Abstract
Two groups of 129I and 127I targets were analyzed using a gas quadrupole mass spectrometer (QMS) to determine the transmutation rates via the melting method. Sodium iodide was chosen to make the target. The iodine composition in the 129I targets is 82.7% 129I and 17.3% 127I. The transmutation rate of the 129I(n, γ)130I reaction was determined by measuring the 130Xe with QMS. An equivalent corrective method was brought out to correct the 129I(n, 2n)128I branch which is interfered with by the 127I(n, γ)128I reaction. And the correction formula was deduced in theory. For very little 128Xe from the 129I(n, 2n)128I reaction, the equivalent corrective method could not be suitable here. However, it is suitable for the mass of 128Xe from 129I(n,2n)128I reaction that reaches the accurately detective level of the mass spectrometry.
1. Introduction
Artificial transmutation of 129I is an alternative way to reduce its radiotoxic risk [Citation1–Citation4]. Stable xenon isotopes are the main products of this artificial work. 127I is the stable iodine isotope of 129I and usually chosen instead of 129I in relevant transmutation experiments in early researches[Citation5,Citation6]. In 1997, researchers from the Netherlands Energy Research Foundation (ECN) who gave their long-duration (192.95 days) transmutation results of 127I which had been irradiated in the high flux reactor at Petten[Citation5]. In 2004, Japanese researchers and their Dutch co-workers used 127I in another long-duration (271.23 days) transmutation experiment at Petten[Citation6]. However, in the two experiments above, the xenon isotopes releasing action was not carefully researched. In 2010, a target-melting method was brought forward and adopted by our team where it was observed that 99.9% of the 128Xe had been released from the target and thus an accurate measurement result of the transmutation rate was obtained[Citation7]. And this method was adopted again to measure the 129I transmutation rate this time.
Additionally, the iodine isotopic composition corresponds to 85–87% 129I and 13–15% 127I, depending on the sample. Moreover, the 129I(n,2n)128I reaction and the 127I(n,γ)128I reaction have the same product: 128I. Thus when taking the double isotopic nature of the iodine samples into account, the relevant question arises: what proportion do the two reactions form the 128I, respectively? In 2002, using the 127I(n,γ)128I and 129I(n,γ)130I cross sections from the JENDL-3 and EXFOR libraries, Henzl et al. calculated the 127I(n,γ)128I reaction product on the basis of the mass of127I and 129I under spallation neutron conditions[Citation8]. They concluded that 93–95% of the total 128I had been produced from the reaction 129I(n, 2n)128I. However, their calculation method contained a lot of parameters and the result had been large error inevitably.
In this work, an equivalent corrective method was derived to deduct the 128I from 127I(n,γ)128I reaction on the basis of the accurate measurement of 128Xe using QMS.
2. Experimental
2.1. Iodine targets preparation
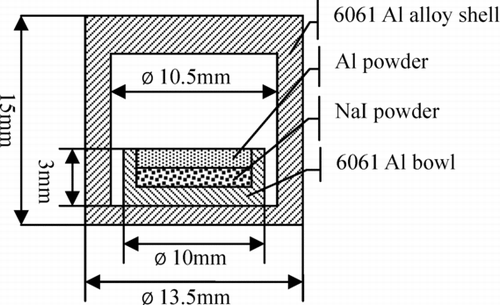
As shown in , the target core was sealed by 6061 Al alloy and eligible in leakage check. The core included three parts: 6061 Al bowl, NaI powder, and Al powder (99.99%). The granule diameter of NaI and Al powder was about 50 μm. The Na127I crystal was produced by General Research Institute for Nonferrous Metals. It was a national standard material. The purity of Na127I was 99.99%. The mixture powder of Na127I and Na129I was obtained from the solution, which had been heated at 353 K until changed to powder.
The Na127I used in 127I target was directly weighted. When preparing the 129I target, two piles of NaI (including Na127I and Na129I) powder were weighted at the same time. One pile was used to prepare the target and the other was used to measure the mass of 127I and 129I with the ICP-MS (Inductively Coupled Plasma – Mass Spectrometer) by using isotope dilution method. The Nu Plasma MC-ICP-MS produced by Nu Instruments Company was employed to do this work and two ion receiving channel was used to measure the ions of 127I and 129I at the same time. To lessen the interference from Xe, the high pure Ar gas was used and the 129I concentration of the solution was set to a high level. At the same time, decreasing the plasma power was a good method to decrease the interference for the high ionization energy of xenon. The mass discrimination factor was obtained from another national standard material Te. The factor of 128Te/130Te was used as that of 127I/129I for very close mass number. To get fine data, the ratio of the sample signal and the background signal is more than 300. Moreover, the 131Xe signal was used to deduct the 129 amu background signal. The probability of producing H126Xe+ or H128Xe+ in the ion source was at 10−5 grade and could be neglected. The total determination error was about 0.5%. The measured iodine isotopic composition in 129I targets was 82.7 % of I-129 and 17.3% of I-127 in mass. The mass of 127I and 129I in the 129I target was obtained from two weighted values and the measured mass ratio.
Two groups of targets were made in this experiment. Each group includes one 127I target and one 129I target. All targets had the same structure as shown in Figure . The 127I target was used to be the equivalent target of the 129I target. All target cores were pressed under 400 MPa pressure. lists the details of four targets in this experiment.
Table 1 Details of four targets.
2.2. Irradiation
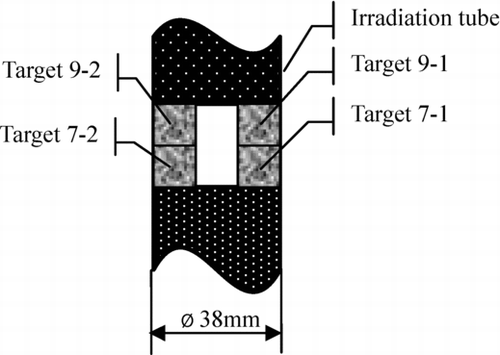
All targets were irradiated in No. 8 channel of the center water cavity of the Xi’an pulsed reactor[Citation9]. As shown in , four targets were separated to two groups. Group 1 included 9-1 and 7-1. Group 2 included 9-2 and 7-2.
The 129I target was above the 127I target. Two groups of targets lied in the middle of irradiation tube where there was steady neutron flux. This arrangement ensured that the transmutation rates of 127I in two kinds of targets could be as same as possible. That was the equivalent correction condition.
Neutron conditions in center water cavity are: thermal neutron flux is about 5.0 × 1013n/(cm2·s) and epithermal neutron flux is about 7.7 × 1011n/(cm2·s). All targets were irradiated for 6 hours.
2.3. Postirradiation analysis
2.3.1. Online isotope dilution mass spectrum analysis
GAM400 QMS (produced by IPI Corp. in 2003 in Germany) was used to measure the xenon isotopes. The lower detection limit of GAM400 QMS for xenon is 2 × 10−12 mol. The error of measuring 10−10 mol Xenon is 1%[Citation10]. Two standard gases are adopted in our experiment. One is the xenon isotopes standard gas which was bought from IRMM (Institute of Reference Materials and Measurement, Belgium). The other is the dilution gas calibration standard gas which was produced by the IPI Corp. allying with NINT.
All targets were measured with melting method after 30 cooling days. The details of melting method were described in [Citation7]. Online isotope dilution mass spectrum method was used to analyze the 128Xe and the 130Xe. The 129Xe was selected as the xenon dilution isotope. The isotope ratio correction coefficients of 129Xe/128Xe, 129Xe/130Xe, and 129Xe/132Xe were measured with the xenon isotopes standard gas before measurement. Then the dilution isotope 129Xe was calibrated by the calibration gas at first. When measuring, sample a part of the released xenon gas and the 129Xe dilution gas, respectively. And mix two kinds of gases and get rid of non-noble gas by a gas getter pump which was an alloy of Zr-V-Fe and worked at 923 K to adsorb over 99% non-noble gas including hydrogen. For the 1000 Pa pressure limitation of the inlet channel, this purification process could remarkably increase the sensitivity and decrease the interference including from the hydrogen. After that, the leaving mixed gas was inlet in the analyzer to measure the ratios of 129Xe/128Xe and 129Xe/130Xe. At last, the number of 128Xe atoms in the released xenon can be calculated by formula (1).
128 N S: the number of atoms of 128Xe in sample gas, unit, mol.
129 N D: the number of atoms of 129Xe in dilution gas, unit, mol.
η D: sampling proportion of dilution gas.
η S: sampling proportion of sample gas.
129/128 R D: isotopic ratio of 129Xe and 128Xe in dilution gas.
129/128 R S: isotopic ratio of 129Xe and 128Xe in sample gas.
129/128 R MS: isotopic ratio of 129Xe and 128Xe in mixed gas.
129/128 ρ: the isotopic ratio correction coefficients of 129Xe/128Xe.
129/128 R S was very small compared with 129/128 R MS because of the very little 129Xe in the background gas in the target. So 129/128 R S can be neglected. Formula (1) changes to a brief one:
At the same time, the number of 130Xe atoms could be also obtained by formula (2) after change of the up-marker “128” with “130”. That was:
In order to validate what percent of the released xenon proportioned in melting-cooling process, we pumped the stainless steel container to 10 Pa after measuring the released xenon and melted the target 9-2 again. The second released gas from the target 9-2 was measured again by QMS. Detail data were shown in .
Table 2 Determination results of four targets after the irradiation.
Deducting the background, the targets were made in air and the pressure was 96 ± 1 kPa. The air volume in each target was evaluated about 0.8 ml. The number of 130Xe atoms in each target was calculated as 1 × 10−13 mol. The number of 128Xe atoms in each target was calculated as 4.6 × 10−14 mol. The values above were too little compared with the transmutation product 128Xe and 130Xe. So they could be neglected.
2.3.2. 129I(n,2n)128I reaction rate correction with equivalent target
The 129I transmutation rate ρ T9 included two parts: 129I(n,γ)130I reaction rate ρ T9-0 and 129I(n,2n)128I reaction rate ρ T9-8. So
ρ T9-8 can be obtained from formula (5),
128 N S9-7: the number of atoms of 128Xe produced by 127I(n,γ)128I reaction in irradiated 129I target, unit, mol.
ρ T9-7: the transmutation rate of 127I(n, γ)128I reaction in irradiated 129I target.
Pβ 8: the β − emission probability of 128I, that is, 0.931.
N I-7: the total number of atoms of 127I in 129I target, unit, mol.
N I-9: the total number of atoms of 129I in 129I target, unit, mol.
Define that ρ T7 was the transmutation rate of 127I(n,γ)128I reaction in the irradiated 127I target. For the equivalent transmutation rates of 127I in two kinds of targets, there was a proximate formula: ρ T7 = ρ T9-7. ρ T7 could be obtained from the 127I target by analyzing 128Xe.
So formula (5) could be proximately changed to formula (6):
Additionally, 129I(n,γ)130I reaction rate ρ T9-0 can be calculated as:
So formula (4) was changed to formula (8):
This was the corrected formula for the total transmutation rate of 129I.
3. Results and discussions
Our measurement results are listed in Table .
As shown in Table , the proportion of release 130Xe in the second melting process was only 0.1% (last row, column 5). So 99.9% of the xenon isotopes had been released from the target in the first melting and cooling process. This result accorded with the data of [Citation7] very well. The data of column 3 and 4 could be directly used to calculate the transmutation rates. This calculation contributes only 0.1% for the total error.
The data of column 6 were the 129I(n,2n)128I reaction correction 128Xe. We could see that the correction values were evidently larger than the theory value. Because there were very few high energy neutrons in Xi’an pulse reactor which could induce the (n, 2n) reaction, 128Xe from 129I(n,2n)128I reaction was very small in theory. Therefore, a very small correction value would be obtained by using formula (8). However, the data of column 6 were not small. It was not reasonable. Why? A critical reason was ρ T7≠ ρ T9-7. The distance of Target 7-1 and 9-1 was about 15 mm. They were not in a plane. So the neutron conditions could not be regarded as same. Maybe this was the main reason. At the same time, Target 7-1 and 7-2 were in the same plane and their reaction rates were in accordance with 2%. So do Target 9-1 and 9-2. The neutron conditions could be therefore regarded as the same when targets were in the same plane and their distances to center line were equal.
Although we could not achieve the correction results this time, the equivalent correction method was really useful to the practice of 129I(n,2n)128I correction for the fast neutron transmutation experiment in which much more 129I(n,2n)128I reaction could be happened to produce much more 128Xe.
4. Conclusion
The sodium iodide was used to make four targets which had been irradiated in the Xi’an pulse reactor. The transmutation rates of four targets were determined by the xenon isotope analysis with a QMS. From the experiment, we could conclude as follows:
| 1. | 99.9% of all produced xenon in the pressed target could be released by using the melting method. | ||||
| 2. | Good error of the transmutation rates could be obtained by using the gas mass spectrum method. | ||||
| 3. | The equivalent method was brought forward firstly. But the correction results were invalid in this experiment. However, it was a feasible method to the practice of 129I(n,2n)128I correction for the fast neutron transmutation experiment. | ||||
Notes
aThis error is for the determination of 130Xe from the second melting.
References
- Wolkenhauer , W C , Leonard , B R Jr and Gore , B F . 1973 . Transmutation of high-level radioactive waste with a controlled thermonuclear reactor: report BNWL-1772 , Richland, Washington , DC : Battelle Pacific Northwest Labs .
- Smith , C F and Cohen , J J . 1980 . A critique of rationale for transmutation of nuclear waste: report BNL-28282 , Livermore , CA : Lawrence Livermore National Laboratory .
- Attrep , M J . 1992 . Accelerator transmutation of 129I: report LA-UR–92-64 , Los Alamos , NM : Los Alamos National Lab .
- Wootan , D W , Jordheim , D P and Matsumoto , W Y . 1991 . Irradiation test of 99Tc and 129I transmutation in the fast flux test facility . Trans Am Nucl Soc , 64 : 125 – 130 .
- Konings , R JM . 1997 . Transmutation of iodine: results of the EFTTRA-T1 irradiation test . J Nucl Mater , 244 ( 1 ) : 16 – 21 . doi: 10.1016/S0022-3115(96)00729-5
- Ichimura , E , Takaki , N , Schram , R PC , Meulekamp , R K and Bakker , K . 2004 . Iodine transmutation studies using metal iodide targets . J Nucl Mater. , 334 ( 2-3 ) : 149 – 158 . doi: 10.1016/j.jnucmat.2004.05.012
- Li , X , Ouyang , X , Wei , G , Tu , J , Pan , X , Zhang , W , Zhang , Z and Liu , B . 2012 . Gas mass spectrum analysis of I-127 transmutation targets irradiated by Xi’an pulse reactor . J Nucl Sci Technol. , 49 ( 1 ) : 90 – 95 . doi: 10.1080/18811248.2011.636545
- Henzl , V , Henzlova , D , Kugler , A , Wagner , V , Adam , J , Caloun , P , Kalinnikov , V G , Krivopustov , M I , Pavliouk , A V , Stegajlov , V I , Tsoupko-sitnikov , V M and Westmeier , W . 2002 . Transmutation of 129I with high energy neutrons produced in spallation reactions induced by protons in massive target . J Nucl Sci Technol , 2 ( Suppl. ) : 1248 – 1251 .
- Jing-ye , A , Zhang , W -s , Wang , W -s , Chen , W -s and Zhong , Y -h . 2002 . Parameters measurement for experimental equipments of Xi’an pulsed reactor . Nucl Power Eng , 23 ( 6 ) : 85 – 88 . [in Chinese]
- Zhang , Z -b , Adolf , G , Wei , G -y , Li , X , Gerken , H and Chang , Y . 2004 . A bench top quadrupole mass spectrometer system for accurate analysis of trace noble gas . J Chin Mass Spectrom Soc , 25 ( 0z1 ) : 194 – 203 . [in Chinese]