Abstract
A novel new sorbent for the separation of krypton from off-gas streams resulting from the reprocessing of used nuclear fuel has been developed and evaluated. A hydrogen mordenite powder was successfully incorporated into a macroporous polymer binder and formed into spherical beads. The engineered form sorbent retained the characteristic surface area and microporosity indicative of mordenite powder. The sorbent was evaluated for krypton adsorption capacities utilizing thermal swing operations achieving capacities of 100 mmol of krypton per kilogram of sorbent at a temperature of 191 K. A krypton adsorption isotherm was also obtained at 191 K with varying krypton feed gas concentrations. Adsorption/desorption cycling effects were also evaluated with results indicating that the sorbent experienced no decrease in krypton capacity throughout testing.
1. Introduction
Reprocessing of used nuclear fuel will result in the release of volatile radioactive gas species. These gases are typically composed of primary fission product isotopes including krypton (85Kr), iodine (129I), tritium (3H), carbon (14C) and xenon (Xe). Although Xe isotopes have very short half-lives and become stable during normal fuel cooling times, they have a high commercial economic value and will negatively impact the adsorption of Kr. The resultant radioactivity emissions released in off-gas streams are addressed by the US Environmental Protection Agency in 40 CFR 190 by establishing annual dose limits resulting from nuclear fuel cycle activities in the commercial sector [Citation1]. According to the regulation, emissions of both 129I and 85Kr will need to be limited to meet the lower value of (1) their specific fuel-cycle-based emission limit and (2) the value needed to limit the combined dose equivalent (from all radioactive material discharges) to any member of the public. This paper addresses a retention technique of Kr to meet these requirements.
Cryogenic distillation is considered the most mature process used to partition Kr from nuclear fuel reprocessing off-gas streams [Citation2]. However, due to the high capital and operating costs in addition to cryogenic distillation process complexity, the physical adsorption process is being evaluated as a potential candidate for the capture of Kr from off-gas streams. Physical adsorption processes can be operated in either thermal swing adsorption (TSA) or pressure swing adsorption operations or a combination of both. However, isotherms generated from previous physical adsorption work suggests that the capacity for Kr is linearly dependant using pressure swing operations but exponentially dependant with thermal swing operations [Citation3]. Therefore, TSA operation is considered to be more advantageous and was selected for preliminary evaluations.
Although activated charcoal is a well known and highly selective Kr adsorbent, reports of explosion incidents caused by the reaction of NOx and charcoal at the elevated desorption temperatures dictate that alternative non-combustible materials be explored [Citation4]. Literature reviews into sorbent materials for Kr capture revealed that various synthetic zeolites have the proper cage structure and frameworks that result in high surface areas and a micropore size conducive for Kr capture. Hydrogen mordenite (HZ) was selected as a candidate adsorbent based on these literature reviews [Citation2,Citation5–11]. However, it became evident that the ability to acquire high surface area material in an appropriately engineered bulk form proved difficult. When available materials were acquired, the measured surface area was found to be quite low. This low surface area was assumed to be a result of the pelletization manufacturing process and will inhibit the adsorption of Kr. Although mordenite powders are readily available commercially and exhibit surface areas up to 500 m2/g, their use in process columns would result in potential high differential operating pressure. The possibility of preparing a novel engineered material using a macroporous polymer known for its chemical, thermal and radiolytic stability was investigated to bind or encapsulate the mordenite powder.
2. Experimental
2.1. Chemicals and materials
Hydrogen mordenite powder CBV90A was acquired from Zeolyst International (Conshohocken, PA). Dralon X 100 Polyacrylonitrile (PAN) fibers were obtained from Dralon GmbH (Dormagen, Germany). Dimethyl sulfoxide (DMSO) was obtained from Sigma Aldrich (St. Louis, MO). 150 and 2500 μL/L Kr in helium were obtained from Air Liquide (Houston, TX). All chemicals were used as received.
2.2. Sorbent preparation
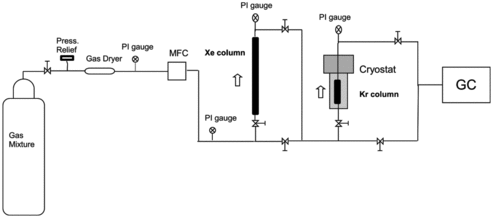
Ten grams of the HZ powder was mixed with 30 mL of DMSO. The resulting slurry was heated to 80 °C and 2 g of PAN was added to the slurry. This combination results in ∼80 weight% loading of the HZ in the PAN binder. The heated slurry was allowed to mix until the PAN had completely dissolved and a homogenous mixture was obtained. The mixture was then transferred into a specially designed glass apparatus as shown in and allowed to drip into a continuously stirred nanopure water reservoir where the mixture drops formed insoluble mordenite/PAN solid sorbent particles while the DMSO was retained in the water bath.
The final sorbent particles, herein referred to as HZ-PAN, were filtered away from the excess water/DMSO mixture and allowed to dry overnight in a vacuum oven at 60 °C. The dried sorbent particles were sieved to a particle size range of 0.425–1.4 mm in diameter. Brunauer–Emmett–Teller (BET) surface area and the microporosity via the density functional theory (DFT) of the HZ-PAN and the initial powder were obtained from N2 adsorption isotherms utilizing a Micromeritics ASAP 2020 surface area analyzer. Scanning electron microscope (SEM) images of the HZ-PAN particles for observation of its porosity were also obtained. A patent application for this sorbent development has been filed [Citation12].
2.3. Kr capacity evaluations
The HZ-PAN's Kr capacity was evaluated utilizing an experimental setup incorporating a custom-designed cryostat system. The system enables sorbent capacity (SC) evaluations at temperatures ranging from 150 to 500 K with sorbent bed masses ranging from 2 to 600 g. includes a schematic of the experimental setup including the cryostat. 5.02 grams of HZ-PAN was placed into the cryostat cold column and subsequently activated at 150 °C with He flowing at 50 sccm for 12 hours. Following the activation, the cryostat was allowed to cool to 191 K with He flowing. Once the cryostat reached the experimental temperature, a feed gas of 150 μL/L Kr with a helium balance was introduced with an MKS (MKS Instruments, Inc.) mass flow controller (MFC) at a flow rate of 50 sccm. This feed gas was directed through a column containing Drierite prior to being introduced into the cryostat to ensure a dry gas stream. The gas effluent from the cryostat was analyzed with an Agilent Technologies model 7890A gas chromatograph (GC) utilizing a Supelco carboxen 1010 plot fused silica capillary column with a thermal conductivity detector to complete Kr breakthrough.
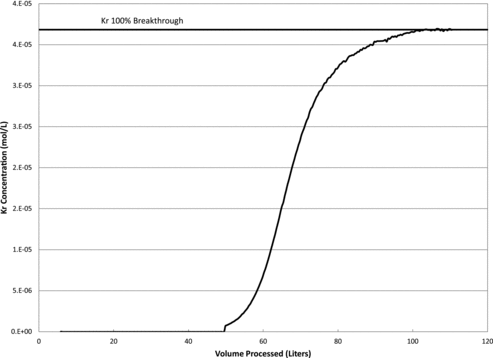
Breakthrough curves were generated by plotting the concentration of the constituent of interest (C) in the column effluent as a function of the volume (v) of gas processed. The collected breakthrough curves were used to calculate the Kr SC. The SCs were calculated from Equation (1):
(1) where V is the total volume processed at breakthrough (L), C0 is the concentration in feed (mmol/L), C is the concentration in effluent at v (mmol/L) and M is the sorbent mass (kilograms).
Because C0 and M are constant, Equation (1) can be rewritten as Equation (2):
(2) The statistical software TableCurve®, from Jandel Scientific, was used to find the best fit for equations representing the breakthrough curves. The software was used to define the area below the breakthrough curve to obtain the value for the last term in Equation (2). This result was then subtracted from the total area C0V to give the amount of Kr adsorbed on the material. The adsorbed amount was then divided by the sorbent bed mass to give a final SC in mmol Kr per kilogram of sorbent.
2.4. Kr adsorption isotherm
Kr adsorption isotherms were generated with the HZ-PAN at191 K, with feed concentrations ranging from 75 to 2544 μL/L Kr in helium. Kr adsorption capacities were calculated as previously discussed at each Kr concentration tested. The Kr feed concentration was varied by adding a second MFC to the system shown in and diluting the 2544 μL/L Kr feed gas using helium to the desired concentration.
The isotherms provide experimentally measured values for equilibrium parameters to be used as input data for modeling. It was determined that the Langmuir equilibrium isotherm was the best fit for the data-set. The Langmuir equilibrium model is written as
(3) where q is the SC at equilibrium with feed concentration (C), qmax is the predicted maximum capacity of the sorbent at the specified temperature and Keq is the Langmuir adsorption equilibrium constant. The values for qmax and Keq were determined by performing a Langmuir linear regression of the data obtained from the 191 K tests.
3. Results and discussion
3.1. Sorbent characterization
The BET surface area of the initial hydrogen mordenite powder was found to be 493 m2/g. The DFT N2 isotherm analysis of the powder resulted in a cumulative surface area of 649 m2/g and a pore volume of 0.175 cm3/g in the 0.4–1.0 nanometer (nm) pore size range. The BET surface area of the newly prepared engineered sorbent was found to be 336 m2/g. Taking into account the 80% weight loading of the HZ in PAN, 15% of the mordenite powder BET surface area was lost during the preparation of the sorbent beads. A cumulative surface area of 250 m2/g and a pore volume of 0.06 cm3/g in the pore size range of 0.4–1.0 nm were obtained utilizing DFT on the new sorbent. The porosity in the 0.4–1.0 nm range is important because the relative diameter of Kr is within this range increasing adsorption potential. SEM photos taken of the HZ-PAN material included in – show the uniformity of sorbent beads, the porosity of the interior of the beads and the composition of the interior.
The analysis of the HZ-PAN demonstrates the ability to incorporate a mordenite powder into a suitable engineered form utilizing a macroporous binder. A large portion of the powder's surface area and porosity were retained and a promising engineered form sorbent was developed.
3.2. HZ-PAN Kr capacities
Kr adsorption capacities were obtained in the same manner for all tests performed. The feed gas at the desired concentration was introduced into the cryostat column containing the activated HZ-PAN at the appropriate temperature. The column effluent was monitored over time via the GC until the Kr concentration exiting the column was equal to the Kr concentration entering the column. This condition indicates complete equilibrium with the sorbent and 100% breakthrough. An example of a Kr breakthrough curve utilized to calculate the sorbent's Kr capacity can be seen in .
Once breakthrough had occurred, the feed gas was stopped and helium was flowed through the cryostat and the temperature was increased to 100 °C for desorption. The column effluent was monitored until Kr was no longer detected. The cryostat was then cooled down to the desired temperature for the next test. This adsorption/desorption cycle was repeated for each test performed and took an average of 35 hours to complete for each test. A total of 24 adsorption/desorption tests were performed on this material in this manner.
Periodically, during the testing scheme, the sorbent's Kr capacity was re-evaluated with a 150 μL/L Kr feed gas at 191 K as a “baseline” to ensure that the sorbent crystalline structure withstood the thermal cycling from multiple adsorption/desorption processes. contains the number of thermal cycles previously performed, the calculated capacities of Kr for each baseline test and the percent of Kr breakthrough obtained at the end of the baseline test. It is clear from the data that the thermal cycling did not impact the sorbent's capacity during the testing scheme.
Table 1. Kr capacities of sorbent baseline tests, 150 μL/L Kr at 191 K.
In addition to the capacity measurements, the HZ-PAN was removed from the cryostat after testing. The sorbent visually appeared to be in the same condition as when it was first placed in the system and had retained its original mass. BET surface area analysis was performed again on the final sorbent resulting in a surface area of 341 m2/g. The DFT analysis resulted in a cumulative surface area of 250 m2/g and a pore volume of 0.06 cm3/g in the pore size range of 0.4–1.0 nm indicating no change when compared with the initial analysis. These results demonstrate that the HZ-PAN was not negatively affected by the thermal cycling over the course of testing.
3.3. Kr adsorption isotherms
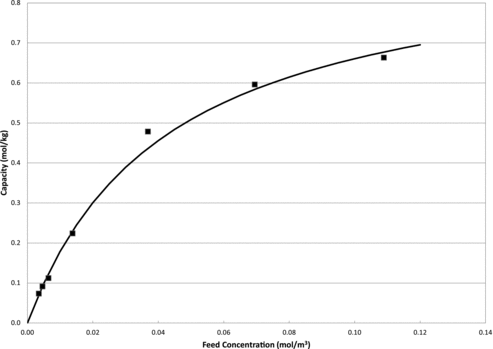
Kr capacities were determined with HZ-PAN at seven Kr concentrations ranging from 75 to 2544 μL/L. The Langmuir parameters Keq and qmax were determined from a linear regression plotting C/q vs. C, utilizing the experimental data where the slope is equal to 1/qmax and the intercept is equal to 1/(Keqqmax). From the regression, Keq was found to be 23.4 m3/mol and qmax was 0.94 mol/kg. These parameters were incorporated into the Langmuir adsorption equation and plot of the experimental data and the Langmuir prediction can be seen in .
It can be seen from the graph in that the Langmuir model predicts the adsorption data quite well. The Langmuir parameters can be utilized to develop models for the scale-up and process design calculations for implementation into a future fuel reprocessing off-gas treatment process.
4. Conclusions
A new engineered form sorbent has been developed that incorporates a mordenite powder into a macroporous polymer binder. The sorbent retained the characteristic surface area and microporosity of the initial powder. Initial Kr capacity evaluations indicate that a capacity of 100 mmol/kg sorbent can be achieved at 191 K, and this result compares reasonably well with the Munakata et al. [Citation10] reported value of approximately 35 mmol/kg for hydrogen mordenite at 195 K. Thermal cycling of the sorbent showed no detrimental effects over multiple TSA operations, demonstrating the sorbent's resiliency. An experimental Kr adsorption isotherm was obtained and compared to a Langmuir adsorption model with promising results. Future evaluations will be performed utilizing the HZ-PAN to determine the effects of varying temperature, concentration and competing species (e.g. N2, O2 and Xe). Additional sorbents will be prepared with varying cation mordenites and evaluated in the same manner as the HZ-PAN.
Acknowledgements
This work was supported by the US Department of Energy, Assistant Secretary for Nuclear Energy, under the Fuel Cycle Research and Development Off-gas Sigma Team; DOE Idaho Operations Office Contract DE-AC07-05ID14517.
This material is published by permission of the Idaho National Laboratory for the U.S. Department of Energy under Contract No. DE‐ACO7‐051D14517. The U.S. Government retains for itself, and others acting on its behalf, a paid‐up, non‐exclusive, and irrevocable worldwide license in said article to reproduce, prepare derivative works, distribute copies to the public, and perform publicly and display publicly, by or on behalf of the Government.
References
- Environmental Protection Agency. Protection of environment 2000: Chapter I, Environmental Protection Agency (Continued). Part 190_Environmental Radiation Protection Standards for Nuclear Power Operations (40CFR190.10). Washington, DC: US Environmental Protection Agency; 2010.
- Monson P. Krypton retention on solid adsorbents. Paper presented at: Proceedings of the 16th DOE Nuclear Air Cleaning Conference; 1980 Oct 20–23; San Diego, CA.
- Ianovski D, Munakata K, Kanjo S, Yokoyama Y, Koga A, Yamatusuki S, Tanaka K Fukumatsu T, Nishikawa M, Igarashi Y. Adsorption of noble gases on H-mordenite. J Nucl Sci Technol. 2002;39:1213–1218.
- Benedict M, Pigford TH, Levi HW. Nuclear chemical engineering. New York (NY): McGraw-Hill; 1981. Chapter 10, Fuel reprocessing; p. 481–484.
- Pence D. Critical review of noble gas treatment systems. Paper presented at: Proceedings of the 16th DOE Nuclear Air Cleaning Conference; 1980 Oct 20–23; San Diego, CA.
- Pence D, Chou C, Paplawsky W. An integrated off-gas treatment system design for nuclear fuel reprocessing plants using selective adsorption – Final Report SAI/00979-2. San Diego (CA) Science Applications, Inc.; 1979.
- Pence D, Paplawsky W. Noble gas separation from nuclear reactor effluents using selective adsorption with inorganic adsorbents. Paper presented at: Proceedings of the 16th DOE Nuclear Air Cleaning Conference; 1980 Oct 20–23; San Diego, CA.
- Ruthven D, Devgun J, Tezel F, Sridhar T. Removal of Kr from N2 by selective adsorption. Paper presented at: Proceedings of the 16th DOE Nuclear Air Cleaning Conference; 1980 Oct 20–23; San Diego, CA.
- Munakata K, Fukumatsu T, Yamatsuki S, Tanaka K, Nishikawa M. Adsorption equilibria of krypton, xenon, nitrogen and their mixtures on molecular sieve 5A and activated charcoal. J Nucl Sci Technol. 1999;36:818–829.
- Munakata K, Yamatsuki S, Tanaka K, Fukumatsu T. Screening test of adsorbents for recovery of krypton. J Nucl Sci Technol. 2000;37:84–89.
- Munakata K, Kanjo S, Yamatsuki S, Koga A, Ianovski D. Adsorption of noble gases on silver-mordenite. J Nucl Sci Technol. 2003;40:695–697.
- Garn T, Law J, Greenhalgh M, Tranter T, Battelle Energy Alliance, LLC. A composite media for fluid stream processing, a method of forming the composite media, and a related method of processing a fluid stream. United States patent application 20130116112. 2011 2011 Nov 8.