Abstract
During the process of core cooling at Fukushima Daiichi nuclear power plants accident, large amount of contaminated water was accumulated in the basements of the reactor buildings at Units 1–4. The present study estimated the quantities of I-131 and Cs-137 in the water as of late March based on the press-opened data. The estimated ratios of I-131 and Cs-137 quantities to the core inventories are 0.51%, 0.85% at Unit 1, 74%, 38% at Unit 2 and 26%, 18% at Unit 3, respectively. According to the Henry's law, certain fraction of iodine in water could be released to atmosphere due to gas–liquid partition and contribute to increase in the release to environment. A lot of evaluations for I-131 release have been performed so far by the MELCOR calculation or the SPEEDI reverse estimation. The SPEEDI reverse predicted significant release until 26 March, while no prediction in MELCOR after 17 March. The present study showed that iodine release from accumulated water may explain the release between 17 and 26 March. This strongly suggests a need for improvement of current MELCOR approach which treats the release only from containment breaks for several days after the core melt.
1. Introduction
One of the features unique to the Fukushima Daiichi NPP (nuclear power plants) accident in March 2011, hereafter Fukushima accident, was the accumulation of large amount of contaminated water in the basements of the R/B (reactor building), T/B (turbine building), RW/B (radioactive waste disposal building) and so on, as a result of tsunami invasion, precipitation and unknown leak of the core cooling water from somewhere of the CV (containment vessel) boundary [Citation1]. Schematic of the contaminated water accumulation in the basements of R/B, T/B and trench is shown in .
Figure 1. Schematic of contaminated water accumulation in the basements of R/B, T/B [Citation1] and release from accumulated water considered in present simplified model calculation.
![Figure 1. Schematic of contaminated water accumulation in the basements of R/B, T/B [Citation1] and release from accumulated water considered in present simplified model calculation.](/cms/asset/fe8d1de7-0439-43fd-9d10-b940212c2040/tnst_a_881725_f0001_oc.jpg)
Since the soluble iodine such as I2 have an equilibrium condition between gas and liquid phases according to the Henry's law in an infinite time for a closed system, their masses in each phase could reach to a state of equilibrium. On the other hand, the upper parts of the R/B, T/B and RW/B are not enclosed space completely, for example due to hydrogen burn at Units 1 and 3, and therefore gaseous iodine in the building could be released to the environment, while the decreased amount by release is continuously compensated by the gas–liquid partition.
This paper describes the estimated radioactive quantities of I-131 and Cs-137 in the accumulated water in the basements of R/B, T/B, RW/B and so on at Units 1–4 as of late March based on the press-opened data by NISA (Nuclear and Industrial Safety Agency). Using this estimation, the influence of iodine release from accumulated water due to gas–liquid partition on the release to environment, hereafter source terms, was studied for the late phase (from CV failure to 2 or 3 weeks after that) of the Fukushima accident. At the start of estimation, a simplified model on iodine chemistry was prepared and the application results were compared with the reverse estimation from atmospheric dispersion simulation with SPEEDI [Citation2] using the monitoring data [Citation3].
Regarding the iodine chemistry issues such as re-volatilization from the sump water inside CV under severe accident conditions, a lot of experimental and analytical studies have been globally performed so far [Citation4]. For the Fukushima accident, an analytical study focusing on the gaseous iodine release from the water pool inside S/C (suppression chamber) at Unit 2 was conducted with the Kiche (kinetics of iodine chemistry) code [Citation5] developed at JAEA (Japan Atomic Energy Agency) within the same analytical framework as previous studies. In the Kiche analysis, the iodine concentration of the water pool inside CV was reversely estimated from the quantities of the accumulated water and the cumulative amount of released gaseous I-131 was estimated to be about 3.0×1016 Bq on about 17 March [Citation6].
The previous studies including the Kiche analysis treated the iodine release only from contaminated water inside CV, while the present study tried to deal with the direct release from the contaminated water leaked outside of CV and accumulated in the basements of R/Bs of which volumes are quite larger and their radioactive concentrations are lower than those of the water pool inside CV.
In addition, the necessity of further modeling for current severe accident analysis codes was pointed out through the comparison of estimated source terms between severe accident analyses with MELCOR [Citation7] and the reverse estimation with SPEEDI.
2. Estimation of radionuclide quantities in accumulated water
2.1. Concentration, volume, and location of accumulated water
The radioactive concentrations of I-131 and Cs-137 in accumulated water and their volumes in the basements of Units 1–4 were measured and estimated, respectively, by Tokyo Electric Power Company (TEPCO) and the results (see ) were press opened from the previous NISA on 3 June 2011 [Citation8]. The locations of R/B, T/B, RW/B, trench at Units 1–4, central RW/B and the flow of accumulated water in the basements between the buildings considered in the present study are shown in .
Table 1. Estimated volumes and measured radioactive concentrations of accumulated water in the basements of R/B, T/B, RW/B, trench at Units 1–3 and central RW/B [Citation8].
Figure 2. Locations and flow of accumulated water in the basements of R/B, T/B, RW/B, trench and central RW/B considered in the present study [Citation8].
![Figure 2. Locations and flow of accumulated water in the basements of R/B, T/B, RW/B, trench and central RW/B considered in the present study [Citation8].](/cms/asset/c1a08e03-49ce-4862-849d-e753960edb50/tnst_a_881725_f0002_oc.jpg)
It was assumed that the contaminated water was not exchanged at the RW/B between Units 1 and 2, and the contaminated water of Unit 3 flowed into the basement of T/B at Unit 4 because no fuel assemblies were loaded at the core of Unit 4 as of 11 March and the integrity of spent fuel in the pool was confirmed later. Therefore, the radioactive quantities in the buildings at Unit 4 are treated, hereafter, as those at Unit 3. It is noted that the accumulated water in the basements at Units 2 and 3 was partially transferred to the main process and high-temperature incinerator buildings of the central RW/B, respectively, between mid-April and 31 May as one of the mitigation countermeasures. This transported amount was also considered in the present estimation of I-131 and Ce-137 quantities in the accumulated water.
2.2. Estimation methods
The core inventories of I-131, Cs-137 of Units 1–3 on 11 March [Citation9] calculated by ORIGEN2 [Citation10] are shown in . The radioactive concentrations in accumulated water on 11 March were reversely estimated from the measured ones considering the radioactive decay from 11 March to the sampling date. The total volume of accumulated water at each unit was calculated by summing the volumes of accumulated water at R/B, T/B, RW/B and trench. In Unit 2, the volume of accumulated water at central RW main process building that was transferred as countermeasure was further added to the volume of Unit 2 as described above, while the volume of accumulated water at central RW high-temperature incinerator building was further added in Unit 3.
Table 2. Inventories of I-131, Cs-137 at Units 1–3 of the Fukushima Daiichi nuclear power plants on 11 March 2011 calculated by ORIGEN2 [Citation9].
The total amount of radionuclides in accumulated water was estimated by multiplication of the radionuclides concentration by the volume assuming the uniform concentration in the accumulated water, and then the ratio of radionuclides quantities in accumulated water to the core inventories was calculated for each unit.
2.3. Results of radionuclide quantities in accumulated water
Estimated amount of I-131 and Cs-137 in the accumulated water at Units 1–3 are shown in –, respectively, together with their calculation processes. Estimated fractions of I-131 and Cs-137 quantities in the accumulated water to each core inventory in late March are summarized in .
Table 3. Estimated amount of I-131 in accumulated water at Unit 1.
Table 4. Estimated amount of I-131 in accumulated water at Unit 2.
Table 5. Estimated amount of I-131 in accumulated water at Unit 3.
Table 6. Estimated amount of Cs-137 in accumulated water at Unit 1.
Table 7. Estimated amount of Cs-137 in accumulated water at Unit 2.
Table 8. Estimated amount of Cs-137 in accumulated water at Unit 3.
Table 9. Estimated fractions of I-131 and Cs-137 in accumulated water to each core inventory in late March.
shows that the fraction of I-131 becomes roughly twice compared with that of Cs-137 at Units 2 and 3, while the opposite trend was found at Unit 1. The fractions of I-131 and Cs-137 in the accumulated water at Units 2 and 3 became higher by about two orders of magnitude than that of Unit 1. This indicates that in case of Units 2 and 3, the cooling water which directly contacted the degraded fuels in reactor pressure vessel flowed into the basements of the R/Bs, while most of such contaminated water did not in Unit 1.
Possible reason for higher values at Units 2 and 3 is that the thermal-induced break could have occurred somewhere of the RCIC (reactor core isolation cooling system) or HPCI (high-pressure core injection system) line which is operated by the turbine driven by main steam from the reactor core. Since the obvious decrease in the reactor pressure was not found in all the units from earthquake to the tsunami arrival on 11 March, the likelihood of RCIC/HPCI lines failure due to earthquake is considered to be excluded. The leak from somewhere of the RCIC/HPCI lines due to superheated steam could be more probable because these lines are not designed for severe accident conditions. However, it is impossible to identify the leak paths because of no evidences at present.
The highest fractions of I-131 and Cs-137 were estimated at Unit 2. There is a possibility that the S/C room and the S/C boundary partially failed. In Unit 3, almost half amount of the two radionuclides was transferred to R/B, T/B and RW/B compared with that of Unit 2. It is reported that the dose rate inside S/C at Unit 3 was kept high [Citation1]. In Unit 1, small amount of radionuclides was transferred to R/B, T/B, RW/B and trench, and the boundaries between CV and the related buildings were considered to be mostly kept intact during the accident.
2.4. Comparison with previous study
Similar study has been already published [Citation11] and showed almost the same tendencies in radioactive quantities in the accumulated water as the present study. However, small differences in the ratios of I-131 and Cs-137 quantities in the accumulated water to each core inventory were found between the previous and present studies. For example, the ratio of I-131 quantities in accumulated water at Unit 2 in the previous study is 52%, while that of the present study is 74%. The reason for differences was that the two studies used almost the same concentration data but the previous study used the concentration data at central RW/PB (radioactive waste treatment building/main process building) measured directly by themselves on 29 April. On the other hand, the present study used the same concentration data between T/B at Unit 2 and central RW/PB measured by TEPCO on 27 March because the water at central RW/PB was delivered from T/B at Unit 2 after mid-April.
In addition, to be exact, the volumes of accumulated water measured on the same date as the concentration measurement had to be used for this estimation but both of the previous and present studies used the volumes measured on 31 May. This is because it was difficult to estimate the contributions of precipitation and tsunami, and it is also considered that the water supply to the core and steam generation by the decay heat may have been mostly balanced during the late phase of Fukushima accident. Taking into account the uncertainties of accumulated water volumes and concentration measurement, the present estimation may include several tens of percent error.
3. Influence of iodine in accumulated water on source term
It turned out from that the quantities of I-131 at Units 2 and 3 cannot be ignored compared with their core inventories. Since it is considered that the iodine concentration of the gas phase above the water could increase based on the Henry's law even if the ambient conditions are different from those inside CV on which the previous studies focused, the effects of iodine release from the accumulated water on the total release to environment from the Fukushima Daiichi NPP accident were investigated in this chapter.
3.1. Discrepancy of iodine source terms between current severe accident analyses and reverse estimation from atmospheric dispersion simulation using monitoring data
A lot of analyses on the source terms have been tried mainly using the two methods so far. One is the reverse estimation using the atmospheric dispersion simulation code such as SPEEDI [Citation2] combined with the environmental monitoring data [Citation3]. The other is the estimation from thermo-hydraulic and radionuclides release and transport analyses [Citation12–14] within the reactor coolant system and CV using the severe accident analysis codes such as MELCOR [Citation7], MAAP [Citation15], THALES-2 [Citation16] and so on.
The comparison of I-131 release rate and its cumulative release between MELCOR analysis [Citation12] and reverse estimation from SPEEDI [Citation3], hereafter the SPEEDI reverse, are shown in and , respectively. Comparing the results between the two methods before 17 March, no large difference was found in the trend of I-131 release rate but the cumulative amount of I-131 release predicted by MELCOR became larger by about one order of magnitude compared with the SPEEDI reverse. Since the source terms of the SPEEDI reverse were estimated taking into account the release toward all the wind directions, that is, estimated from the concentration of radioactive materials deposited onto land and sea surface, the results of the SPEEDI reverse are directly comparable with the MELCOR calculation. Therefore, almost single-digit difference is considered to be too large even if various uncertainties in monitoring and the SPEEDI reverse calculation were taken into account. In addition, if the iodine release from accumulated water described after Section 3.2 was treated in MELCOR in future, calculated source term before 17 March could increase further. Thus, the current MELCOR calculation may overestimate the source term.
Figure 3. Comparison of I-131 release rate between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].
![Figure 3. Comparison of I-131 release rate between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].](/cms/asset/30d9f2d6-ba77-4444-b2f1-5c372b361884/tnst_a_881725_f0003_b.gif)
Figure 4. Comparison of cumulative I-131 release between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].
![Figure 4. Comparison of cumulative I-131 release between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].](/cms/asset/48434ac3-80bd-4ab4-a20b-f90abdc5f29d/tnst_a_881725_f0004_b.gif)
On the other hand, regarding the release after 17 March, the SPEEDI reverse predicted continuous release more than 1 month, while no prediction in MELCOR. The SPEEDI reverse also showed that about 40% of total amount of I-131 source term of 1.2×1017 Bq was released before 17 March and most of the rest was released by about 26 March when the fresh water was injected into the core.
The current MELCOR treats the release to environment only from dry well or S/C and does not model the release from accumulated water of R/B, T/B or RW/B although the code considers the gas–liquid partition at S/C. The reason for termination of the MELCOR calculation after 17 March is due to almost no more release prediction after that because of decrease in the core temperatures and lack of modeling for release from accumulated water in addition to enormous computation time.
It is guessed that the release between 17 and 26 March predicted by the SPEEDI reverse can be roughly explained by the release from accumulated water. To support this hypothesis, related iodine chemistry models are described in Sections 3.2 and 3.3. In addition, since the release of radioactive iodine from accumulated water could become a large uncertainty for the consequence of Fukushima accident, preparation of simplified iodine chemistry model and comparison of the application results with the SPEEDI reverse are written in Sections 3.4 and 3.5 or later, respectively.
3.2. Gas–liquid partition of iodine
Since the soluble radionuclides such as I2 have an equilibrium condition between gas and liquid phases according to the Henry's law, in an infinite time for a closed system, their masses in each phase could reach to a state of equilibrium. Most of iodine dissolved into water becomes I− and some of I− species could be changed to I2. The mass transfer equation for I2 from water to atmosphere in MELCOR is written as [Citation7]
(1) where [I2 atm] is the concentration of I2 in atmosphere (kmol/m3), [I2 water] is the concentration of I2 in water (kmol/m3), Awater is the water–atmosphere surface area (m2), kwater is the mass transfer coefficient from water surface to atmosphere (m/s),
is the gas–liquid partition coefficient for I2 (–) defined by [I2 water]/[I2 atm] under an equilibrium condition, and Vatm is the volume of atmosphere (m3).
The transfer rate of I2 from water to atmosphere is proportional to the contact area between water and atmosphere. It is considered that the contact surface area is mostly equal to the effective floor area (see ) of each building. Taking into account that the total surface area of the accumulated water in the basements is very large and that the upper parts of the R/B, T/B and RW/B are not enclosed space completely, iodine release from the accumulated water is expected to play an important role in the source term evaluation during the late phase of Fukushima accident.
Table 10. Effective floor area of each building [Citation8].
3.3. Radiolytic conversion of I− to I2 and its dependency on pH
The release of I2 to the gas phase above the contaminated water could depend mainly on the pH levels in the water, the dose rate and so on [Citation5,Citation17]. The representative dependency, that is, the fitting curve of radiolytic conversion of I− to I2 on pH prepared by Oak Ridge National Laboratory is shown in , where the conversion ratio of I− to I2 in accumulated water, is expressed by Equation (2) as a function of pH
(2)
Figure 5. Dependency of radiolytic conversion of I− to I2 on pH [Citation17] (data from [Citation25]).
![Figure 5. Dependency of radiolytic conversion of I− to I2 on pH [Citation17] (data from [Citation25]).](/cms/asset/855cbb39-8bf8-46fd-9772-1b8017ce64cd/tnst_a_881725_f0005_oc.jpg)
It is noted that the test solution had been irradiated at ambient temperature for 1 hour at 45 kGy/hour. In the case of pH below 7, I2 concentration could increase due to the radiolytic conversion of I− to I2. It is reported that the pH level of water in CV has a tendency to fall below 7 during the late phase of severe accidents because of the formations of nitric acid due to radiolysis in atmosphere and/or hydrochloric acid due to radiolysis of plastic wire insulation.
Previous study showed that the following two reactions as shown in Equations (3) and (4) are strongly related to this radiolytic conversion although the phenomena are very complex and cannot be explained only by these reactions,
(3)
(4) where h is the Planck constant (J s), ν is the frequency of γ ray (1/s), and e− is the electron.
When the I2 concentration in water increases gradually by radiolysis as shown in Equation (3), both of H+ and I2 concentrations could increase so that the equilibrium of Equation (4) might be maintained. On the other hand, if pH increases (H+ concentration decreases) abruptly by injection of pH buffering products, the equilibrium of Equation (3) is shifted to the right hand to compensate for the decrease in H+ concentration. As a result, I2 concentration in water could also decrease.
In the Fukushima accident, the core cooling water was changed from sea water to fresh water at 15:37 on 25 March at Unit 1, at 10:10 on 26 March at Unit 2 and at 18:02 on 25 March at Unit 3, respectively. The government's report described that the boric acid solution was added to the fresh water only at Unit 2 to prevent the re-criticality [Citation1] while no description on its chemical form. Our inquiry to TEPCO clarified that the pure boric acid (B(OH)3) was added on 26 March instead of the sodium pentaborate (Na2B10O16·10H2O) which is usually used in the standby liquid control system (SLCS) of Japanese BWR.
If the sodium pentaborate was injected, there was a possibility of pH neutralization in accumulated water on 26 March due to addition of strongly basic sodium. However, the fact of pure boric acid injection denied the possibility of pH change and suggested that the pH of accumulated water had been kept at the same level before and after 26 March. shows the pH and NaCl concentration in accumulated water at T/B of Unit 2 measured on 27 March [Citation18]. From this table, it can be said that pH of accumulated water had been maintained at 7.1.
Table 11. Measured pH and NaCl concentration in accumulated water at T/B of Unit 2 [Citation18].
3.4. Preparation of simplified model on I2 release from accumulated water
A simplified I2 release model from the accumulated water was prepared based on experiences regarding the experimental analysis on iodine chemistry [Citation19]. Schematic of considered phenomena and release paths of the simplified model is shown in .
The major analytical assumptions which were determined based on the conditions of safety analysis for Japanese construction permit [Citation20] are described in .
Table 12. Major assumptions of simplified model for iodine release from accumulated water.
The instant equilibrium between gas and liquid phases, that is, infinite mass transfer coefficient and/or infinite water–atmosphere surface area in Equation (1) was assumed. Any chemical reactions and organic iodide formation were not considered. The deposition of I2 onto inner surface of buildings was considered but no re-vaporization or resuspension from the wall surface was assumed for simplification. The treated chemical species in water were I2, I−, and only I2 in atmosphere.
The release rate of I-131 from Unit j to the environment at elapsed time Ti (day) from shutdown, Rj,Ti (Bq/hour) and the cumulative release from Units 1–3, total release (Bq) are given as follows:
(5)
(6) where Bj,Ti is the ventilation rate of related buildings (1/day),
is the conversion ratio of I− to I2 in accumulated water (–), D is the fraction of deposition onto inner wall of related buildings (–), Si,j is the amount of I-131 in accumulated water at Unit j at elapsed time Ti (Bq) and the initial amount, S0,j (Bq) at accident initiation, T0 = 0 (day) was assumed to be equal to 6.9 × 1015 at Unit 1, 1.7 × 1018 at Unit 2, 6.1 × 1017 at Unit 3, respectively, Vatm,j is the volume of atmosphere at Unit j (m3), and Vwater,j is the volume of accumulated water at Unit j (m3).
The release from accumulated water was assumed to start from 17 March. The release from trench was ignored because of small surface area. For simplification, it was also assumed for all the units that during the whole calculation has the constant value equal to 0.0022 which is calculated by substituting pH = 7.1 into Equation (2). The ventilation rates of related buildings, Bj,Ti were calculated by Equation (7) considering steam generation rate on the assumption that all the decay heat at each unit is transferred to the accumulated water at 323 K,
(7)
where Bd is the design value of ventilation rate for related buildings = 50%/day, Cp is the specific heat of water = 4186 (J/(kg K)), Hj,Ti is the decay heat at Unit j at elapsed time Ti (W) from shutdown, Mw is the molecular weight of water = 0.018 (kg/mol), and λv is the heat of vaporization for water = 40,800 (J/mol).
For example, the ventilation rates of related buildings of Unit 2 on 17 March and 26 March were equal to approximately 680%/day and 500%/day, respectively. Comparison of the mass transfer coefficients of iodine from accumulated water to atmosphere between previous studies [Citation21] and conditions described above showed that the assumption of infinite mass transfer coefficient in the present simplified model is still valid for this relatively large ventilation rate because of large surface area of accumulated water as shown in .
It is noted that all the values used in the present simplified model were preliminary determined and need to be carefully validated for more detailed evaluation.
3.5. Comparison of simplified model calculation with SPEEDI reverse estimation
The comparison of I-131 release rate between the simplified model calculation, hereafter the simplified model, and the SPEEDI reverse is shown in . The results of the simplified model were obtained by summing the release rates from all the accumulated water at Units 1–4. Based on Equation (4), the contribution of Unit j to the total release is roughly proportional to the initial amount of I-131 in accumulated water, S0,j as described in Section 3.4 although it depends on the volumes of atmosphere and accumulated water.
Figure 6. Comparison of I-131 release rate between simplified release model and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].
![Figure 6. Comparison of I-131 release rate between simplified release model and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].](/cms/asset/d0330286-ae0f-4dc3-a26a-fce3adb90031/tnst_a_881725_f0006_oc.jpg)
The SPEEDI reverse showed a decreasing trend in release rate as a whole and abrupt decrease on 26 March when the core cooling was changed from seawater to freshwater. Significant peaks were found on around 22–24 March and 30 March which may be caused by remelting of the cores due to shortage of core cooling water supply [Citation22].
The simplified model predicted well the tendency of I-131 release rate before and after 26 March by considering reduction of ventilation rate of related buildings due to decrease in steam generation rate commensurate with the decay heat and radioactive decay of I-131.
Abrupt decrease on 26 March was reproduced assuming that I2 concentration at near surface of accumulated water after fresh water injection became 20th (1/20) of that before injection due to stratification of the accumulated sea water caused by difference in the specific gravity between freshwater and seawater. The decrease in I2 concentration was simulated by expediently changing the partition coefficient from 100 to 2000. The value of 20th was tentatively adopted so that the simplified model might agree with results of the SPEEDI reverse. Preliminary study on the possibilities of decrease in I2 concentration at near surface of accumulated water at time of freshwater injection is described in Section 3.6.
Although the simplified model did not predict the significant peaks on around 23 March and 30 March, it can be said that the simplified model reproduced well the overall release baseline predicted by the SPEEDI reverse during the late phase after 17 March. This supports that the release between 17 and 26 March was dominated by the release from accumulated water.
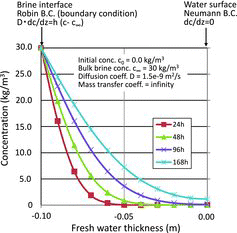
3.6. Study on stratification of accumulated water at time of freshwater injection
To obtain the prospects for decreased I2 concentration caused by stratification of freshwater and seawater at near surface of the accumulated water, a preliminary calculation was performed within use of an analytical solution method. In the calculation, the Fick's second law (one-dimensional unsteady state diffusion equation) for infinite plane as shown in Equation (8) was applied under the condition of no convection flow, and upper freshwater and lower seawater considering the difference in specific gravity between the two waters,
(8)
where C is the concentration of NaCl in water (kg/m3), D is the diffusion coefficient of NaCl in water (m2/s), and z is the vertical distance (z = 0 at upper surface of freshwater) (m).
The initial and boundary conditions (BC) are as follows:
c0 = 0 at t = 0, initiation of NaCl diffusion,
at z = 0, upper surface of fresh water (Neumann BC),
at z = −0.1, interface between freshwater and seawater (Robin BC) where h is the mass transfer coefficient (kg/s) and c∞ is the NaCl concentration in seawater.
Since the concentration of I2 in accumulated water at T/B of Unit 2 was smaller by about seven orders of magnitude than that of NaCl, the present study looked at the NaCl diffusion. It is noted that the diffusion coefficient of I2 in water is smaller by about 20% than that of NaCl and therefore the behavior of NaCl and I2 in water could be almost the same.
The upward diffusion of NaCl from underlying seawater to freshwater (0.1 m in depth) located on top of the seawater was estimated by solving Equation (8) using the infinite series. The depth of 0.1 m was determined from the injection rate of freshwater per day and effective floor area of the buildings. The mass transfer coefficient between freshwater and seawater is assumed to be infinite while that between atmosphere and freshwater is 0 for simplification.
Calculated NaCl concentrations to a depth of 0.1 m are shown in . The calculation showed that it takes several tens of hours for NaCl to be diffused sufficiently into upper lying freshwater. This suggested the strong possibility of stratification at near surface of accumulated water after injection of freshwater although further detailed simulations are needed considering the more realistic conditions.
shows the Na and Cl concentrations in accumulated water at T/B of Unit 2 measured on 27 March, next day of fresh water injection. Measured Cl concentration is 14,000 mg/L which is smaller by 5000 mg/L than that of seawater. This corresponds to the NaCl concentration of 14.0 kg/m3×(22.99 + 35.45)/35.45 = 23.1 kg/m3 in , where 22.99 and 35.45 are the atomic weights of Na and Cl, respectively. If the complete mixing between freshwater and seawater was assumed, the NaCl concentration on 27 March could become mostly equal to or slightly larger than that of seawater (30 kg/m3). This is because it is considered that most of the injected freshwater of 627 m3 between 26 and 27 March was vaporized by the decay heat and the accumulated seawater before 26 March was gradually concentrated due to vaporization and injection although the contribution of precipitation is unknown.
Our inquiry to TEPCO revealed that the sample water was taken by throwing a bucket and scooping near surface of accumulated water, but at that time, they did not pay attention to know how deep the sample was scooped from the surface of accumulated water because of limited sampling time under high irradiation condition. However, this data supports the possibility of stratification at near surface of accumulated water.
3.7. Comparison of cumulative release between I-131 and Cs-137
The comparison of cumulative Cs-137 release between severe accident analyses and reverse estimation from atmospheric dispersion simulation is shown in . There is an analogy in the cumulative releases between I-131 and Cs-137 before 17 March, while a difference was found in the gradient from 17 March to 26 March between them. The cumulative release of I-131 became 2.4 times for 9 days between 17 March and 26 March, while that of Cs-137 became 1.5 times.
Figure 8. Comparison of cumulative Cs-137 release between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].
![Figure 8. Comparison of cumulative Cs-137 release between severe accident analyses and reverse estimation from atmospheric dispersion simulation [Citation3,Citation12].](/cms/asset/de935cbe-dd8a-497f-85b4-316d67e98de9/tnst_a_881725_f0008_b.gif)
This discrepancy might be explained by the difference in contribution of radiolytic conversion and gas–liquid partition between iodine and cesium. It is considered that the increase in cesium release during that time was mainly due to the remelt of fuels on 22–24 March and 30 March, or re-vaporization/resuspension of once-deposited cesium onto the wall, and small quantities of cesium was released by the gas–liquid partition. On the other hand, if the same ratio of I-131 is assumed to be released as the chemical form of CsI in analogy with Cs-137 of which chemical forms are CsI and CsOH, except for the radiolytic conversion and gas–liquid partition, 2.4 − 1.5 = 0.9 times of the I-131 cumulative release as of 17 March (= 40% of total source term) might have been released between 17 March and 26 March due to the gas–liquid partition. If this estimation is correct, the release from the accumulated water may account for about 35% (= 40% × 0.9) of the total I-131 source term of 1.2×1017 Bq.
3.8. Future issues
The previous studies on iodine chemistry focused on the sump water inside CV, while no investigation has been performed for large scale such as R/Bs under conditions of low radioactive concentration and low dose rate. The applicability of existing models to the contaminated water outside of CV and the influence on the late-phase source terms should be carefully investigated in future together with the effect of stratification of freshwater and seawater in accumulated water on the iodine release at the time of freshwater injection.
For more detailed source-term evaluation during the late phase, the effect of re-vaporization or resuspension of radionuclides once deposited on the complicated and huge surface of the facilities should be taken into account. The increase in formation of gaseous iodine [Citation23] such as methyl-iodide due to reaction with organics released from paints or impurities of seawater, existence of B4C control rods in the core [Citation24] should also be investigated in detail in future.
The modification of severe accident analysis codes for consideration of the radionuclides transfer between gas and liquid phases in the basements of R/B, T/B and RW/B is expected to decrease the discrepancy of source terms between the current severe accident analyses with the MELCOR and the SPEEDI reverse estimation, and to contribute also to improvement of the interpretation as to what has taken place in the reactor or CV during early phase of the Fukushima accident.
4. Conclusions
A large volume of contaminated water was accumulated in the basements of R/B, T/B and RW/B of the Fukushima Daiichi Units 1–4. The present study estimated the quantities of I-131 and Cs-137 in the water as of late March based on the volume and radioactive concentration data press opened by NISA. The estimated ratios of I-131 and Cs-137 quantities solved in accumulated water to the core inventories were equal to 0.51%, 0.85% at Unit 1, 74%, 38% at Unit 2 and 26%, 18% at Unit 3, respectively.
Since it is considered that the iodine concentration of the gas phase above the water could increase based on the Henry's law, the effects of iodine release from the accumulated water on the total release to environment were investigated. It has been pointed out that there is a difference in I-131 source-term evaluation after 17 March between severe accident analyses with MELCOR and the reverse estimation from atmospheric dispersion simulation with SPEEDI using the monitoring data.
The present study using a simplified iodine chemistry model showed that this discrepancy could be explained by lack of the release model from accumulated water due to the radiolytic conversion of I− to I2 and the gas–liquid partition based on the Henry's law in MELCOR although the simplified model was preliminary prepared and needs to be carefully validated for more detailed evaluation.
The present study also showed that I-131 release from accumulated water may account for about 35% of the total amount of source term because relatively large ventilation rate of related buildings continued for a few weeks due to large steam generation from accumulated water commensurate with the decay heat despite of small radiolytic conversion ratio from I− to I2 in the water with pH = 7.1. The abrupt decrease in I-131 release rate on 26 March predicted by the SPEEDI reverse may be explained by decrease in I2 concentration of surface water due to stratification of freshwater and seawater at near surface of accumulated water after freshwater injection.
These findings obtained through the present study strongly suggests a need for modeling of iodine release from accumulated water and improvement of current approach for source-term analysis with MELCOR and so on which treats the release only from breaks of dry well or S/C for several days after the core melt. The present study also indicates that the release of radioactive iodine from accumulated water in the basements of R/Bs could become a great concern for the consequence of Fukushima accident.
Acknowledgements
The authors wish to thank Prof. Koji Okamoto of University of Tokyo and Prof. Jun Sugimoto of Kyoto University for their helpful comments to prepare this paper. Special thanks are also due to Mr Harutaka Hoshi of Japan Nuclear Energy Safety Organization (JNES) and Dr Haruyasu Nagai of JAEA for giving the digital results of the MELCOR calculation and the SPEEDI reverse estimation for the Fukushima accident. Dr Shinya Mizokami of TEPCO was very supportive for our confirming the facts of event chronology relating to accumulated water. Authors also would like to thank Dr Tetsuji Yamaguchi of JAEA for his technical comments on possibility of pH change in accumulated water by borate injection and Dr Yasuteru Shibamoto of JAEA for his useful prospects on stratification of freshwater and seawaters in accumulated water.
References
- Nuclear Emergency Response Headquarters. Report of the Japanese government to the IAEA ministerial conference on nuclear safety – the accident at TEPCO's Fukushima Nuclear Power Stations. Tokyo: Government of Japan; 2011. Available from: http://www.kantei. go.jp/foreign/kan/topics/201106/iaea_houkokusho_e.html
- Ministry of Education, Culture, Sports, Science and Technology. SPEEDI system for prediction of environmental emergency dose information. Pamphlet of SPEEDI; 2007.
- Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H. Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. J Nucl Sci Technol. 2011;48(7):1129–1134.
- Working Group on Analysis and Management of Accidents. State of the art report on iodine chemistry ( NEA/CSNI/R(2007)1). Issy-les-Moulineaux (France): Organization for Economic Co-operation and Development; 2007. Available from: http://search.oecd. org/officialdocuments/displaydocumentpdf/?cote=NEA /CSNI/R(2007)1&docLanguage=En
- Moriyama K, Maruyama Y, Nakamura H. Kiche: a simulation tool for kinetics of iodine chemistry in the containment of light water reactors under severe accident conditions ( JAEA-Data /Code 2010-034). Ibaraki-ken: Japan Atomic Energy Agency; 2011.
- Moriyama K, Maruyama Y, Nakamura H. Evaluation of gaseous iodine release during the Fukushima Daiichi Accident. In: L11: Transaction of 2011 Fall Meeting of AESJ; Kita-Kyusyu, Japan. Tokyo: Atomic Energy Society of Japan; 2011. Japanese.
- Gauntt RO, Cash JE, Cole RK, Erickson CM, Humphries LL, Rodriguez SB, Young MF. MELCOR computer code manuals, volume 1: primer and user's guide, version 1.8.6 ( NUREG/CR-6119: Vol. 1: Rev. 3). Albuquerque (NM): Sandia National Laboratory; 2005.
- Nuclear and Industrial Safety Agency [Internet]. News release on 2011 Jun 3. Available from: http://www. meti.go.jppress2011062011060300220110603002.pdf. Japanese.
- Nishihara K, Iwamoto H, Suyama K. Estimation of fuel compositions in Fukushima-Daiichi nuclear power plant ( JAEA-Data/Code 2012-018). Tokai: Japan Atomic Energy Agency; 2012. Japanese.
- Croff AG. ORIGEN2: a versatile computer code for calculating the nuclide composition and characteristics of nuclear materials. Nucl Technol. 1983;62(3):335–352.
- Nishihara K, Yamagishi I, Yasuda K, Ishimori K, Tanaka K, Kuno T, Inada S, Gotoh Y. Radionuclide release to stagnant water in Fukushima-1 nuclear power plant. Trans At Energy Soc Jpn. 2012;11(1):13–19. Japanese.
- Hoshi H, Ogino M, Kawabe R, Fukasawa M. Computational analysis on accident progression of Fukushima Daiichi NPS. In: Proc. Tokyo PSAM2013-1061; Tokyo, Japan. Tokyo: Atomic Energy Society of Japan; 2013.
- Mizokami S. Detailed analysis of the accident progression of units 1 to 3 by using MAAP code. In: Technical Workshop on the Accident of TEPCO's Fukushima Daiichi NPS; 23–24 July; Tokyo, Japan. Tokyo: Nuclear and Industrial Safety Agency; 2012.
- Ishikawa J. Analysis for accident progression with THALES2 code. Technical Workshop on Fukushima Daiichi NPP Accident: Nuclear Regulation Authority; 23–24 July; Tokyo, Japan. Tokyo: Nuclear and Industrial Safety Agency; 2012.
- Electric Power Research Institute (EPRI). MAAP4 application guidance, desktop reference for using MAAP4 software, revision 2. Palo Alto (CA): EPRI; 2010.
- Kajimoto M, Muramatsu K., Watanabe N. Development of THALES-2: a computer code for coupled thermal-hydraulics and radionuclides transport analyses for severe accident at LWRs and its application to analysis of radionuclides revaporization phenomena. In: Proceedings of the International Topical Meeting on Safety of Thermal Reactors; Portland, OR. La Grange Park (IL): American Nuclear Society; 1991.
- Beahm EC, Weber CF, Kress TS, Parker GW. Iodine chemical forms in LWR severe accidents ( NUREG/CR-5732). Oak Ridge (TN): Oak Ridge National Laboratory; 1992.
- Yamagishi I. Issues on treatment of accident-generated water at Fukushima Daiichi Power Station. Paper presented at: 2011 Fall Meeting of AESJ; 2011; Kita-Kyusyu, Japan. Available from: http://www.aesj. or.jp/11fall-symp/presentations/20110919yamagishi.pdf. Japanese.
- Hidaka A. Experimental analyses of iodine behavior under severe accident conditions with ART. J Nucl Mater. 1997;248:226–232.
- Nuclear Safety Commission. Regulatory guide for reviewing safety assessment of light water reactor facilities. Latest issue: 2001 Mar, first issue: 1990 Aug. Available from: http://www.nsr.go.jp/archive/ nsc/NSCenglish/guides/lwr/L-SE-I_0.pdf].
- Ball J, Marchand C. AECL–IRSN International Standard Problem (ISP) no. 41 follow up exercise: phase 2, iodine code comparison exercise against CAIMAN and RTF experiments ( NEA/CSNI/R(2004)16). Issy-les-Moulineaux (France): Organization for Economic Co-operation and Development; 2004. Available from: http://www.oecd-nea.org/nsd/docs/2004/csni-r2004-16. pdf
- Tanabe F. Analyses of core melt and re-melt in the Fukushima Daiichi nuclear reactors. J Nucl Sci Technol. 2012;49(1):18–36.
- Moriyama K, Tashiro S, Chiba N, Maruyama Y, Nakamura H, Watanabe A. Experiment on the gaseous iodine release from irradiated cesium iodide solutions (contact research) ( JAEA-Research 2011–2016). Ibaraki-ken: Japan Atomic Energy Agency; 2011.
- Haste T, Payot F, Dominguez C, March Ph, Simondi-Teisseire B, Steinbrück M. Study of boron behavior in the primary circuit of water reactors under severe accident conditions: a comparison of Phebus FPT3 results with other recent integral and separate-effects data. Nucl Eng Des. 2012;246:147–156.
- Lin CC. Chemical effects of gamma radiation on iodine in aqueous solutions. J. Inorg Nucl Chem. 1980;42:1101–1107.