials in contact with the cooling water and at the same time it is affected by the materials themselves. So, water chemistry control is required to satisfy the need for improved integrity of target materials and at the same time it is required to be optimal for all materials and systems in the plant. Experts on water chemistry in NPPs, who can handle such complicated systems and technologies, should be supported by sufficient knowledge and experiences with plant operation as well as plant chemistry.
In the late 1970s, just after first NPP were operating in Japan, many difficulties were encountered related to water chemistry and structural materials, e.g. defects in fuel cladding, stress corrosion cracking of stainless steel used in boiling water reactors (BWRs), defects in steam generator (SG) tubing in pressurized water reactors (PWRs) and high occupational exposure due to accumulated radioactive corrosion products [Citation1]. Almost all of the technical problems were overcome and structural integrities have been kept for the past three decades but the possible reappearance of these problems under different conditions of plant operation still remains. Details of the technical progress in the period were introduced in the Twentieth Anniversary Issue of the Journal of Nuclear Science and Technology (JNST) [Citation3].
Plant water chemistry is affected by each plant's unique systems, materials used and operational histories, which lead to difficulties in establishing the trio of requirements for water chemistry with conventional water chemistry control. Each plant must be operated with its own optimized water chemistry. For this, sufficient knowledge on plant systems, materials and operation as well as a sufficient technological basis for the chemistry are required for plant chemists and their supervisors. In order for workers to exchange their experiences about plant water chemistry with other workers, the Research Committee on Water Chemistry worked to promote two purposes in the Atomic Energy Society of Japan (AESJ).
| 1. | To improve the technological basis of plant water chemistry control by encouraging frequent discussions among researchers and actual plant workers. | ||||
| 2. | To share knowledge about and experiences with water chemistry control among plant operators and manufacturers and to transfer them to the next generation of plant workers dealing with water chemistry. | ||||
The activities of the committee were succeeded by the Division of Water Chemistry (DWC) of AESJ to organize scientists and engineers in water chemistry and to promote the mission described above.
Experts who acquired sufficient experiences with water chemistry when the problems were first encountered in the 1970s have almost retired [Citation4]. Their experiences should be successfully transferred to a new generation of engineers; these are not subjects in a certain plant but subjects for the whole field of nuclear engineering. To prepare for smooth and successful technical transfer, documents on experiences with water chemistry are being compiled as water chemistry standards, e.g. standards for chemical analysis procedures, and guidance for water chemistry control and guidance for quality assurance related to water chemistry.
In this paper, the historic review of water chemistry activities in Japan, latest activities and future subjects in water chemistry for achieving safe and reliable plant operation are presented.
2. Historical review of activities of water chemistry control in Japanese reactors
2.1. Major roles of water chemistry in NPPs
The most important roles of cooling water in NPPs are as the energy transporting medium and the neutron moderating medium () [Citation2]. The former role is the same as that of cooling water in fossil fuel power plants (FPPs) but the later role is unique to NPPs. The initial energy of neutrons generated by the fission of a 235U nucleus is too high to allow their capture by other 235U nuclei and therefore to sustain the chain reaction. The energy is reduced to about a millionth of the initial value by elastic collisions with moderator nuclei such as those of hydrogen atoms in water. The efficiency of the moderator for slowing down neutrons to energies suitable for nuclear chain reactions is affected by the mass and neutron-absorbing properties of the nuclei of the moderating atoms and the density of the bulk moderator. The nucleus of the hydrogen atom in light water (H2O) has a very effective mass for moderating neutrons but it also has an affinity for absorbing them. Light water NPPs (i.e. LWRs, such as PWRs or BWR) use H2O as the combined moderator and coolant.
Figure 1 Major water chemistry monitoring items related to safe and reliable NPP operation. (a) In BWRs and (b) in PWRs
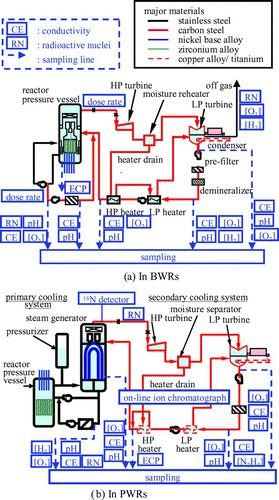
Water chemistry in NPPs has many similarities with that in FPPs and aspects of control in the former are often based on those in the latter. Specifying chemistry involves recognition that, while chemistry affects the materials contacting the cooling water, the materials also affect the chemistry. Monitoring of the chemistry is important not only for preventing future problems in materials, but also for indicating problems occurring at the early stages of operation.
The major purposes of water chemistry control in NPPs are divided into two categories.
| 1. | Reactivity control (essential power output control) and | ||||||||||||||||||||||
| 2. | Mitigation of adverse effects which includes
| ||||||||||||||||||||||
Some of the purposes are achieved actively by adding chemicals, while others are achieved passively without additives. For both categories, water chemistry is monitored and if the data are out of the range of the target values suitable countermeasures are adopted to restore the plant to the correct values.
The process control parameters for plant operation, e.g. temperature, pressure, flow rate and neutron flux, are continuously monitored in the high-temperature water systems and the data transmitted to the central control room of the plant. Some water chemistry sensors, e.g. for conductivity, pH, [O2] and [H2], cannot tolerate high temperatures, pressures and so are installed on cooled depressurized sampling streams. Other parameters, e.g. concentrations of boron, lithium and metallic species, as well as radionuclides, are monitored by off-line measurements of sampled water [Citation5].
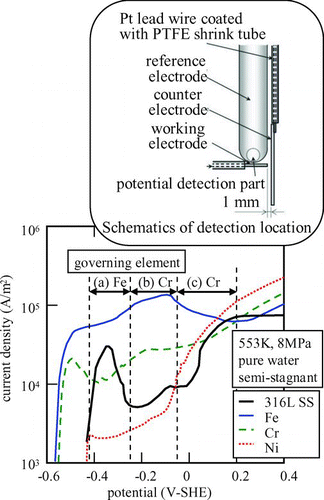
Locations for on-line monitoring and sampling for off-line measurement in BWRs are shown in Figure (a) [Citation6]. The cooled and depressurized samples are taken to the plant chemical laboratory for analysis.
In PWRs, water chemistry differs in the primary and secondary cooling systems. Major concerns of water chemistry in the primary cooling system are to control boron concentration so as to maintain the reactor reactivity and then so as to control pH to minimize radioactive corrosion products and keep shutdown dose rates low. The main concern in the secondary cooling system is to keep a suitable pH, which mitigates flow-accelerated corrosion (FAC) of carbon steel piping, and to minimize oxygen and nonvolatile contaminants such as sulfate, sodium and chloride, which maintains sufficient integrity of the SG tubing. Locations for on-line monitoring and sampling in PWRs are shown in Figure (b) [Citation6].
2.2. Organized activities of water chemistry research
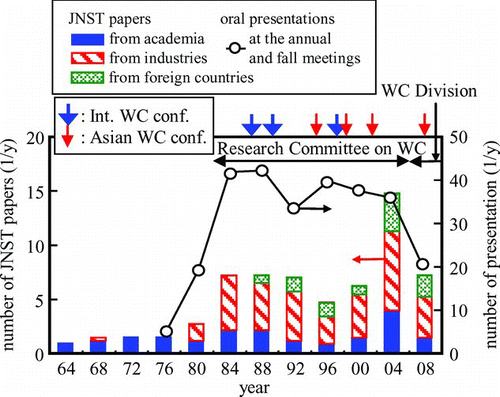
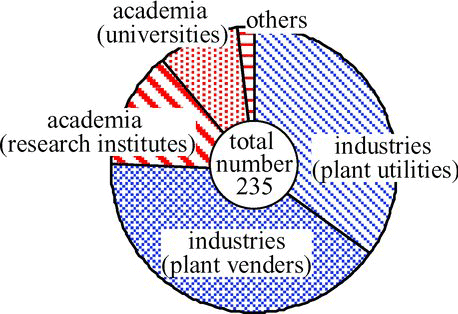
The Research Committee on Water Chemistry of AESJ was which firstly established in 1982, and continuously played important roles to facilitate technical discussions and information exchanges on water chemistry of NPPs until it was succeeded by the DWC (). In June 2012, there were 235 members of the DWC, respecting 3% of the total number of the AESJ members (6000).
Table 1 Historical aspects of activities of AESJ Research Committee on Water Chemistry
With passing years, the number of technical presentations at annual and fall meetings of the AESJ steadily increased () and more time for further discussions and information exchanges was often required in the periodically held committee on water chemistry (5–6 times per year), which was the first reason for establishing the committee. Significantly, the features of the committee were that the number of members from industrial bodies was much larger than that from academia ().
Voluntary activities of the committee members resulted in successful promotion of the International Conference on Water Chemistry and also the Asian Water Chemistry Symposium and also contributed to compiling and publishing water chemistry data bases as a water chemistry handbook [Citation7]. Competition of the members from industrial bodies enhanced to improve water chemistry technologies, while their cooperation richly contributed to world-wise technical transfer.
2.3. Historic trends of major topics of JNST papers related to water chemistry
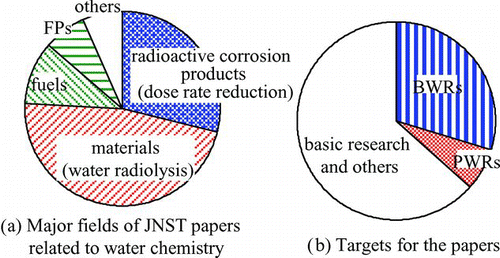
Historic trends of major topics of JNST papers related to water chemistry are summarized in . The number of JNST papers related to water chemistry is about 260, which represents 5% of all JNST papers. The first JNST paper related to water chemistry was on in-pile loop experiments for fission product (FP) release into water [Citation8]. By 1980s, just before the Research Committee on Water Chemistry was organized, various papers on basic research studies were published. It is noteworthy that papers on basic research on water radiolysis were published, while papers on radioactive corrosion product behaviors in sodium cooling systems for liquid metal cooled fast breeder reactors (LMFBR) were published prior to those for LWRs [Citation9,10]. In the early 1980s, many papers on occupational exposure, e.g. radioactive corrosion product behaviors in BWR cooling water were published, which enhanced discussions on corrosion product behaviors in the LWR cooling water [11].
Table 2 Historic trends of major topics of JNST papers related to water chemistry
Major investigated topics were strongly affected by the needs of each period. Major fields and purposes of the publications are shown in .
3. Water chemistry of NPPs
3.1. Key parameters of water chemistry of NPPs
Distributions of materials in contact with the coolant and materials themselves differ in BWR and PWR systems, which complicate treatment of corrosion problems. Corrosion behaviors are much affected by water qualities and differ according to the combinations of water qualities and the materials themselves. The different combinations of materials and different water chemistry controls, in turn, cause different interactions between materials and cooling water and then, consequently different material problems. The interactions of materials and cooling water in the BWR primary cooling system and the PWR cooling systems have been detailed in reference [Citation6,7]. High temperature water causes corrosion of structural materials, which leads to adverse effects in the plants, e.g. increasing shutdown radiation, generating defects in materials of the major components and fuel claddings, and increasing the volume of radwaste sources [Citation1].
To control the adverse effects, it is essential to understand corrosion behaviors of structural materials and then to control them in BWR and PWR systems. Corrosion behavior is much affected by the combinations of water qualities and materials. To minimize the adverse effects, optimal water chemistry control has been proposed [Citation12].
3.2. Structural material integrity
3.2.1. BWR primary cooling system
One of the most serious problems encountered with structural materials in BWRs is intergranular stress corrosion cracking (IGSCC) of stainless steel components [Citation13]. Corrosive conditions related to IGSCC are determined by corrosive radiolytic species. To mitigate water radiolysis and then corrosive conditions, hydrogen has been injected into the feed water line. With increasing concentration of injected hydrogen, oxygen concentration measured at a sampling point decreases rapidly and, at the same time, the main steam line dose rate increases gradually ( (a)) [Citation14]. The optimal hydrogen injection amount is determined to suppress oxygen concentration ([O2]) without serious increase of main steam dose rate. From the viewpoint of IGSCC mitigation, the threshold electrochemical corrosion potential (ECP) has been proposed as −230 mV-SHE [Citation15]. However, in the optimal hydrogen concentration determined based on [O2], the ECP was still too high to mitigate IGSCC. Nobel metal is quite effective in decreasing the ECP under the addition of small amounts of H2 in the feedwater. There are several procedures to plate noble metal on the surface of structural materials, e.g. noble metal coating and noble metal chemical addition (NMCA). An example of NMCA is shown in Figure (a), where an excess amount of H2 to a stoichiometric ratio of H2O can reduce the ECP below the threshold.
Figure 5 Optimal water chemistry control (BWR primary cooling system). (a) Measured at a BWR plant and (b) calculated for a BWR
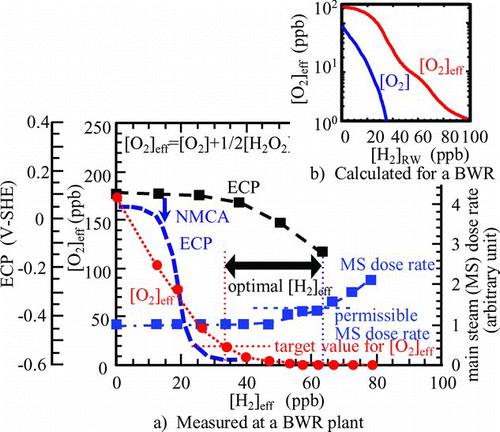
The gap between the measured ECP and measured [O2] is explained by the contribution of H2O2 to ECP especially at high H2 injection rate (Figure (b)) [Citation16]. The [H2O2] and [O2] have been calculated and for both normal water chemistry (NWC: without H2 injection) and hydrogen water chemistry (HWC: with H2) conditions, [H2O2] alone was high enough to determine ECP [Citation17]. From the viewpoint of structural integrity of BWRs, monitoring and control of [O2], [H2], ECP and main steam dose rate are important. To explain the gap quantitatively and to extrapolate the measured values to any location of interest, water radiolysis models have been applied [Citation18–Citation20]. The models were extended to understand 16N behaviors in the reactor water and main steam line [Citation21].
3.2.2. PWR primary cooling system
As a result of full suppression of water radiolysis in the PWR primary system, it has been reported that the major water chemistry factor to determine primary water stress corrosion cracking (PWSCC) is not oxygen but hydrogen [Citation22]. For this, the optimal [H2] to mitigate PWSCC and at the same time to suppress oxidant concentration has been proposed. Crack growth rate showed a peak around 20 Ncm3/kg of [H2] ( (a)) [Citation23]. The latest data on PWSCC initiation showed that the crack initiation time decreased monotonously with [H2] (Figure (b)) [Citation24]. To mitigate PWSCC initiation and at the same time mitigate crack growth rate, [H2] should be decreased below the peak value. The water radiolysis model has been developed for PWR conditions to explain ECP for different [H2] (Figure (c)) [Citation25].
3.2.3. PWR secondary cooling system
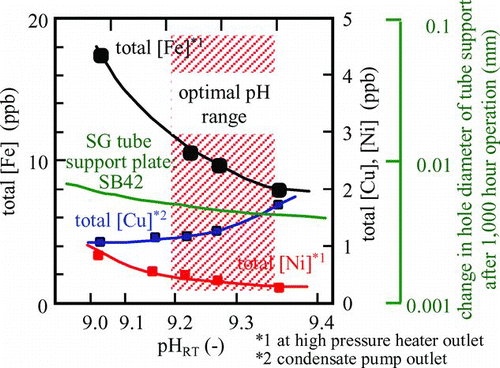
Major countermeasures for FAC of carbon steel at FPPs (examples at US plants reported by EPRI) have been shown in reference [Citation26]. As a countermeasure for FAC, increasing pH above 9.5 is often applied for copper material-free plants. However, some PWR plants still use copper alloy for low-pressure heater tubes, which prevents increasing pH above 9.5. The relationships between pH and material responses are shown in [Citation7]. High pH of possibly more than 9.2 resulted in lower [Fe] (most of the iron is from FAC of carbon steel) and lower [Ni] from stainless steel and then suppressed corrosion of the SG support plate, while it increased [Cu] from corrosion of feed water heater tubes. When pH was higher than 9.35, [Cu] was thought to increase rapidly. So, pH for the plants using low-pressure heater tubes of copper alloy should be kept between 9.2 and 9.35.
Another candidate water chemistry control measure to mitigate FAC is oxygen injection, which has been successfully applied in BWRs and FPPs. A slight increase in feed water [O2] without increasing O2 input into the SG is also considered. For this, it is essential to determine [O2] exactly in the location of interest. Oxygen concentration is kept at a sufficiently low level at the condenser hot well and hydrazine is injected at the outlet of the condensate demineralizer to decrease [O2] as low as possible and to mitigate the effects of oxygen on SG tubing. As a result of careful selection of the oxygen injection point and injection amount, oxygen injection can mitigate FAC without any serious effects on the SG tubing and this has been confirmed at the Tsuruga-2 PWR plant [Citation27].
3.3. Fuel integrity
It was reported that crud, especially copper ion, depositing on the fuel surface might enhance cladding corrosion in BWRs, while Li in the primary water might enhance cladding corrosion in PWRs. So, copper and lithium concentrations in the water have been controlled carefully to mitigate fuel cladding corrosion and no serious corrosion problems have been reported [Citation28].
Corrosive conditions of BWR fuel cladding have been studied based on water radiolysis models, while its effects on corrosion behaviors were studies based on accelerated corrosion experiments [Citation29, Citation30].
3.4. Occupational exposure reduction
3.4.1. BWRs
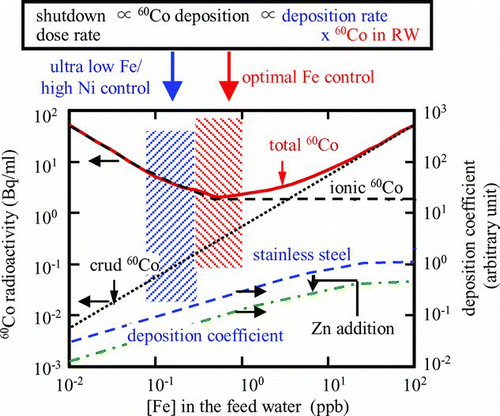
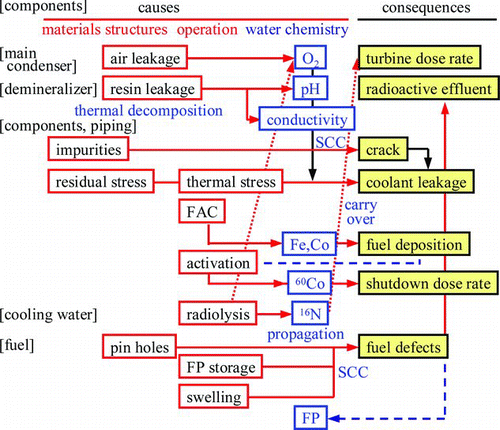
The major radioactive nuclide to determine the shutdown dose rate in the BWR primary cooling system is 60Co, which is fed through the feed water into the reactor water, activated on the fuel surface, and then released into the reactor water again to deposit on primary piping and to cause the shutdown dose rate. Major sources of corrosion products are condensate water and feed water for iron crud (oxide particles) and feed water for cobalt ions. Ferrous ions in the water are easily oxidized to become oxide particles and fed into the reactor water with cobalt ions to deposit on fuel surface. Iron crud enhances cobalt deposition as cobalt ferrite (CoOFe2O3) on the fuel surface and then generation of 60Co, while too low an amount of iron crud causes cobalt deposition as cobalt mono-oxide (CoO) on the fuel surface, which easily dissolves into the water, and then ionic 60Co radioactivity increases [Citation31]. To minimize shutdown dose rate, two approaches have been proposed for this competition ().
One is optimal iron crud concentration ([Fe]) control and the other is ultra low iron crud and higher nickel ion concentration ([Ni]) control. When [Fe] is controlled in a suitable region to suppress crud 60Co and ionic 60Co at the same time, total 60Co radioactivity is suppressed at the minimum value and then 60Co deposition can be controlled at the minimum level. When it is suppressed as low as possible, ionic 60Co radioactivity increases but at the same time [Ni] also increases, which causes a lower cobalt deposition rate on the piping inner surface and then 60Co deposition can be controlled at the minimum level. These different approaches for dose rate reduction with the same final goal are applied for operating power plants [Citation31].
Cobalt behaviors on the fuel surfaces were confirmed by experiments related to effects of surface roughness on crud deposition rates, boiling-induced deposition of ionic species on fuel surfaces, and chemical reaction of ionic species and crud on the fuel surfaces [Citation12,Citation32,Citation33]. The effects of nickel ion on 60Co deposition on stainless steel piping were confirmed by surface characterization of exposed specimens [Citation34].
Another choice for dose rate reduction for the BWR primary cooling system is Zn injection to suppress 60Co deposition, which has been applied in a lot of US BWRs [Citation13] and some of Japanese BWRs [Citation35].
3.4.2. PWRs
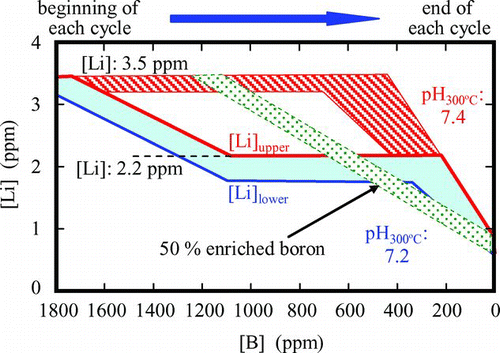
Radioactive corrosion products in PWR primary cooling water and then shutdown dose rate around SGs are much affected by pH in the primary water. Higher pH is used for getting lower radioactive corrosion product concentrations. However, boron concentration ([B]) control is one of the key technologies to provide for stable thermal output. Boron concentration is decreased during plant operation. To keep pH at the constant level, Li concentration ([Li]) should follow [B]. A typical pattern of pH control is shown in [Citation36]. pH300 discussed here is not measured directly in the water but calculated by using [B] and [Li]. pH control shown in the figure is complicated due to restriction of the upper limit for [Li] to prevent the effects of Li on corrosion of fuel claddings.
For a long time, the upper limit of [Li] accepted for integrity of fuel cladding was 2.2 ppm, but recently that was increased to 3.5 ppm to extend the optimal pH region as shown in Figure [Citation37]. Much higher [Li] around 7 ppm has been discussed for a future upper limit. Increasing fuel cycle period requires higher [B] at the beginning of each cycle, which results in lower pH and narrows the optimal pH region. Natural abundance of 10B is 19.9%. When 10B enriched around 50% is applied for reactivity control, [Li] can be suppressed and the optimal pH region can be extended as shown in Figure [Citation38].
Application of enriched 10B is one option for extending the optimal pH region for application of high burn-up fuels, which is the target application in Japan. One of the key technologies for applying enriched 10B is suitable instrumentation to measure the isotopic ratio of boron.
Another choice for dose rate reduction for the PWR primary cooling system is Zn injection, which has been applied in a lot of US and European PWRs and also some PWRs in Japan. Usually both optimal pH control and Zn injection are applied to reduce dose rate [Citation39].
3.5. Radwaste source reduction
For water polishing, large amounts of ion exchange resins and filter elements are used. Spent resins and spent filter elements as well as filter and resin back wash water and resin regeneration solution should be treated as radwaste. To keep the primary cooling water clean, frequent back wash and regeneration processes are required and volumes of spent resins and filter elements must increase to keep clean-up ability. Their volume should be cut as much as possible and then they can be put into drums for final deposition.
Many ways have been tried for reducing total radwaste volume. To simplify radwaste volume reduction procedures (e.g. powdered and pelletized treatment of condensate liquid waste, volume reduction of spent resin and volume reduction of miscellaneous solid waste), systems have been back-fitted for operated BWRs and cuts if radwaste sources have been achieved [Citation12,Citation40].
Lowering corrosion product generation is the most essential countermeasure for radwaste source reduction. Corrosion resistance materials, i.e. low alloy steel with small amounts of chromium, have been applied to suppress corrosion product generation, so that the input of iron crud at the condensate demineralizer has been reduced. In addition to application of corrosion resistant materials, pre-filters have been installed at the inlet of the condensate demineralizer to improve total removal efficiency of the condensate clean-up system, which not only lowers radioactive corrosion product concentration in the reactor water but also cuts radwaste source by extending resin life and minimizing frequency of regeneration and back wash operations of condensate resin. Non-regeneration operation of the condensate demineralizer (one time resin use) is becoming popular as a way to eliminate amounts of condensated liquid waste [Citation12].
3.6. Optimal water chemistry
3.6.1. Experiences with optimal water chemistry control
The function of water chemistry control in NPPs and the corresponding control items are shown in [Citation2]. Major control targets have been described in the literature [Citation2,Citation6,Citation41–Citation44]. Water chemistry under BWR operating conditions used to be essentially pure, neutral water of low conductivity with no additives. Over the last 20 years, however, injections have been made into the feed water: oxygen to prevent carbon steel corrosion, hydrogen to moderate water radiolysis in the recirculation water and zinc to mitigate accumulation of radioactive corrosion products [Citation45–Citation47]. In PWRs, the reactivity and resulting thermal output of the core are controlled by adjusting the boric acid concentration in the primary cooling water. Primary coolant chemistry under PWR operational conditions is generally for low [O2] (by H2 addition) and high pH (by Li addition to offset the boric acid). Lately, however, Zn has been added in the several parts per billion ranges to some plants to reduce activity transport the same as in BWRs.
Table 3 Purposes of water chemistry control and control items in NPPs
3.6.2. Option of suitable water chemistry control as measure for plant reliability
Optimal water chemistry control for reliable plant operation has been introduced in operating plants. Water chemistry control is not only a measure for establishing targets on plant reliability; at the same time any adverse effects should be carefully considered to prevent additional problems in the plants. Alternative water chemistry control procedures are often prepared for the same purposes, while other hardware measures can be applied instead of water chemistry improvement (). The target values for water chemistry control are not definitely determined; rather target values should be strictly kept under several sets of conditions.
Table 4 Parallel water chemistry controls for achieving plant reliability
4. Latest achievements
4.1. Basic researches
4.1.1 Water radiolysis
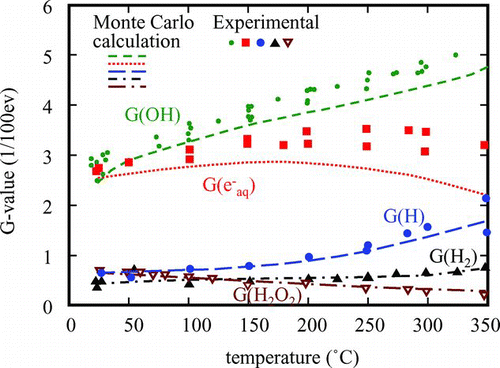
Before establishment of water chemistry in Japan, a water radiolysis experiments were done and radiolysis products; O2, H2 and H2O2, were determined in gamma-ray radiolysis at ambient temperature [Citation9]. Thanks to developments of both pulse radiolysis and analytical techniques, many data on rate constants and G-values of water decomposition products at elevated temperatures have been accumulated. Furthermore, Monte Carlo calculation of water radiolysis with gamma rays has been carried out and the calculated results reproduced the experimental results fairly well, as shown in [Citation48]. Based on basic data of water radiolysis, simulation codes have been developed to predict the concentration of miscellaneous oxidants at different points in the primary circuit of the reactor.
4.1.2. Hydrogen peroxide behaviors
It is difficult to measure hydrogen peroxide, which is easily decomposed in water at elevated temperature. High temperature dissolved oxygen detectors were developed to determine O2 and H2O2 concentrations separately in the reactor water but they could not be applied at BWR plants [Citation49]. Hydrogen peroxide was estimated in the reactor primary cooling water using water radiolysis models but it was first determined in an operation BWR in Sweden in the late 1980s.
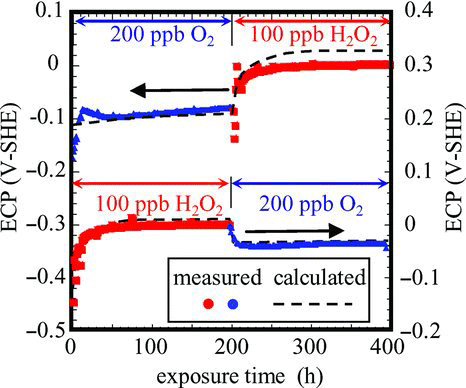
Material tests could be carried out with controlled H2O2 concentration using a poly-tetra-fluoroethylene (PTFE) lined autoclave and sampling lines [Citation50]. Hydrogen peroxide detection systems based on luminol chemiluminescence spectroscopy made it possible to determine subparts per billion level of [H2O2] [Citation51]. As basic studies to support corrosive condition control in the BWR primary cooling system, numerous papers on H2O2 behaviors under elevated temperature were published (see [Citation52] and references therein). The effects of [H2O2] and oxide film formed on the surface on ECP of stainless steel () as well as sensitive H2O2 determination tools have also been described [Citation53]. The ECP evaluation model was developed to have good agreement of calculated and measured ECP behaviors under combined changes in [H2O2] and oxide films (Figure ) [Citation54].
To understand H2O2 behaviors, polarization curves under high temperature pure water should be determined. Usually polarization curves have been measured in high conductivity water by adding electrolyte [Citation55], but new technologies based on the adjacent reference electrode method were successfully applied to measure those under highly purified water at elevated temperature () [Citation56].
4.1.3. Crud and ion deposition on fuel surface
As one of the key parameters to determine radioactive corrosion product behaviors as well as fuel performance, crud deposition on the boiling surface of fuel rods has been studied. As a result of careful observation of the crud deposition process and analogy to heat transfer models under nuclear boiling, a microlayer evaporation and dry out model has been developed to analyze crud deposition under boiling at BWR fuel surfaces [Citation57].
The model was extended from insoluble crud deposition on boiling surface to those of soluble species. Typical patterns of crud and ionic species deposition on the fuel surface are summarized in [Citation58]. The results were used to develop the microlayer evaporation and drying out model, which was applied not only to crud and ion deposition on BWR fuels but also to boron deposition under subcooled boiling and boron accumulation on PWR fuel to evaluate axial offset anomaly phenomena [Citation59].
Table 5 Scheme of chemical change of deposited Ni (Co), Li and B
4.2. Research on water chemistry control
4.2.1. Decontamination measures and prevention of recontamination
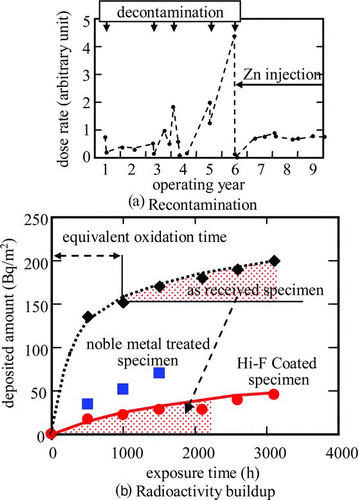
Several decontamination measures with larger decontamination factor (DF) and, at the same time, with minimized radwaste input, e.g. CORD by Siemens, HOP by Hitachi and T-OZON by Toshiba, have been developed [Citation60–Citation62]. One of the difficulties of decontaminations is even if a sufficient DF is obtained during the decontamination processes, dose rate might easily return its previous level or sometime even more than the previous level ((a)). So even if the DF is suppressed at a reasonably low level, recontamination is mitigated by some remaining oxide film on the decontaminated surfaces. Zn injection after decontamination has been seen to be beneficial to mitigate recontamination [Citation13].
One of the latest achievements for mitigating recontamination is Hi–F Coat, in which a ferrite film coated on the decontaminated surfaces during serial decontamination procedures in plants [Citation63]. The Hi–F Coat technology is a kind of pre-oxidation treatment for mitigating radioactive corrosion product build-up (Figure (b)), which was awarded the 2010 AESJ Technical Progress Award in connection with HOP decontamination.
4.2.2. PWR SG cleaning
With long-time operation of power stations, corrosion products are deposited at the SGs as sludge or scale, which results in SG degradation, even when the secondary water contains a small amount of corrosion products from the equipment and tubes in the secondary system. Chemical cleaning or sludge lancing has been adopted to remove the sludge or scale to reduce the SG degradation, but these methods also have problems. A basic study on chemical cleaning was carried out to establish effective procedures without large amount of secondary waste, long process time and serious adverse effects [Citation64]. Advanced technologies have resulted in a new chemical cleaning procedure (ASCA), in which dilute chemicals (EDTA) without inhibitors and with suitable pH (Fe-ASCA: 10,000 ppm for pH 9; Cu-ASCA: 2,300 ppm for pH 10) were applied to dissolve sludge even in crevices without serious adverse effects, e.g. carbon steel corrosion. ASCA has been successfully applied at operating PWR plants [Citation65].
4.2.3. Flow accelerated corrosion
In 2004, the feed water piping of the secondary cooling system in the Mihama-3 PWR plant suddenly ruptured due to FAC, in earlier occurrence of which was seen previously in the Surry-2 plant in 1986 [Citation26].
There are two approaches to detect FAC affected zones for further wall thinning evaluation, one is inspection-based detection and the other is prediction-based detection. The thousands of possible FAC zones cause long and costly inspection procedures for plants, even if the zones are narrowed down based on temperature and flow velocity. To further narrow down the inspection zones, suitable prediction or estimation procedures for FAC occurrence should be applied. At the same time, prediction of FAC occurrence zones with computer programs can be tuned by supplying plenty of inspection data. Coupling of the estimation and inspection procedures might be beneficial for effective and reliable preparation against FAC occurrence and propagation.
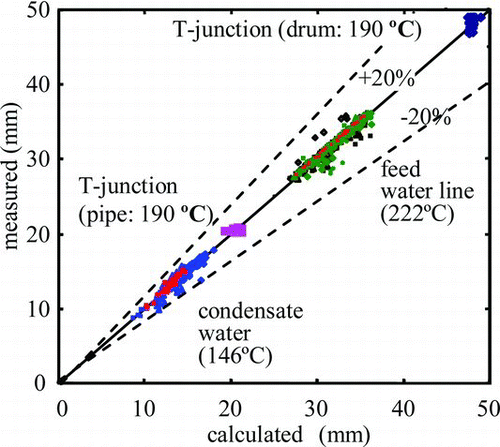
Wall thinning rates have been calculated with the coupled model of electrochemical analysis and oxide-layer-growth analysis by applying the corrosive conditions and the mass transfer coefficients. From comparison of the calculated wall thinning rates with hundreds of measured results at the secondary piping of an actual PWR plant, it was confirmed that the calculated wall thinning rates agreed with the measured ones within a factor of 2 and the accuracy of the evaluation model for residual pipe wall thickness was less than 20% () [Citation66].
In PWR secondary water, hydrazine is injected to suppress [O2]. Oxygen injection is a promising candidate to mitigate FAC. For this, precise [O2] control in the secondary cooling system is required to mitigate any impact on SG tube corrosion, which was supported by results obtained with the N2H4-O2 reaction model [Citation67]. Reaction constants of N2H4 and O2 were from basic research study for N2H4 and H2 co- injection into BWRs [Citation68].
4.3. Future technologies
The concept of an advanced water chemistry diagnosis system for detection of anomalies and preventive maintenance of system components was proposed and put into a concrete form. Using the analogy to a medical inspection system, analyses of water chemistry change would make it possible to detect symptoms of anomalies in system components. Then, correlations between water chemistry change and anomaly occurrence in the components of the BWR primary cooling system were analyzed theoretically. These fragmentary correlations were organized and reduced to an algorithm for the on-line diagnosis system using on-line monitoring data, pH and conductivity. By using actual plant data, the on-line diagnosis model system was verified to be applicable to early and automatic finding of the anomaly cause and for timely supply of much diagnostic information to plant operators () [Citation69].
5. Ongoing subjects
5.1. Roadmap of water chemistry research
Water chemistry has played important roles in improvement of fuel and structural materials integrities, reduction of occupational exposure, and reduction of radwaste sources. Additional contributions of water chemistry to upgraded fuel development and plant aging management as well as future power rate up are required. Water chemistry affects all materials contacting with cooling water and at the same time it is affected by the materials themselves. So, water chemistry control is required to satisfy improved integrity of target materials and at the same time it is required to be optimal for all materials and systems in the plant.
To establish suitable scenarios and strategic plans for R&D programs on water chemistry under restricted human and financial resources, collaborations of government, academic and industrial groups have been discussed to propose road maps of R&D plans for water chemistry of nuclear power systems. Major targets and a schedule of R&D projects are listed in [Citation70].
Table 6 Major targets and subjects of R&D projects related to water chemistry
Major subjects set on the way toward the final goal “establishing safer and more reliable plants due to water chemistry control” are divided into four categories as follows.
| 1. | Water chemistry control related to reduction of occupational exposure and radwaste source | ||||
| 2. | Water chemistry control related to materials integrities in aging plants | ||||
| 3. | Water chemistry control related to fuel cladding materials | ||||
| 4. | Common and basic technologies related to water chemistry | ||||
Thirteen subjects have been proposed within the categories.
The detailed strategies for approaching the targets of the subjects and R&D scenarios are described as road maps in which major targets, major procedures for R&D, major contributors for R&D, major founders and time schedules are proposed.
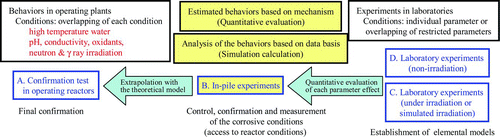
In aging NPPs, radiation-induced or accelerated material problems should be given attention and suitable countermeasures for them should be prepared. Many laboratory experiments on these material problems have been carried out; however their experimental results should be confirmed by in-pile loop experiments under mixed neutron and gamma-ray irradiation conditions.
5.2. JMTR In-pile loop experiments
Behaviors of FP were studied in the FP loop of the Japan Material Test Reactor (JMTR) in the early 1970s, while piping cracks due to IGSCC were observed at the experimental reactor, JPDR in the late 1970s [Citation71, Citation72]. In the SCC loop of the JMTR, IGSCC experiments under different water chemistry conditions were carried out but before sufficient amounts of data were obtained, the JMTR was stopped due to refurbishment of all reactor systems [Citation73]. The refurbishment is almost finished and it is expected that new projects using the JMTR in-pile loops will be started soon after.
The in-pile loops installed in the JMTR, i.e. IGSCC test loops and a water chemistry test loop, will permit experiments to be run for confirming the effectiveness of lifetime management measures for aging LWRs.
The effects of direct and indirect irradiations on water chemistry and materials behaviors are also to be confirmed in the JMTR in-pile loop experiment () [Citation74].
5.3. Challenges following the Fukushima Daiichi NPP meltdown accident
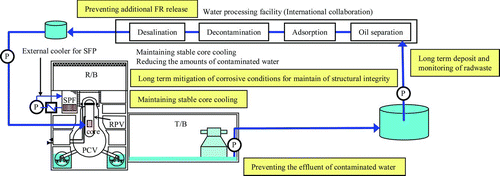
For the Fukushima Daiichi NPP, termination of the accident with stable cooling and radioactivity containment, decontamination of residential areas around the plant and finally plant decommissioning will be ongoing tasks to be carried out based on the utility's restoration road map [Citation75].
The targets for phase 1, e.g. stable cooling of the primary containment vessel (PCV) and spent fuel pools (SFPs) and secure storage of accumulated water in the turbine and reactor buildings (R/B and T/B), had been achieved as of July 2011. Temperatures in the PCV and SFP are being controlled below 100 °C and water processing systems consisting of oil separation, cesium adsorption, decontamination and desalination systems are being operated for recirculation cooling and for decreasing the amounts of accumulated water. Mitigation of corrosive conditions for long-term control of reactor systems is also being carried out () [Citation76]. To meet the targets for phase 2, much more work is required.
At the same time, to increase safety for all other LWR units, long-term R&D needs to be continued. Future plant operation in Japan will be much affected by the plant operation plan shown in the utility's road map.
6. Conclusions
In Japan, scientists and engineers working in water chemistry in LWR plants as well as researchers in academia have been involved in a single society to share and exchange their technologies, their knowledge and their experiences and this has contributed to safe and reliable plant operation. However more efforts on publishing their output from inside the group to persons outside it are required. Publication is not the only means to present their achievements and also those reinforce their technical bases. It is strongly recommended that more collaboration between industries and academia should be undertaken to enhance the achievements and more publications should provide for smooth and timely exchange of technical bases, which would be beneficial for technical transfer from old generations of workers to young workers.
For this, research activities and publications must move toward the same direction.
Abbreviations
| AESJ: | = |
Atomic Energy Society of Japan |
| BWR: | = |
boiling water reactor |
| DWC: | = |
Division of Water Chemistry |
| ECP: | = |
electrochemical corrosion potential |
| FAC: | = |
flow-accelerated corrosion |
| FPP: | = |
fossil fuel power plants |
| HWC: | = |
hydrogen water chemistry (with H2 injection) |
| IGSCC: | = |
intergranular stress corrosion cracking |
| JMTR: | = |
Japan Material Test Reactor |
| JNST: | = |
Journal of Nuclear Science and Technology |
| LMFBR: | = |
liquid metal cooled fast breeder reactors |
| LWR: | = |
light water reactor |
| NPP: | = |
nuclear power plant |
| NWC: | = |
normal water chemistry (without H2 injection) |
| PCV: | = |
primary containment vessel |
| PTFE: | = |
poly-tetra-fluoro-ethylene |
| PWR: | = |
pressurized water reactor |
| PWSCC: | = |
primary water stress corrosion cracking |
| SG: | = |
steam generator |
| SHE: | = |
standard hydrogen electrode |
| SFP: | = |
spent fuel pools |
| [Fe], [Ni], [O2], [H2]: | = |
concentrations of iron curd, nickel ion, oxygen and hydrogen, respectively (ppb) |
Acknowledgements
Drafts of article have been reviewed by key members of the DWC. The authors wish to express their sincere thanks to the members for their supports and encouragements.
Notes
Note:*ethanol amine.
Note: opt: optimal; *1 primary target; *2 high temperature sensor required; *3 [H2]/[O2] > 2.
References
- Uchida , S . 2006 . Latest experience with water chemistry in nuclear power plants in Japan . Power Plant Chem , 8 : 282 – 292 .
- Uchida , S , Takiguch , H and Lister , D H . 2010 . Instrumentation for monitoring and control of water chemistry for light-water-cooled nuclear power plants . Power Plant Chem , 12 : 278 – 295 .
- Ishigure , K , Abe , K , Nakajima , N , Nagao , H and Uchida , S . 1989 . Water chemistry experience of nuclear power plants in Japan . J Nucl Sci Technol. , 26 : 145 – 156 .
- Uchida , S , Otoha , K and Ishigure , K . 2006 . “ Water chemistry – one of the key technologies for safe and reliable nuclear power plant operation ” . In Proceedings of the 15th Pacific Basin Nuclear Conference Sydney , , Australia Oct 15–20; Accompanied by CD-ROM.
- Uchida , S , Naitoh , M , Okada , H and Suzuki , H . 2012 . Water chemistry guidance in nuclear power plants in Japan . Power Plant Chem. , 14 : 5 – 17 .
- Takiguchi , H , Takamatsu , H , Uchida , S , Ishigure , K , Nakagami , M and Matsui , M . 2004 . Water chemistry data acquisition, processing, evaluation and diagnosis systems in light water reactors . J Nucl Sci Technol. , 41 : 214 – 225 .
- Atomic Energy Society of Japan . 2000 . Handbook of water chemistry of nuclear reactor systems , Tokyo : Corona Publishing Co. . Japanese
- Motojima , K and Ishiwatari , N . 1965 . Determination of microamount of aluminum, chromium, copper, iron, manganese, molybdenum and nickel in pure water by extraction photometry - determination of microamount of aluminum, chromium, copper, iron, manganese, molybdenum and nickel in pure water by extraction photometry . J Nucl Sci Technol. , 2 : 13 – 17 .
- Koike , M , Tachikawa , E , Hashimoto , H and Ohkubo , T . 1973 . Gamma-radiolysis of water using a specially designed gamma-irradiation loop . J Nucl Sci Technol. , 10 : 111 – 117 .
- Sagawa , N , Iba , H , Urata , M and Ozawa , Y . 1976 . Transport and deposition of metals in sodium-stainless steel systems, (VI) diffusion of deposited radioisotopes into stainless steel . J Nucl Sci Technol , 13 : 358 – 364 .
- Uchida , S , Asakura , Y , Kitamura , M , Matsushima , Y , Meguro , Y , Ohsumi , K and Miki , M . 1980 . Estimation of shutdown dose rate around recirculation pipes during operating life of boiling water reactors . J Nucl Sci Technol. , 17 : 119 – 128 .
- Otoha , K , Uetake , N and Uchida , S . 1997 . Improvements of water chemistry for moderating environmental impacts of BWR plants . J Nucl Sci Technol. , 34 : 948 – 956 .
- Cowan , R L , Indig , M E and Kass , J N . 1986 . “ Experience with hydrogen water chemistry in boiling water reactors ” . In Proceedings of the International Conference on Water Chemistry Nuclear Reactor Systems, Water Chemistry 4 29 – 36 . Bournemouth , UK Oct 13–17
- Takiguchi , H , Sekiguchi , M , Abe , A , Akamine , K , Sakai , M , Wada , Y and Uchida , S . 1999 . Evaluation of effectiveness of hydrogen water chemistry for different types of boiling water reactors . J Nucl Sci Technol. , 36 : 179 – 188 .
- Cowan , R L . 1996 . “ Mitigation of IGSCC of BWR internals with hydrogen water chemistry ” . In Proceedings of the International Conference on Water Chemistry Nuclear Reactor Systems, Water Chemistry 7 196 – 206 . Bournemouth Oct 13–17
- Wada , Y , Uchida , S , Nakamura , M and Akamine , K . 1999 . Empirical understanding of the dependency on BWR designs for HWC effectiveness . J Nucl Sci Technol. , 36 : 169 – 178 .
- Uchida , S , Satoh , T , Sugama , J , Yamashiro , N , Morishima , Y , Hirose , T , Miyazawa , T , Satoh , Y , Iinuma , K , Wada , Y and Tachibana , M . 2005 . Effects of hydrogen peroxide on corrosion of stainless steel (III) - evaluation of electric resistance of oxide film by equivalent circuit analysis for frequency dependent complex impedances . J Nucl Sci Technol. , 42 : 66 – 74 .
- Ibe , E , Nagase , M , Sakagami , M and Uchida , S . 1987 . Radiolytic environments in boiling water reactor cores . J Nucl Sci Technol. , 24 : 220 – 226 .
- Ichikawa , N and Takagi , J . 2003 . Precise evaluation of corrosion environments of structural materials under complex water flow condition, (II) . J Nucl Sci Technol. , 40 : 941 – 950 .
- Yeh , T K and McDonald , D D . 2006 . The efficiency of noble metals in reducing the corrosion potential in the primary coolant circuits of boiling water reactors operating under hydrogen water chemistry operation . J Nucl Sci Technol. , 43 : 1228 – 1236 .
- Ibe , E , Karasawa , H and Uchida , S . 1991 . Radiation chemistry of radioactive nitrogen species in BWR reactor cores . J Nucl Sci Technol. , 28 : 347 – 355 .
- Pathania , R S and McIlree , R . 1988 . “ A reviw of the effect of hydrogen on stress corrosion cracking of alloy 600 in 360 C water ” . In Proceedings of Third Internatinal Symposium On Environmental Degradation of Materials in Nuclear Power Systems – Water reactors 551 – 526 . Warrendale , , USA August 30-September 3
- Wilson , L and Hickling , J . 2006 . “ Use of primary water chemistry in pressurized water reactors to mitigate pwscc in nickel base alloys ” . In Proc. International Conference on Water Chemistry of Nuclear Reactor Systems Jeju Island , , Korea October 23–26 [CD-ROM]
- Molander , A , Jenssen , A , Konig , M and Norring , K . 2008 . “ PWSCC initiation and crack growth data for alloy 600 with focus on hydrogen effects ” . In Proc. Int. Workshop on Optimization of Dissolved Hydrogen Content in PWR Primary Coolant Sendai , , Japan Accompanied by Jul 18–19 Accompanied by CD-ROM
- Takiguchi , H , Ullberg , M and Uchida , S . 2004 . Optimization of dissolved hydrogen concentration for control of primary coolant radiolysis in pressurized water reactors . J Nucl Sci Technol. , 41 : 601 – 609 .
- Dooley , R B . 2008 . Flow accelerated corrosion in fissile and combined cycle/HRSG plants . Power Plant Chem. , 10 : 68 – 89 .
- Sugino , W , Ohira , T and Yoneda , K . 2010 . Effect of water chemistry improvement on flow-accelerated corrosion in PWR secondary systems . Power Plant Chem. , 12 : 654 – 664 .
- International Atomic Energy Agency . 2011 . Optimization of water chemistry to ensure reliable water reactor fuel performance at high burnup and in ageing plant (FUWAC) , Vienna : IAEA . IAEA-TECDOC-1666
- Nishino , Y , Yasuda , T , Ibe , E and Endo , M . 1997 . “ Effects of water radiolysis on zircaloy corrosion ” . In Proceedings of the International Topical Meeting on LWR Fuel Performance 232 – 239 . Portland Mar 2–6
- Karasawa , H , Ishida , K , Wada , Y , Endo , M , Nishino , Y , Aizawa , M , Fuse , M , Kadoi , H and Takiguchi , H . 2006 . Hydrazine and hydrogen co-injection to mitigate stress corrosion cracking of structural materials in boiling water reactors (III) – effects of adding hydrazine on zircaloy-2 corrosion . J Nucl Sci Technol. , 43 : 1218 – 1223 .
- Uchida , S , Miki , M , Masuda , T , Nagao , H and Otoha , K . 1987 . BWR plants with low shutdown radiation level-design and construction of plants and current experience with occupational exposure of the plants . J Nucl Sci Technol. , 24 : 593 – 600 .
- Fujimori , H , Asakura , Y , Suzuki , K and Uchida , S . 1988 . Chemical state and deposition rate of nickel ion deposit produced on heated surface in high pressure boiling water . J Nucl Sci Technol. , 25 : 464 – 471 .
- Nishino , Y , Sawa , T , Ohsumi , K and Itoh , H . 1989 . Reaction rates of amorphous iron hydroxide with nickel and cobalt ions in high temperature water . J Nucl Sci Technol. , 26 : 1121 – 1129 .
- Hemmi , Y , Ichikawa , N , Saito , N and Masuda , T . 1994 . Protective oxide film on alloy X750 formed in air at 973 K . J Nucl Sci Technol. , 31 : 552 – 561 .
- Shirao , Y , Nakagami , M and Yotsuyanagi , T . 2005 . “ Experience of water chemistry control at hamaoka nuclear power station. ” . In Proceedings of the 13th International Conference on Nuclear Engineering (ICONE-13/2005) Tokyo , , Japan May 16–20 Accompanied by CD-ROM
- Electric Power Research Institute . 2007 . Pressurized water reactor primary water chemistry guidelines: revision 6, EPRI 1014986 , Palo Alto : EPRI .
- Bojinov , M , Karastoyanov , V , Kinnunen , P and Saario , T . 2010 . Influence of water chemistry on the corrosion mechanism of a zirconium-niobium alloy in simulated light water coolant conditions . Corrosion Sci. , 52 : 54 – 67 .
- Riess , R , Henzel , N and Marchl , T . 1999 . “ Use of 10B enriched boric acid in Siemens PWRs ” . In Proceedings of 4th International Symposium on Primary and Secondary Side Water Chemistry of Nuclear Power Plants Balatonfured , , Hungary Sep 29–Oct 2 Accompanied by CD-ROM
- Stellwag , B , Haag , J , Markgrat , B , Preiksch , D and Wolter , D . 2004 . “ Short-term and long-term effects of zinc injection on RCS chemistry and dose rates at Siemens PWR plants ” . In Proceedings of International Conference on Water Chemistry of Nuclear Reactor Systems San Francisco Oct 11–14 Accompanied by CD-ROM
- Chino , K , Matsuda , M , Kawamura , F , Yusa , H , Horiuchi , S and Kikuchi , M . 1984 . A pelletization model and its application to radioactive waste treatment . Nucl Technol. , 67 : 429 – 436 .
- EPRI . 1999 . PWR primary water chemistry guidelines: Vol. 1: revision 4 Palo Alto , CA EPRI TR-105714-V1R4. EPRI
- EPRI . 1999 . PWR primary water chemistry guidelines: Vol.2: revision 4 Palo Alto , CA EPRI TR-105714-V2R4. EPRI
- EPRI. 2004 . Pressurized water reactor secondary water chemistry guidelines – revision 6 Palo Alto , CA EPRI 1008224. EPRI
- BWRVIP-130 . 2004 . BWR vessel and internals project, BWR water chemistry guidelines – 2004 revision Palo Alto EPRI 1008192
- Izumiya , M , Mizuniwa , F , Ohsumi , K , Kanbayashi , G , Matsushima , Y and Tanno , K . 1976 . Oxygen injection for corrosion suppression of BWR feed water system . Karyoku-Genshiryoku Hatsuden. , 27 : 419 – 426 . Japanese
- Fejes , P P . 1980 . “ Degradation Practice in Swedish BWRs ” . In Proceedings of the Seminar Countermeasures for BWR Piping Cracking Palo Alto Paper No 54, 1980 Jan 22–24
- Cowan , R L . 2004 . “ Modern BWR chemistry operating strategies ” . In Proceedings of the International Conference on Water Chemistry of Nuclear Reactor Systems San Francisco Oct 11–14 Accompanied by CD-ROM
- Sanguanmith , S , Muroya , Y , Meesungnoen , J , Lin , M , Katsumura , Y , Kohan , L M , Guzonus , D A , Stuart , C R and Jay-Gerin , J P . 2011 . Low-liner energy transfer radiolysis of liquid water at elevated temperatures up to 350°C: Monte-Carlo simulations . Chem Phys Lett. , 508 : 224 – 230 .
- Nakayama , N and Uchida , S . 1984 . Pressure balanced type membrane covered polarographic oxygen detectors for use in high temperature-high pressure water, (II) effects of environmental factors on detector performance in water at 285 °C . J Nucl Sci Technol. , 21 : 476 – 483 .
- Uchida , S , Shigenaka , N , Tachibana , M , Wada , Y , Sakai , M , Akamine , K and Ohsumi , K . 1998 . Effects of hydrogen peroxide on intergranular stress corrosion cracking of stainless steel in high temperature water, (I) - effects of hydrogen peroxide on electrochemical corrosion potential of stainless steel . J Nucl Sci Technol. , 35 : 301 – 308 .
- Yamashiro , N , Uchida , S , Satoh , Y , Morishima , Y , Yokoyama , H , Satoh , T , Sugama , J and Yamada , R . 2004 . Determination of hydrogen peroxide in water by chemiluminescence detection, (I) flow injection type hydrogen peroxide detection system . J Nucl Sci Technol. , 41 : 890 – 897 .
- Miyazawa , T , Terachi , T , Uchida , S , Satoh , T , Tsukada , T , Satoh , Y , Wada , Y and Hosokawa , H . 2006 . Effects of hydrogen peroxide on corrosion of stainless steel, (V) characterization of oxide film with multilateral surface analyses . J Nucl Sci Technol. , 43 : 884 – 895 .
- AsakuraY , Uchida S . 1987 . Structural material anomaly detection system using water chemistry Data, (III) in-line monitors for electrical conductivity of high temperature water . J Nucl Sci Technol. , 24 : 632 – 638 .
- Uchida , S , Hanawa , S , Nakamura , T , Tsukada , T , Satoh , T and Kysela , J . Determination of electrochemical corrosion potential along the JMTR in-pile loop (I) evaluation of ECP of stainless steel in high temperature water as a function of oxidant concentrations and exposure time . Nucl Technol , in press
- Wada , Y , Watanabe , A , Tachibana , M , Uetake , N , Uchida , S and Ishigure , K . 2000 . Effects of hydrogen peroxide on intergranular stress corrosion cracking of stainless steel in high temperature water, (III) crack growth rates in corrosive environment determined by hydrogen peroxide . J Nucl Sci Technol. , 37 : 901 – 912 .
- Tachibana , M , Ishida , K , Wada , Y , Shimizu , R , Ota , N and Hara , N . 2012 . Cathodic polarization curves of the oxygen reduction reaction on various structural materials of boiling water reactors in high temperature–high purity water . J Nucl Sci Technol. , 49 : 551 – 561 .
- Asakura , Y , Kikuchi , M , Uchida , S and Yusa , H . 1978 . Deposition of iron oxide on heated surfaces in boiling water . Nucl Sci Eng. , 67 : 1 – 7 .
- Asakura , Y , Nagase , M , Uchida , S , Ohsumi , K , Nishino , Y , Yoshikawa , S , Amano , O and Suzuki , N . 1989 . Deposition of nickel and cobalt ions on heated surface under nucleate boiling condition . J Nucl Sci Technol. , 26 : 1112 – 1120 .
- Uchida , S , Asakura , Y and Suzuki , H . 2011 . Deposition of boron on fuel rod surface under sub-cooled boiling conditions - an approach toward understanding AOA occurrence . Nucl Eng Design. , 241 : 2398 – 2410 .
- Bertholdt , H O and Watson , S L . 1998 . “ A new decontamination process to decommissioning of nuclear stations ” . In Proceedings of the International Conference on Decommissioning and Decontamination Nucl. Hazard Waste Management, SPECTRUM’98 118 Denver Sep 13–18
- Nagase , M , Ishida , K , Uetake , N , Aizawa , M , Nakamura , F , Yoshikawa , H , Tamagawa , T and Furukawa , K . 2001 . Low corrosive chemical decontamination method using pH control, (I) basic system . J Nucl Sci Technol. , 38 : 1090 – 1096 .
- Ichikawa , N , Endo , M , Yoshii , T , Kanasaki , T , Kaneda , M and Yamazaki , K . 2006 . “ Application of T-OZON process to a reactor pressure vessel and its internals decontamination ” . In Proceedings of the International Conference on Water Chemistry of Nuclear Reactor Systems Jeju , , Korea Oct 22–26 Accompanied by CD-ROM
- Hosokawa , H , Nagase , M and Fuse , M . 2010 . Development of a suppression method for deposition of radioactive cobalt after chemical decontamination: (I) effect of the ferrite film coating on suppression of cobalt deposition . J Nucl Sci Technol. , 47 : 531 – 537 .
- Kawamura , H , Fujiwara , K , Kambe , H , Hiorano , H , Takiguchi , H , Yoshino , K , Yamamoto , S , Shibata , T and Ishigure , K . 2006 . Applicability of chemical cleaning process to steam generator secondary side, (III) . J Nucl Sci Technol. , 43 : 655 – 668 .
- Suzuki , M and Oohashi , E . 2012 . Cleaning the secondary side of steam generators of Tomari power station unit 1/2 using ASCA and UEC technology . J. Adv Maint. , 4 : NT44 – 45 .
- Uchida , S , Naitoh , M , Uehara , Y , Okada , H , Hiranuma , N , Sugino , W , Koshizuka , S and Lister , D H . 2009 . Evaluation methods for corrosion damage of components in cooling systems of nuclear power plants by coupling analysis of corrosion and flow dynamics (III), evaluation of pipe wall thinning rate with the coupled model of static electrochemical analysis and dynamic double oxide layer analysis . J Nucl Sci Technol. , 46 : 31 – 40 .
- Uchida , S , Naitoh , M , Uehara , Y , Okada , H , Hiranuma , N , Sugino , W and Koshizuka , S . 2008 . Evaluation methods for corrosion damage of components in cooling systems of nuclear power plants by coupling analysis of corrosion and flow dynamics (II): evaluation of corrosive conditions in PWR secondary cooling system . J Nucl Sci Technol. , 45 : 1275 – 1286 .
- Ishida , K , Wada , Y , Tachibana , M , Aizawa , M , Fuse , M and Kadoi , E . 2006 . Hydrazine and hydrogen co-injection to mitigate stress corrosion cracking of structural materials in boiling water reactors, (I) temperature dependence of hydrazine reactions . J Nucl Sci Technol. , 43 : 65 – 76 .
- Asakura , Y , Nagase , M , Uchida , S and Ohsumi , K . 1992 . Structural material anomaly detection system using water chemistry data . J Nucl Sci Technol. , 29 : 1120 – 1126 .
- Uchida , S , Katsumura , Y , Ikoma , H , Takamori , K , Kadoi , E and Ishigure , K . 2008 . “ Road maps on research and development plans for water chemistry of nuclear power systems in Japan ” . In Proceedings of the International Conference on Water Chemistry of Nuclear Reactor Systems, NPC’08 Berlin , , Germany Sep 15–18 Accompanied by CD-ROM
- Handa , M , Yamagishi , S , Fukuda , T , Shiba , K , Takahashi , Y , Tanifuji , T , Oomori , S and Kondo , A . 1974 . Studies of ceramic fuels with use of fission gas release loop, (IV) fission gas release from uranium carbide in the presence of trace amounts of water vapor . J Nucl Sci Technol. , 11 : 387 – 394 .
- Futamura , Y , Torigai , K and Ogawa , Y . 1979 . Piping cracks in JPDR, (IV)analysis of cause of cracks found near weld joints connecting reactor vessel nozzle safe-end to pipe . J Nucl Sci Technol. , 16 : 137 – 146 .
- Kaji , Y , Ugachi , H , Tsukada , T , Nakano , J , Matsui , Y , Kawamata , K , Shibata , A , Ohmi , M , Nagata , N , Dozaki , K and Takiguchi , H . 2008 . In-core SCC growth behavior of type 304 stainless steel in BWR simulated high-temperature water at JMTR . J Nucl Sci Technol. , 45 : 725 – 734 .
- Hanawa , S , Nakamura , T , Uchida , S , Kus , P , Vsolak , R and Kysela , J . Determination of electrochemical corrosion potential along the JMTR in-pile loop (II) validation of ECP electrodes and the extrapolation of measured ECP data . Nucl Technol , in press
- 2011 . Current status of “roadmap towards restoration from the accident at Fukushima Daiichi nuclear power station , TEPCO . Available from: (Revised edition) http://www.tepco.co.jp/en/press/corpcom/release/betu11_e/images/110817e3.pdf
- Uchida , S , Naitoh , M , Okada , H and Suzuki , H . 2011 . The Fukushima Dai-ichi NPP accident crisis and its influence on energy policy in Japan . Power Plant Chem. , 13 : 544 – 556 .
![Figure 6 Optimal water chemistry control (PWR reactor water). (a) Crack growth rate, (b) crack initiation time and (c) ECP as a function of [H2]](/cms/asset/f71c3fba-b1b3-433b-a277-4bdb0e2d195b/tnst_a_773171_o_f0006g.jpg)