Abstract
The Hitachi ferrite coating film process (Hi-F) has been developed to lower recontamination after chemical decontamination. In this process, the chemical decontamination process is carried out, and a fine Fe3O4 coating film is formed on the surface of stainless steel piping in an aqueous solution. In order to improve the suppression of 60Co deposition further, we combined the original Hi-F with a preoxidation step. We found the deposited amount of 60Co with preoxidized Hi-F coating film (OHi-FC) was 1/10 of that for non-coated specimens. In this study, we investigated the suppression mechanism of 60Co for the OHi-FC. The composition of OHi-FC was changed from Fe3O4 to Fe2O3 and then the crystals in the OHi-FC grew three times larger than those of the original Hi-F coating film. Consequently the corrosion amount of the stainless steel base metal was reduced by getting larger grains in the coating film. Because 60Co was incorporated into the corrosion oxide, the suppression effect of 60Co deposition by preoxidation was attributed to the suppression of the formation of the corrosion oxide by the OHi-FC.
1. Introduction
Chemical decontamination is an effective method to reduce occupational radiation exposure in boiling water reactors (BWRs) when carrying out such large-scale tasks as overhaul of primary recirculation pumps and shroud replacement. In chemical decontamination, the oxides formed on the surface of the stainless steel piping that incorporates the 60Co, are dissolved with reductive and oxidative chemical reagents. The stainless steel base metal of the piping is exposed to reactor water after the chemical decontamination process, and for the oxide film which has incorporated 60Co, its growth rate on the piping during plant operation just after the decontamination is higher than that just before it. Then, there is a possibility that the deposition amount of 60Co on the piping just after decontamination is higher than that just before the chemical decontamination process. Actually, rapid deposition amount increases of 60Co within a few operating cycles have been observed in some nuclear power plants [Citation1–Citation3]. Therefore, we developed the Hitachi ferrite coating film process (Hi-F) to reduce the recontamination by 60Co after the chemical decontamination [Citation4–Citation6]. Following the chemical decontamination, a fine Fe3O4 coating film is formed on the stainless steel base metal of the piping. The oxide film growth rate on the piping during the plant operation is suppressed by this fine coating film that blocks diffusion of both oxidant in the reactor water to stainless steel base metal and metal ions in the oxide film to reactor water. Consequently, the amount of 60Co deposition was decreased by reducing the formation of the oxide film that incorporates 60Co.
In our previous studies [Citation4–Citation7], we confirmed that the Fe3O4 film is effective to suppress 60Co deposition after chemical decontamination under normal water chemistry condition (NWC) and hydrogen water chemistry condition (HWC). For the Hi-F, laboratory experiments confirmed that the 60Co deposition reduction effects under NWC and HWC were 1/5 and 1/4, respectively, compared to non-treatment for up to 3100 h, and about 60 μg/cm2 was a sufficient amount of film formation to get a lower 60Co deposition. To further improve this process in terms of its reduction effect on the 60Co deposition amount, the Hi-F was combined with the preoxidation step under the NWC. By combining the Hi-F with the preoxidation step, the amount of 60Co deposition was reduced to 3/10 compared to the reduction in deposition due to the Hi-F coating film (Hi-FC) under HWC. The suppression effect of 60Co deposition with the preoxidized Hi-F coating film (OHi-FC) was three times higher than that with the Hi-FC alone.
In order to determine the preoxidation time for the suppression of the 60Co deposition by OHi-FC, it is important to investigate the oxidation mechanism of the Hi-FC on stainless steel in an aqueous solution at 553 K and the suppression mechanism of 60Co by OHi-FC. In the present study, we studied the oxidation mechanism of the Hi-FC and the suppression mechanism of 60Co by the OHi-FC by X-ray diffraction, transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDX) measurements. Furthermore, we determined a requirement for the OHi-FC to suppress the deposition amount of 60Co.
2. Experimental
2.1. Specimen preparations
2.1.1. Formation of Hi-FC specimens
Type 316 stainless steel (316SS) specimens (surface area: 8 mm×15 mm; thickness: 1.5 mm) were polished with 600-grit emery paper, and degreased with acetone. To coat them with ferrite film, the specimens were put in 1-L flasks filled with water through which nitrogen gas was bubbled to remove oxygen. The water and specimens in the flasks were heated to 363 K and then Fe(HCOO)2 solution was added to the hot water. Three chemical reagents are needed to form a Hi-FC: Fe(HCOO)2 solution, H2O2, and N2H4. The Fe(HCOO)2 solution was produced by dissolving Fe metal in formic acid solution, with nitrogen bubbling to remove the oxygen. The formic acid concentration was 0.12 mmol/dm3.
After the Fe(HCOO)2 solution had been completely mixed with the water, H2O2 was added. The pH of this mixed solution was adjusted to 7 at 363 K by addition of N2H4. The concentrations of Fe2+, H2O2, and N2H4 in the flask solution were 3.58, 0.13, and 2.05 mmol/dm3, respectively. Ferrite formation occurred by the following equation. Ferric ion was produced from ferrous ion by the Fenton reaction with hydrogen peroxide according to the following equation.
2.1.2. Formation of OHi-FC specimens
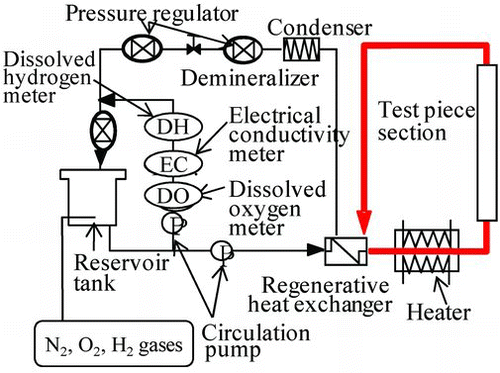
The Hi-FC specimens were oxidized under the NWC to be used for a preoxidation effect test. A flow diagram of the experimental apparatus for preoxidized specimens under the NWC is shown in . The gases N2 and O2 were bubbled into the reservoir tank water to keep dissolved oxygen concentration at 200 ppb and the soaking time was 0.1, 6, 24, 50, 100, 200, or 500 h. In the apparatus, the temperature and pressure were controlled at 553 K and 7.8 MPa, respectively, to simulate actual plant conditions.
2.2. 60Co deposition behavior test
The 60Co deposition behavior test was conducted under the HWC to examine the preoxidation effect. The non-coated, the Hi-FC, and the OHi-FC specimens, with preoxidation time of 50, 100 or 500 h, were set in the autoclave. To simulate the HWC that has been applied to older nuclear plants, the gases H2 and N2 were bubbled into the reservoir tank water to keep their concentrations at the desired levels of under 5 ppb for dissolved oxygen and 50 ppb for dissolved hydrogen. 60Co was added (activity: 6.7 Bq/kg) and also cobalt sulfate (cobalt ion concentration: 0.1 ppb). The temperature and pressure were controlled at 553 K and 7.8 MPa, respectively. The same experimental apparatus of Figure was used for the 60Co deposition test of specimens under the HWC. The amounts of 60Co on the test specimens were measured using a germanium gamma-ray detector (Seiko EG&G Co. Ltd., GEM15P-70) with a multichannel pulse height analyzer.
2.3. Oxide film analysis
The Hi-FC and OHi-FC specimens were analyzed by X-ray diffraction (Rigaku Co. Ltd., RINT-2500HL), TEM (Hitachi, HF-2000) and EDX (Hitachi, HF-2000). The wavelength and incident angle for X-ray diffraction were 0.15418 nm and 0.1°. The TEM was operated at 200 kV, and beam spot and camera length were 9 and 29 nm, respectively.
After the surface analysis, the oxide film formed in the high-temperature water under the experimental conditions was separated into two parts of outer and inner layers [Citation6]. The outer layer of the oxide could be dissolved in a reductive condition and was separated by cathodic electrolysis into constituent compounds. The test specimen, with the inner layer, was electrolyzed in an electrolyte solution of 5 wt% H2SO4 and 0.5 wt% hexamethylentetramine with 100 A/m2. The inner oxide film layer was separated by alternately soaking in an oxidizing potassium permanganate (300 ppm KMnO4) solution and a reducing 10 wt% ammonium citrate dibasic solution at 368 K. The amounts of 60Co in the separated oxide films were measured using the same germanium gamma-ray detector as used for the 60Co deposition behavior test. The counting rates of the gamma rays from the 60Co deposited on the test specimens and the deposition amounts were corrected for natural decay during the experimental period. The amounts of the 60Co on the test specimens were also measured using the same germanium gamma-ray detector as above.
3. Results and discussion
3.1. The suppression effect of OHi-FC on 60Co deposition
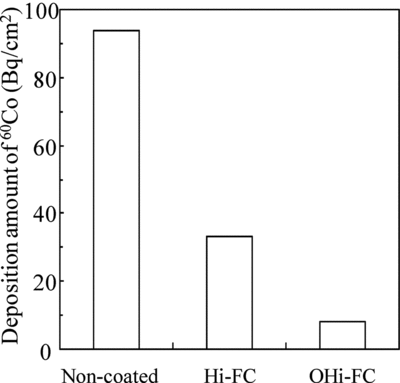
The 60Co deposition behavior test was conducted under the HWC to examine the preoxidation effect. The average deposition amounts of 60Co on non-coated,&break; Hi-FC, and OHi-FC (preoxidation time: 500 h) specimens used in the preoxidation effect test are shown in . The deposition amounts of 60Co on the non-coated, Hi-FC, and OHi-FC specimens were 94, 33, and 8 Bq/cm2, respectively. We found that the Hi-FC and OHi-FC specimens lowered the amount of 60Co to about 1/3 and 1/10 of that for non-coated specimens. In other words, by combining the Hi-F with the preoxidation step, the suppression effect of 60Co deposition was three times higher than that of the Hi-F alone. To investigate the suppression mechanism of 60Co by the OHi-FC, we analyzed these specimens by X-ray diffraction, TEM, and EDX. Furthermore, the distribution of 60Co deposition in these samples was also observed.
3.2. Investigation of the suppression mechanism of 60Co by OHi-FC
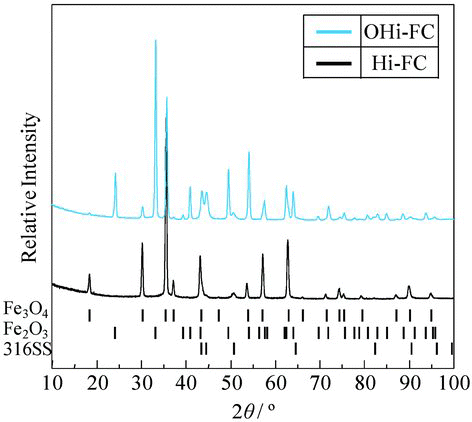
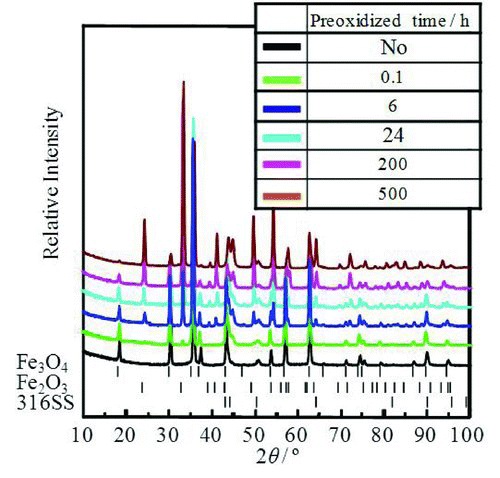
In order to identify the chemical form and morphology of the Hi-FC and OHi-FC formed on a 316SS surface, we used X-ray diffraction, TEM, and EDX. shows X-ray diffraction pattern of these specimens. The peak positions of the Hi-FC were identical to those of a standard Fe3O4 polycrystalline form and the Fe3O4 film formed on the stainless steel surface was concluded to have a single spinel polycrystalline phase [Citation8,Citation9]. This phase might be attributable to a cubic spinel-type structure in the Fe3O4. On the other hand, the peak positions of the OHi-FC were identical to those of Fe3O4 and Fe2O3 polycrystalline phases [Citation8–Citation10]. The OHi-FC formed on the stainless steel surface was concluded to have two polycrystalline phases: Fe3O4 polycrystalline and Fe2O3 polycrystalline. This result means that a part of the Fe3O4 in the Hi-FC was changed to Fe2O3 by preoxidation. shows the TEM and EDX results of the cross-sectional morphologies, electron diffraction patterns of the surface film, and the depth profile of elements of the Hi-FC specimen and the OHi-FC specimen with preoxidation time of 500 h after the 60Co deposition test. The TEM images and EDX measurements of the Hi-FC showed a close-packed double-layer thin film. The electron diffraction pattern of the surface Hi-FC agreed with the pattern of the (111) plane of Fe3O4. The outer layer was mainly Fe3O4 single phase and crystal size was about 150 nm. The inner layer contained not only Fe but also Cr and Ni and its thickness was about 400 nm. This indicated that the outer layer was mainly the Hi-FC and the inner layer was the corrosion oxide of 316SS base metal, because the Fe, Cr, and Ni ions, which are constituent elements of 316SS, were included in the inner oxide layer. The same analytical results for the preoxidized OHi-FC also showed a close-packed double-layer thin film. The electron diffraction pattern of the surface OHi-FC agreed with the pattern of the (182) plane of Fe2O3. The surface of the outer layer for this was mainly Fe2O3 film and the crystal size was about 450 nm. The accompanying inner layer was the corrosion oxide film and its thickness was about 80 nm.
Figure 4 The TEM and EDX results of the cross-sectional morphologies and the depth profile of elements of the Hi-FC and the OHi-FC with preoxidation time of 500 h
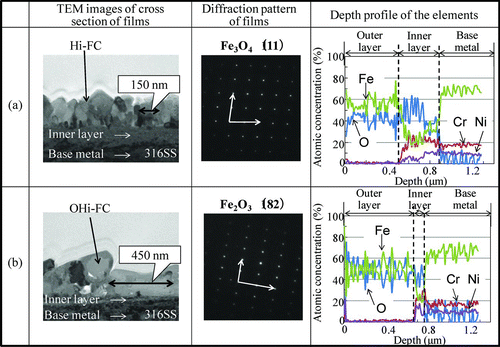
The TEM results indicated that the crystal size in the OHi-FC was three times as large as that of the Hi-FC. This meant that the Fe3O4 crystals grew in horizontal direction and became three times larger, as their composition was changed from Fe3O4 to Fe2O3 by preoxidation. In other words, the coverage of the Hi-FC was increased with increasing the crystal size by the preoxidation step.
shows the EDX results of average atomic concentration for each layer. The atomic concentrations of Fe ions in the inner layer and the base metal for all specimens were 20% and 70%, respectively. This difference means that there was diffusion of the Fe ions in the base metal to the aqueous solution. Because the atomic concentration of the Fe ions in the inner layer for these specimens and the size of the test pieces were the same, the dissolution amount of the Fe ions depended on the thickness of the inner layer. From the TEM results, the thickness of the inner layer for the OHi-FC was 1/5 of that for Hi-FC. In other words, the amount of Fe dissolution from the base metal with the OHi-FC was suppressed to 1/5 of that of Hi-FC by blocking diffusions of both the oxidant and Fe ions with a high coverage of the base metal with OHi-FC.
Table 1 Atomic concentration in the outer layer, inner layer, and base metal as obtained by EDX
In previous reports [Citation11], Fe ions which dissolved from the base metal to the aqueous solution, were reprecipitated on the base metal as oxides that incorporated 60Co. In this study, the dissolution amount of the Fe ions from the base metal was changed by the coverage of the coating films. We consider that the difference of the dissolution amount of the Fe ions for these specimens possibly affected the deposition amount of 60Co.
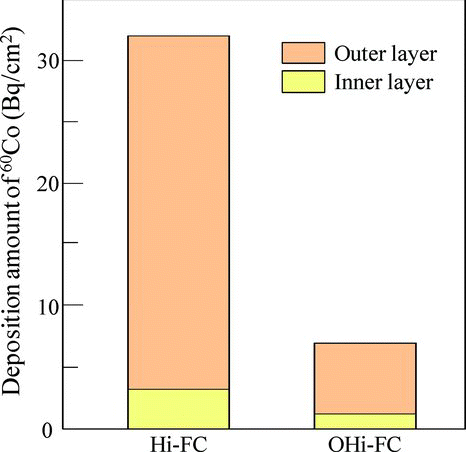
In order to understand the 60Co deposition mechanism of these specimens, we analyzed the 60Co deposition amount of the inner and outer layers. shows the deposition amount of 60Co in the inner and outer layers of Hi-FC and OHi-FC specimens. The deposition ratios of 60Co in the inner and outer layers of Hi-FC and OHi-FC specimens were 1/10 and 1/7, respectively. These results indicated that 60Co was mainly deposited in the outer layer and the reason for this was that the Fe ion, which dissolved from base metal to the aqueous solution, was reprecipitated on the Hi-FC as oxide that included 60Co from the aqueous solution. Consequently, the suppression of the deposited amount of 60Co, which was incorporated in the oxide by preoxidation, was due to the suppression of the dissolution amount of the Fe ions by the increasing coverage of the OHi-FC. The relation between the deposition amount of 60Co and formation of the inner layer was able to explain the TEM, EDX, and previous results [Citation11]. In addition, the X-ray and electron diffraction patterns of these specimens in Figures and indicated that the specimens did not include Co-related oxides.
Figure 5 The deposition amounts of 60Co in the inner and outer layers of the Hi-FC and the OHi-FC with preoxidation time of 500 h
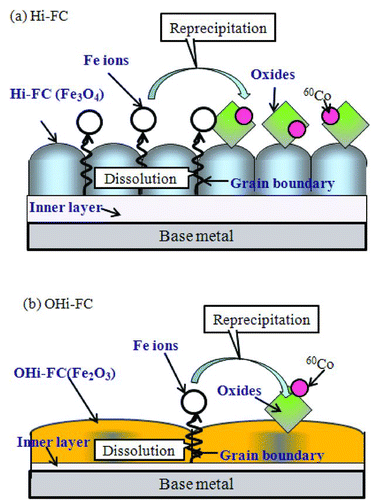
From these results, we considered the suppression mechanism of 60Co by the OHi-FC, using the above results of X-ray diffraction, TEM images, EDX, and 60Co deposition behavior test. illustrates the deposition of 60Co on the film surface of the (a) Hi-FC and (b) OHi-FC. In the deposition mechanism of the Hi-FC, Fe ions dissolved from base metal into the aqueous solution, pass through the grain boundary of the Hi-FC and then Fe ions reprecipitate on the surface of the Hi-FC with incorporation of 60Co from the aqueous solution. The main dissolution route of the Fe ions from the base metal into the aqueous solution is through the grain boundary of the Hi-FC because the diffusion of Fe ion in the Fe3O4 crystals of the Hi-FC is very slow [Citation12]. On the other hand, the deposition mechanism of the OHi-FC is shown in Figure (b). The Hi-FC was changed from Fe3O4 to Fe2O3 by preoxidation and the crystals grew three times larger with the composition change; consequently, the coverage of the OHi-FC was increased, when compared with the Hi-FC. Because the OHi-FC, with increased coverage, had a film that better blocked both diffusion of oxidant in the water to the base metal and of metal ions in the oxide film to water compared with the Hi-F, the dissolution amount of Fe ions from the base metal to the aqueous solution with the OHi-FC was suppressed. Consequently, the suppression of the deposited amount of 60Co, which was incorporated in the reprecipitated oxide film on the coating film by&break; preoxidation, was due to the suppression of the dissolution amount of Fe ions from the base metal to the aqueous solution by the OHi-FC.
Figure 6 The illustrations of the deposition of 60Co on the surface of the (a) Hi-FC and (b) the OHi-FC
In order to adopt this OHi-FC to piping in BWR plants, we next had to clarify the relationship between the composition change of the OHi-FC by preoxidation and the deposition amount of 60Co.
3.3. The oxidation mechanism of the Hi-FC
In order to investigate the composition change of the OHi-FC, X-ray diffraction patterns of the OHi-FC specimens, formed with the preoxidized times of 0, 0.1, 6, 24, 200, and 500 h, were observed and are shown in . The X-ray diffraction results indicated that a part of the Fe3O4 single phase in the Hi-FC was changed to a double phase of Fe3O4 and Fe2O3 by the preoxidation step. With increasing preoxidation time, the peak intensity of Fe2O3 was increased and that of Fe3O4 was decreased.
Figure 7 The X-ray diffraction patterns of Hi-FC with preoxidation times of 0, 0.1, 6, 24, 200, and 500 h
According to previous crystal growth studies&break; [Citation13–Citation17], Fe3O4 changes to Fe2O3 immediately after deposition of O2 on the surface. The formation of Fe2O3 in Fe3O4 was calculated as per Equation (Equation2), which is as follows:
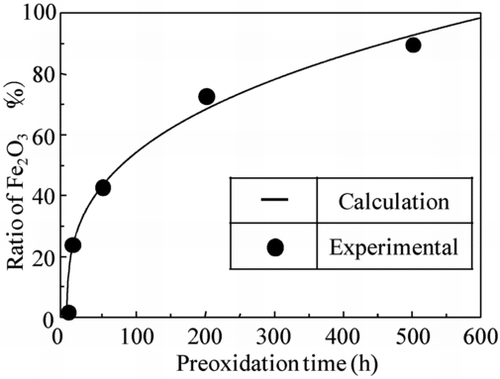
shows preoxidation time dependency of the crystal size of the coating film which was obtained by the TEM images. The crystals in the coating films grew with time and the crystals were about 150, 280, and 450 nm in size at 0, 50, and 500 h, respectively.
Figure 9 The preoxidation time dependency of the crystal size of OHi-FC with preoxidation time of 0, 50, and 500 h in aqueous solution
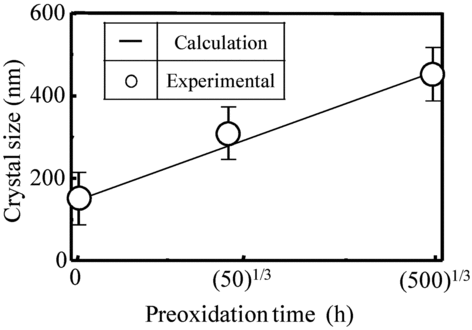
The solid line in Figure is the fitting value of Equation (Equation3) calculated with the crystal growth coefficient (k 2). In this calculation, the crystal growth coefficient (k 2) was a parameter. There was reasonable agreement between the lines and the measurements when the crystal growth coefficient was set as 38.5. From these results, we concluded that the crystal growth of the OHi-FC followed the crystal growth theory.
From the results in Sections 3.2 and 3.3, the crystal size in the OHi-FC was three times as large as that of the Hi-FC. This meant that the Fe3O4 crystals grew in the horizontal direction and became three times larger, as their composition was changed from Fe3O4 to Fe2O3 by preoxidation. This crystal growth of the OHi-FC&break; followed the crystal growth theory.
3.4. The relation between the deposition amount of 60Co and the preoxidation time of Hi-FC
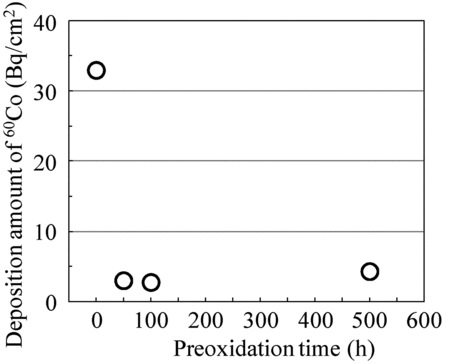
In order to determine the appropriate preoxidation time for the suppression of 60Co deposition by OHi-FC, we carried out the 60Co deposition test with Hi-FC and OHi-FC specimens for preoxidation times of 50, 100, and 500 h. shows the preoxidation time dependency of the deposition amount of 60Co. The deposition amount of 60Co in OHi-FC decreased and the amount of 60Co in OHi-FC was saturated at about 4 Bq/cm2. We determined that it was necessary to continue the OHi-F&break; for more than 50 h to reduce the deposition amount of 60Co to 3/10 compared to the Hi-FC alone. In other words, the crystal size of 300 nm in OHi-FC, which was twice that of Hi-FC, was enough to suppress the 60Co deposition.
4. Conclusion
The Hi-F has been developed as a new suppression method for recontamination by 60Co after chemical decontamination. In this study, we investigated the oxidation mechanism of Hi-FC on stainless steel in an aqueous solution at 553 K and proposed the suppression mechanism of 60Co with the OHi-FC based on X-ray diffraction, TEM, EDX, and 60Co deposition behavior test results. Furthermore, we determined the necessary preoxidation time of the Hi-F to suppress the deposition amount of 60Co.
The X-ray diffraction results indicated that a part of the Fe3O4 in the Hi-FC was changed from Fe3O4 to Fe2O3 and the OHi-FC crystals were three times larger than those of Hi-FC. This meant that the coverage of Hi-FC was increased with larger film crystals. The dissolution amount of the Fe ions from the base metal with OHi-FC was decreased with increasing coverage of the film. Consequently, suppression of the deposited amount of 60Co, which was incorporated in the oxide, was due to the suppression of the dissolution of the Fe ions from the base metal by OHi-FC. Furthermore, we determined the required preoxidation time of the Hi-FC to get a film that suppressed the deposition amount of 60Co. The experimental results indicated it was necessary to preoxidize the Hi-FC more than 50 h to reduce the deposition amount of 60Co to 3/10 compared to Hi-FC alone.
References
- Miyazawa , A , Shibano , T , Sato , T , Shimura , M and Iida , K . 2005 . The operation experience and the simple method to predict dose rate of primary loop recirculation system piping&break; after 1 year chemical decontamination . Symposium on Water Chemistry and Corrosion in Nuclear Power Plants in Asia . October 11–13 2005 , Gyeongju , Korea. pp. 38 – 45 .
- Cowan , R L and Wood , C J . 2002 . Control of radiation fields in BWRs after noble metal chemical addition . 9th International Conference on Water Chemistry in Nuclear Reactors Systems . April 22–26 2002 , Avignon , France. pp. 21 – 26 .
- Wood , C J . 1996 . Advances in water chemistry control for BWRs and PWRs . Water chemistry for nuclear reactor systems 7 . 13–17 October 1996 , Bournemouth , UK. Vol. 1 , pp. 1 – 10 . BNES .
- Hosokawa , H , Nagase , M and Fuse , M . 2010 . Development of a suppression method for deposition of radioactive cobalt after chemical decontamination: (I) the effect of the ferrite film coating on suppression of cobalt deposition . J Nuclear Sci Tech , 47 : 531 – 537 .
- Hosokawa , H , Morisawa , S , Nagase , M , Sakashita , M and Fuse , M . 2007 . “ Development of a suppression method for deposition of radioactive cobalt after chemical decontamination ” . In Symposium on Water Chemistry and Corrosion of Nuclear Power Plants in Asia 225 – 232 .
- Ito , T , Hosokawa , H , Nagase , M and Fuse , M . 2010 . Development of a suppression method for deposition of radioactive cobalt after chemical decontamination: (II) consideration of Fe3O4 plating mechanism on stainless steel in aqueous solution at 363 K . J Nuclear Sci Tech , 47 : 698 – 704 .
- Hosokawa , H , Nagase , M and Fuse , M . 2010 . Development of a suppression method for deposition of radioactive cobalt after chemical decontamination: enhancement of suppression of performance for ferrite film coating , 3 – 10 . Quebec , Canada : Water chemistry for nuclear reactor systems 10 .
- Stine , R and Pishko , M V . 2005 . Comparison of glycosphingolipids and antibodies as receptor molecules for ricin detection . Anal Chem , 77 : 2882 – 2887 .
- Abe , M and Tamaura , Y . 1984 . Ferrite plating in aqueous solution: new technique for preparing magnetic thin film . J Appl Phys , 55 : 2614 – 2618 .
- Geballe , T H , Matthais , B T , Compton , V B , Croenzwit , E , Hull , G W and Longinotti , L D . 1965 . Superconductivity in binary alloy systems of the rare earths and of thorium with Pt-group metals . Phys Rev , 137 : 119 – 127 .
- Hosokawa , H , Nagase , M , Kajitani , H and Kaneoka , T . 2010 . Development of suppression method for deposition of radioactive nuclide after decontamination-(X)-suppression effect of co deposition with pre-oxidized ferrite film . Fall Meeting of the Atomic Energy Society of Japan , G45 Japanese
- Ishigure , K . 2000 . Water chemistry of nuclear power plants , 1 – 83 . Tokyo : Coronasha . Japanese
- Satoh , K . 2002 . Crystal growth in aqueous solutions , 1 – 46 . Tokyo : Koudansha . Japanese
- Kamata , H . 1975 . Chemical reaction in solids , 61 – 86 . Tokyo : Nihon Kagakukai . Japanese
- Dinov , K , Ishigure , K , Matsuura , C and Hiroishi , D . 1993 . Solubility of magnetite in high temperature water and an approach to generalized solubility computations . Journal of Nuclear Materials , 207 : 266 – 273 .
- Kitahara , H. 1994 . Fundamental of surface science for colloid , 10 – 67 . Tokyo : Koudansha . Japanese
- Kubota , N and Mullin , J W . 1995 . A kinetic model for crystal growth from aqueous solution in the presence of impurity . J. Crystal Growth , 152 : 203 – 212 .