?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel liquid–liquid extraction system was investigated for the selective separation of lithium isotopes using ionic liquids (ILs = C8mim+PF6−, C8mim+BF4−, and C8mim+NTf2−) as extraction solvent and 2,2′-binaphthyldiyl-17-crown-5 (BN-17-5) as extractant. The effects of the concentration of lithium salt, counter anion of lithium salt, initial pH of aqueous phase, extraction temperature, and time on the lithium isotopes separation were discussed. Under optimized conditions, the maximum single-stage separation factor α of 6Li/7Li obtained in the present study was 1.046 ± 0.002, indicating the lighter isotope 6Li was enriched in IL phase while the heavier isotope 7Li was concentrated in the solution phase. The formation of 1:1 complex Li(BN-17-5)+ in the IL phase was determined on the basis of slope analysis method. The large value of the free energy change (−ΔG° = 92.89 J mol−1) indicated the high separation capability of the Li isotopes by BN-17-5/IL system. Lithium in Li(BN-17-5)+ complex was stripped by 1 mol L−1 HCl solution. The extraction system offers high efficiency, simplicity, and green application prospect to lithium isotope separation.
1. Introduction
Naturally occurring lithium consists of 6Li and 7Li, two stable isotopes. 6Li can be cracked into tritium (T) and helium (He) by neutron bombarding, thus 6Li is the important material for nuclear fusion reactor to rapidly absorb neutron and proliferate T. 7Li can be used as coolants and ideal heat transfer agent of fusion power reactor and neutral medium of thorium-based fused salt reactor. Therefore, lithium isotopes will play increasingly important role in new energy industry in the future. However, separating lithium isotopes was extremely difficult as 6Li and 7Li only have tiny mass differences with the same extra nuclear structure.
The only method that was currently utilized to large-scale lithium isotope separation is the amalgam method [Citation1]. It offered a large and compelling single-stage separation factor (αmax = 1.054 ± 0.002), but the use of toxic mercury brought serious biological and environmental problems. In order to avoid these hazards, many new techniques were investigated to separate lithium isotope, including laser [Citation2], electromigration [Citation3], chromatography [Citation4], and membrane separation [Citation5]. Since the solvent extraction has the advantages of high selectivity, high recovery, high capacity, simple equipment, and easy automatic control, liquid–liquid extraction systems of lithium isotopes separation were reported in literatures [Citation6]. However, as Li+ has large free energy of hydration, lithium isotope separation effect of the ordinary organic extractant system was very feeble [Citation7]. In addition, high toxicity caused by the use of volatile organic solvents in liquid–liquid extraction process limits its application to large-scale lithium isotope separation. Therefore, there is an urgent need to develop efficient and green liquid–liquid extraction method for lithium isotope separation.
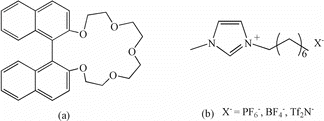
Crown ethers and cryptands, with high affinity and size selectivity for lithium ion, have been investigated with large separation factors of 6Li/7Li in solvent extraction processes [Citation8,9]. For instance, Li isotopes have been separated successively by liquid–liquid extraction using cryptand(2, 2, 1) [Citation10] and benzo-15-crown-5 [Citation11] with αmax of 1.041 and 1.035, respectively. However, they were not suitable for the isotope separation in the industrial scale because of the large water solubility of cryptand (2, 2, 1) and the relatively small separation factor of benzo-15-crown-5. To overcome the above problems, a new kind of macrocyclic extractant, 2,2′-binaphthyldiyl-17-crown-5 (BN-17-5) ((a)) was designed in this paper. Due to the introduction of naphthalene ring, the rigidity of the extractant greatly increased, therefore the water solubility significantly reduced. Besides, the rigid cavity of BN-17-5 matches well with Li ion and achieves elaborate per-organization before coordination with lithium ion. Consequently, BN-17-5 will show strong bonding ability, wonderful selectivity, and relatively large separation factor when coordinated with lithium ion.
Room-temperature ionic liquids (ILs), as a new class of burgeoning green solvents, have attracted considerable interest in many fields of chemistry and industry [Citation12,13]. Their non-inflammability and non-volatility provided advantages for using them as a replacement to volatile organic solvent in solvent extraction process. Imidazolium-based ILs ((b)), one of the commonly used ILs, were considered to be a prospective solvent for metal ions separarion [Citation14,15]. In recent years, several imidazolium-based ILs have been employed for lithium isotope separation with remarkably high extraction performance [Citation16,17].
In this study, an efficient and green liquid–liquid extraction system was investigated for selective separation of lithium isotope using imidazolium-based ILs as extraction solvent and BN-17-5 as extractant. We studied the extraction performance from many aspects including the extraction efficiency, separation factor, thermodynamic, and kinetic considerations. The large single-stage separation factor and the environmental friendliness of BN-17-5/IL system show the potential of surpassing the amalgam method for the industrial separation of lithium isotopes.
2. Experimental
2.1. Instrumentation
Infrared (IR) spectra were measured with a FTLA 2000-104 spectrometer (ABB Bomem, Canada) with KBr pellets in the 500–4000 cm−1 region. Nuclear magnetic resonance (NMR) spectrum was recorded with Avance III 400 MHz Digital NMR Spectrometer (Bruker Biospin, Switzerland) in deuteriochloroform, and chemical shifts are reported in parts per milion (ppm) downfield from tetramethylsilane. Element analyses were conducted on elementar corporation vario EL III analyzer (EL III, Germany). The mass spectra of extractant and ILs were recorded on a mass spectrometer (LCZ/2690 XE/996, USA). A SHA-2 fully automatic multifunction thermostatic water bath oscillator (Beijing, China) was performed for extracting lithium isotope separation. A TG16-WS high-speed centrifuge (Hunan, China) was employed for sufficient disengagement of the extracting phases and aqueous phases. A Varian Spectra AA atomic absorption spectrophotometer (AAS, Varian, USA) was employed for the determination of lithium concentration. Lithium isotopic ratio was determined by Thermo Scientific Element 2 inductively coupled plasma mass spectrometry (ICP-MS, Thermo Fisher Scientific, USA) using sample-standard bracketing method. The radio frequency (RF) generator was operated at 1300 W, and the argon gas flows were as follows: nebulizer gas 0.85 L min−1, cooling gas 13.0 L min−1, auxiliary gas 0.82 L min−1, carrier gas 0.933 L min−1, integration time 0.5 s, sampling time 0.01 s, hits of every peak 100, peak width is 100%, and integral width 10%. One percent HNO3 solution and Li2CO3 were used as blank and lithium isotope standard substance for correcting machinery bias, respectively. The determination was performed in triplicate and results reported as mean ± standard deviation.
2.2. Materials and reagents
Lithium salts, 1-methylimidazole, 1-bromooctane, and l,l′-bi-2-naphthol were purchased from Sigma-Aldrich Ltd, Shanghai, China. All other chemicals used were of analytical grade and used without further purification. Demineralized water (18.2 MΩ cm) purified from a Milli-Q purification system was used throughout the experiment.
BN-17-5 used in the present experiment was synthesized by appropriate modified literature procedures [Citation18,19]. A 250 mL round bottom flask was evacuated, filled with nitrogen and charged with l, l′-bi-2-naphthol (2.87 g, 0.010 mol) followed by 100 mL of dry acetonitrile. To this solution, anhydrous cesium fluoride (7.60 g, 0.050 mol) was added, and the mixture was stirred 25 °C for 1 h. A solution of tetraethylene glycol ditosylate (0.010 mol) in acetonitrile (50 mL) was added, and the mixture was stirred at 65 °C for 48 h. After filtration, the solvent was removed in vacuo, and the residue was dissolved in 100 mL CH2Cl2, and washed with water (4 × 100 mL). After drying with MgSO4 and evaporation of the solvent, the crude product was obtained, which was further purified by column chromatography on alumina with petroleum ether–ethyl acetate (2:1) as eluent to give an 85.1% yield of pure product as a light yellow waxy solid. Elemental analysis (%), Calcd for C28H28O5: C, 75.32; H, 6.47. Found: C, 75.68; H, 6.31. IR (KBr, cm−1): 2959, 2933, 2860, 1468 (C–H); 1690–1650 (C = C); 1120 (C–O). 1HNMR (400 MHz, CDCl3): 3.3–3.7(m, 12H), 3.98–4.26(m, 4H), 7.11–7.48(m, 8H), 7.89 (d, 2H, J = 7.7 Hz), 7.92(d, 2H, J = 8.8 Hz). MS: m/e 445.2 (M+; base peak). MP: 103–105 °C.
The ILs 3-octyl-1-methylimidazole hexafluorophosphate (C8mim+PF6−), 3-octyl-1-methylimidazole tetrafluoroborate (C8mim+BF4−), and 3-octyl-1-methylimidazole bistrifluoromethylsulfonylimide (C8mim+NTf2−) were synthesized according to published procedure with minor variations [Citation20,21]. The yields of C8mim+PF6−, C8mim+BF4−, and C8mim+NTf2− were 93.5%, 91.3%, and 89.7%, respectively. C8mim+PF6−: IR (cm−1): 3100 (=C–H); 2950, 1180 (C–H); 1600 (C = C); 1115 (C–N); 837 (P–F). 1HNMR (400 MHz, CD3CN): 8.46 (s, 1H), 7.38 (s, 1H), 7.33 (s, 1H), 4.14–4.08 (t, 2H, J = 7.6 Hz), 3.82 (s, 3H), 1.83–1.78 (m, 2H, J = 6.8 Hz), 1.29–1.26 (m, 10H), 0.89–0.86 (t, 3H, J = 6.8 Hz). MS: m/e (cation) 195.33, m/e (ation) 144.96. C8mim+BF4−: IR (cm−1): 3060 ( = C–H); 2960, 1170 (C–H); 1640 (C = C); 1120 (C–N); 1060 (B–F). 1HNMR (400 MHz, CD3CN): 8.39 (s, 1H), 7.36 (s, 1H), 7.33 (s, 1H), 4.12–4.08 (t, 2H, J = 7.6 Hz), 3.82 (s, 3H), 1.82–1.79 (m, 2H, J = 6.8 Hz), 1.29–1.26 (m, 10H), 0.88–0.85 (t, 3H, J = 6.8 Hz). MS: m/e (cation) 195.33, m/e (ation) 96.8. C8mim+NTf2−: IR (cm−1): 3080 (= C–H); 2950, 1180 (C–H); 1650 (C = C); 1121 (C–N); 1197 (C–F); 1137(O = S = O); 1056 (S–N). 1HNMR (400 MHz, CD3CN): 8.41 (s, 1H), 7.36 (s, 1H), 7.32 (s, 1H), 4.13–4.08 (t, 2H, J = 7.6 Hz), 3.82 (s, 3H), 1.82–1.79 (m, 2H, J = 6.8 Hz), 1.29–1.26 (m, 10H), 0.9–0.88 (t, 3H, J = 6.8 Hz). MS: m/e (cation) 195.33, m/e (ation) 362.14.
2.3. Extraction experiments
Extracting phase was prepared by dissolving appropriate amounts of BN-17-5 in 5 mL IL in a 25-mL conical flask. For comparison with the performance of IL, a conventional organic solvent of chloroform (5 mL) containing BN-17-5 was also prepared by the same procedures. Aqueous phases were prepared by dissolving each of the lithium salt in demineralized water. The concentration of lithium salts was various in different extraction experiment (0.2–3 mol L−1). Equal volumes of the aqueous and organic phases were mixed and gently shaken by a fully automatic multifunction thermostatic water bath oscillator. In order to promote adequate disengagement of the two phases, solution samples were centrifuged for 5 min with 7000 r min−1. After centrifugation, the upper aqueous phase was separated and samples were prepared by removing appropriate supernatant solution and diluting proper multiple. Lithium in the extracting phases was back-extracted using 1-mol L−1 HCl solution of equal volumes. In the process of extraction, no third phase was observed at the two-phase boundary and tiny change of phase volume can be ignored. The mass balance of the extraction system is described by the following equation:
(1)
(1) where Co and Ce are the initial and final concentrations of lithium ion in aqueous phase, which were determined by flame atomic absorption spectrophotometry. Corg is the concentrations of extracted lithium ion in organic phase. Vaq is the volume of the aqueous phase and Vorg is the volume of the organic phase. The extraction efficiency of lithium (E) and separation coefficiency (D) were obtained by the following equations:
(2)
(2)
(3)
(3)
3. Results and discussion
3.1. Composition of complex
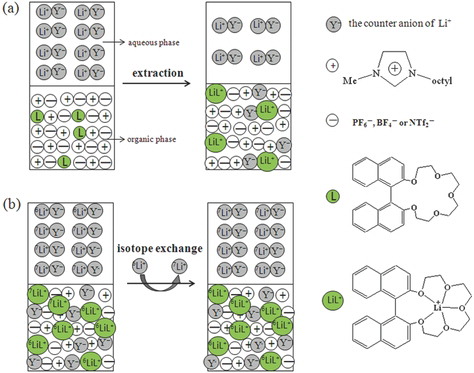
Exploring the composition ratio of lithium complex has important significance for investigating the extraction process of lithium isotopes separation. The Li+ in the aqueous phase are extracted into the IL phase which dissolves BN-17-5 through the process as shown in the following equations:
(4)
(4)
(5)
(5)
The overall reaction of this process is shown as follows:
(6)
(6) where Li+Y−(aq), L(org), n, and (LiLn)+X−(org) denoted the lithium salt in the aqueous phase, the BN-17-5 dissolved in IL phase, the number of crown ether molecules composing a Li-crown complex and formed complexes in IL phase, respectively. The counter anions of the extracted Li(BN-17-5)+ cations were exchangeable with the anions of ILs. A distribution coefficient D of the extraction is shown in the following equation:
(7)
(7) where Ke is the apparent equilibrium constant defined as
(8)
(8)
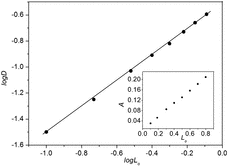
Co and Lo are the initial concentrations of the Li+ in aqueous phase and the BN-17-5 in the IL phase, and A denotes the lithium salt extracted into the IL phase. The activity coefficients of (LiLn)+org and Lorg do not vary and the amount of the Li+ extracted into the IL phase was much smaller than the total Li+ (Lo = 0.1 mol L−1, Co = 1 mol L−1, A = 0.03063 mol L−1) within the concentration range from log Lo = −1 to 0. Thus, it would be reasonable to put Co ≫ A and Lo ≫ A. Then, Equation (7) is abbreviated to
(9)
(9) where C is constant. As shown in , the slope-ratio of lgD vs. lgLo curve is 0.9998. It means Li(BN-17-5)+ 1:1 complex was formed under the experimental conditions. Therefore, the liquid–liquid extraction of lithium isotope separation was mainly due to the coordination of lithium ions with BN-17-5 in IL phase via the ion–dipole bond. The conceptual diagram of reaction mechanism between Li+Y−(aq) and L(org) is described in (a).
3.2. Separation factor
The separating capabilities of BN-17-5 for the Li+ isotopes can be compared by the apparent equilibrium constants (K) of the following chemical exchange equilibrium :
(10)
(10)
where the subscripts (aq) and (org), and X− represent the ions found in the aqueous and in the IL phase, the counter ion of IL, respectively. The conceptual diagram of the above process is depicted in (b).
The K is the same with the single stage separation factor α, as can be seen from the definition of α:
(11)
(11)
(12)
(12)
where ([6Li+]/[7Li+]) represents the isotopic ratio, which was determined by using an inductively coupled plasma mass spectrometry. The maximum single-stage separation factor α of 6Li/7Li of BN-17-5/C8mim+NTf2− system was 1.046 ± 0.002 with TFA− as counter ion. This value is approximately equal to that of cryptand(2B, 2, 1) polymer (αmax = 1.047 ± 0.002) as extractant [Citation22]. Under the same conditions, αmax was 1.040 ± 0.001 with chloroform as solvent. This improvement in the separation factor was attributed to two reasons. On one hand, BN-17-5 achieves elaborate per-organization before coordination, and the forming rigid cavity matches well with Li ion. On the other hand, imidazolium-based ILs used in this experiment were acted as ionic associated agent and extraction solvent. The results show that BN-17-5 dissolved in C8mim+NTf2− was able to extract lithium ions more effectively than that in conventional organic solvent of chloroform.
3.3. Effect of the lithium salts concentration
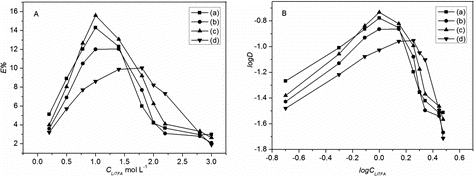
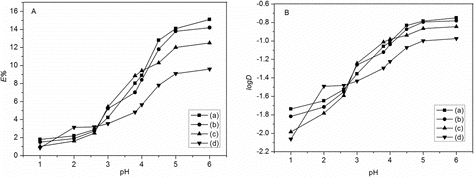
The initial concentration of lithium salts in aqueous phase was one of the significant characteristics in the separation process. With fixed concentration of BN-17-5 (0.186 mol L−1) and fixed volume of aqueous (5 mL) and organic phase (5 mL), a series of concentrations of trifluoroacetic acid lithium (LiTFA) from 0.2 to 3 mol L−1 were tested for Li+ extraction (). The data showed that with the increase of LiTFA concentration, the extraction efficiency changed greatly. The maximum extraction efficiency of Li+ removal was 15.1% in 1 mol L−1 LiTFA with C8mim+NTf2− as solvent, 14.2% in 1 mol L−1 LiTFA with C8mim+PF6− as solvent, 12.5% in 1.4 mol L−1 LiTFA with C8mim+BF4− as solvent, and 9.6% in 1.8 mol L−1 LiTFA with CHCl3 as solvent, respectively. The effect of LiTFA concentration on distribution coefficient also was studied for better comparison. The curve of log D vs. log CLiTFA did not present linear correlation because the concentration of the extracted lithium ion was non-ignorable compared with the concentration of BN-17-5. When the concentration of LiTFA was higher than the corresponding optimum value, the extraction efficiency and distribution coefficient decreased sharply.
3.4. Effect of aqueous phase initial pH
In order to search the optimal pH value of the extraction process, the solutions of LiTFA with optimum concentration were extracted by 0.186 mol L−1 BN-17-5 in different solvents with pH ranging from 1 to 6 for 8 h. The pH of the aqueous phase was adjusted by adding the appropriate hydrochloric acid solution. The extraction behavior of Li+ as a function of pH in the aqueous phase is shown in . The results confirmed that there was an increase in the extraction efficiencies and distribution coefficient with increasing pH from 1 to 6 for each system but not correlations linear. The extraction efficiencies attained maximum at optimum pH 6 for C8mim+NTf2−, C8mim+PF6−, C8mim+BF4−, and CHCl3 systems were 15.1%, 14.2%, 12.5%, and 9.6%, respectively. The extraction efficiencies and distribution coefficient were very tiny in pH below 3. The unpaired electrons of oxygen atoms on BN-17-5 point to inside of the ring forming a rich electronic cavity. Thus, at high concentration of H+, BN-17-5 will be protonated and the formation of lithium complex will be inhibited. Moreover, the higher acidity will cause a gradual loss of [C8mim]+ to aqueous phase. Therefore, hydrochloric acid was prospective for acting as the stripping reagent in back-extraction experiments.
3.5. Effects of counter anion
The complex cation formed was hydrophobic because of the greasy exterior of BN-17-5 used in the present study. In order to preserve electrical neutrality, the complex cation must accompany the anion to be extracted into the IL phase. Therefore, the distribution coefficients were influenced by the affinity of the anion to the ILs. As shown in , the order of the distribution coefficients was I− > Br− > C1− for lithium halides. The order was in agreement with the previous extraction work [Citation23]. For a series of acetic derivate, the order was trifluoroacetate (TFA−) > difluoroacetate (DFA−) > acetate (CH3COO−). The results can be understood by an idea of the hard and soft of acid and base (HSAB) [Citation24,25]. In general, the softer anion has the greater affinity to the organic solvent.
Table 1. Effect of counter anion on the separation of Li+ with BN-17-5.
The isotope separation factors decreased in the order: TFA− > SCN− > I− > DFA− > Br− > Cl− > CH3COO− > CH3COHCOO−. Obviously, the counter anions of lithium salts had great influence on lithium isotope separation. The order of α can also be explained by applying the HSAB theory with the anions of softness orders SCN− > I− > Br− > Cl− for halides, and TFA− > DFA− > acetate for acetate series. However, there was an exception for SCN− and TFA−. SCN− has more softness order than TFA−, while the value of α(LiSCN) was smaller than α(LiTFA). The SCN− gave the second-large separation factor following TFA− in the present experiment. Therefore, LiTFA was most suitable for the industrial separation among a variety of lithium salts. In order to achieve maximum extraction performance, the extraction efficiency, distribution coefficient, and the single-stage separation factor for the lithium isotopes extraction were investigated under the optimum conditions and listed in .
3.6. Kinetic consideration
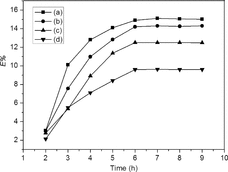
The kinetics of Li+ extraction is indispensable for designing and selecting the operating conditions for metal removal process. Under the optimum operating conditions at room temperature, the extraction was carried out by shaking mechanically the organic phase of 0.186 mol L−1 BN-17-5 and the lithium ion aqueous solution for 2–10 hours (). The results showed that there was a similar trend for ILs or CHCl3 as solvent. It is evident that the rate of removal was very fast initially and became slower with the lapse of time, then attained gradual stabilization and the extraction equilibrium of Li+ by BN-17-5 was achieved within 6 h. However, the lithium isotope exchange equilibrium between organic phase and aqueous phase quickly reached within 1 min [Citation16]. The extraction equilibrium was a relative long-term process in this experiment. The configuration of pre-organized BN-17-5 is not easy to be adjusted when forming active intermediates, thus the formation of Li(BN-17-5)+ complexes required slow kinetics of bonding.
3.7. Thermochemical consideration
To further understand the extraction performance of the extraction system with BN-17-5, various thermodynamic parameters were obtained by studying the effect of different temperatures on the lithium separation factor [Citation26,27]. According to the Bigeleisen–Mayer theory: (1) ln α is reciprocally proportional to T2 for high temperatures (above 100 °C), (2) ln α is proportional to 1/T for low temperatures (below 50 °C), and (3) the relationship between ln α and T is complicated for intermediate region. Considering a separation factor can be regarded as an equilibrium constant for an isotope exchange reaction, the manner in which the separation factor depends on the temperature can be derived by thermodynamics [Citation1]. Assuming that ΔH° is independent of the temperature in the temperature range investigated, the following van't Hoff equation (13) holds for the separation factor:
(13)
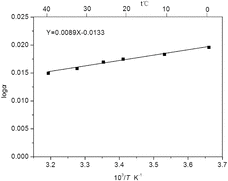
(13) where R is the gas constant. Accordingly, ΔH° (6Li/7Li) can be obtained from the slope of the line determined by plotting log α vs. 1/T (). The free energy change ΔG° (6Li/7Li) and entropy change ΔS° (6Li/7Li) could be calculated by applying the well-known thermochemical equations (14) and (15):
(14)
(14)
(15)
(15)
The change of thermodynamic parameters of isotopic exchange is listed in . The data indicated that the extraction process was a spontaneous exothermic process. The separation factor was decreased with the increase of operating temperature. Thus, 25 °C of operating temperature was used for lithium isotope separation to obtain a relatively high separation factor.
Table 2. Thermodynamic parameters of lithium isotope extraction process.
The −ΔG° of the system of Li+-L-TFA− is bigger than that of the reported system of Li+-crown-I− and Li+-CR-I−, but slightly smaller than the system of Li+-cryp-I−. The larger −ΔG° is beneficial to the formation of stabile complexes and the extraction separation of the lithium isotope. There are two main factors contributing to the remarkably high extraction performance of Li+-L-TFA− system. Frist, BN-17-5 has the rigid cavity and elaborate per-organization before coordination with lithium, which greatly improved the compatibility and bonding ability between extractant and lithium ion. Namely, this extraction system has low energy of rearrangement and high lithium ion bonding ability. Second, the high polarity of ILs solvent influences the extraction performance and presents the large separation factor. Consequently, the extraction system developed a wonderful extraction performance with green, high efficiency, large separation factor.
3.8. Back-extraction of lithium ion
The recovery of extracted metal ions into a receiving phase is also important for the separation and concentration of a target metal ion. There has, however, been little progress in striking a balance between improvements in extraction and recovery by back extraction in IL-based extraction systems [Citation28,Citation29]. According to the above discussion, the cation-binding capabilities of BN-17-5/IL system are extremely pH sensitive. At low pH, the binding constant of BN-17-5 for metal cations will be severely reduced due to the protonation and charge repulsion. The binding affinity can accordingly be switched by varying the pH, with metal ion binding taking place at neutral or high pH, and metal ion release taking place at low pH. This switchable binding property forms the main rationale for the development of IL-based separation processes using BN-17-5.
Thus, relatively high concentration of hydrochloric acid (1 mol L−1) was employed for stripping lithium ion in organic phase with o/a of 1. The single-stage back-extraction efficiency of C8mim+NTf2−, C8mim+PF6−, C8mim+BF4−, and CHCl3 systems was up to 34.5%, 21.4%, 15.3%, and 13.2%, respectively. After repeated stripping process for four times, lithium ion in C8mim+NTf2− phase (0.143 mol L−1) was completely transferred into the acid solution. No trace amount of Li ions was detected in the acid of the fifth back-extraction. The experimental results demonstrated the extraction performance of BN-17-5/C8mim+NTf2− system is superior to others. The IL phase containing BN-17-5 was reused for solvent extractions after being processed with ultra-pure water. Compared with the first lithium isotope separation, no significant change of extraction efficiency was observed. The stripping process based on BN-17-5/C8mim+NTf2− system is feasible and BN-17-5 and C8mim+NTf2− are stable under these experimental conditions. These results highlight the great potential of BN-17-5 as extractants for lithium isotopes separation in IL systems.
4. Conclusions
In summary, an efficient and green liquid–liquid extraction system was developed in this paper for the selective separation of lithium isotopes with BN-17-5 as an extractant and ILs as an extraction solvent. The bonding ratio of extractant and lithium ion, the optimal concentration of LiTFA, the best extraction time, the maximum single-stage separation factor, relevant thermodynamic parameters, and back-extraction experiments were investigated. The experiment results demonstrated that the BN-17-5/IL extraction system offers high efficiency, green nature, easier recycling strategy, and good application prospect to lithium isotopes separation.
Additional information
Funding
References
- Okuyama K, Okada I, Saito N. The isotope effects in the isotope exchange equilibriums of lithium in the amalgam-solution system. J Inorg Nucl Chem. 1973;35:2883–2895.
- Saleem M, Hussain S, Rafiq M, Baig MA. Laser isotope separation of lithium by two-step photoionization. J Appl Phys. 2006;100:053111/1-053111/7.
- Black JR, Umeda G, Dunn B, McDonough WF. Electrochemical isotope effect and lithium isotope separation. J Am Chem Soc. 2009;131:9904–9905.
- Kim DW, Kang BM, Jeon BK, Jeon YS. Separation of lithium isotopes by elution chromatography with an AB18C6 bonded Merrifield peptide resin. J Radioanal Nucl Chem. 2003;256:81–85.
- Hoshinoa T, Terai T. High-efficiency technology for lithium isotope separation using an ionic-liquid impregnated organic membrane. Fusion Eng Des. 2011;86:2168–2171.
- Fang SQ, Zhi KZ, Fu LA. [Lithium isotope separation by liquid–liquid extraction using 4-tert-butylbenzene-15-crown-5]. J Radioanal Nucl Chem. 1994;187:25–31. Chinese.
- Taylor TI, Urey HC. On the electrolytic and chemical-exchange methods for the separation of the lithium isotopes. J Chem Phys. 1937;5:597–598.
- Grote Z, Wizemann HD, Scopelliti R, Severin K. Lithium isotope separation by 12-metallacrown-3 complexes. Z Anorg Allg Chem. 2007;633:858–864.
- Otake K, Suzuki T, Kim HJ, Nomura M, Fujii Y. Novel syntheses method of phenol-type benzo-15-crown-5 ether resin and its application for lithium isotope separation. J Novel Nucl Sci Technol. 2006;43:419–422.
- Jepson BE, Cairns GA. Lithium isotope effects in chemical exchange with cryptand (2, 2, 1). Report. 1979; MLM-2622:1–16.
- Nishizawa K, Ishino S, Watanabe H. Lithium isotope separation by liquid–liquid extraction using benzo-15-crown-5. J Nucl Sci Technol. 1984;21:694–701.
- Sun P, Armstrong DW. Ionic liquids in analytical chemistry. Anal Chim Acta. 2010;661:1–16.
- Zuo Y, Liu Y, Chen J, Li D. The separation of cerium(IV) from nitric acid solutions containing thorium(IV) and lanthanides(III) using pure [C8mim]PF6 as extracting phase. Ind Eng Chem Res. 2008;47:2349–2355.
- Hiroyuki O, Atsushi IO, Takumi S, Noboru A, Hirochika N, Naoki H, Shigeo U, Hisanori I, Kojiro S. Specific cooperative effect of a macrocyclic receptor for metal ion transfer into an ionic liquid. J Anal Chem. 2012;84:9332–9339.
- Naoki H, Hiroyuki O, Keiji K, Hisanori I. Ionic liquid synergistic cation-exchange system for the selective extraction of lanthanum(III) using 2-thenoyltrifluoroacetone and 18-crown-6. J Anal Chem Soc. 2008;24:697–699.
- Xu JJ, Li ZJ, Gu ZG, Wang GL, Liu JK. Green and efficient extraction strategy to lithium isotope separation with double ionic liquids as the medium and ionic associated agent. J Radioanal Nucl Chem. 2013;295:2103–2110.
- Heitzman H, Young BA, Rausch DJ, Rickert P, Stepinski DC, Dietz ML. Fluorous ionic liquids as solvents for the liquid–liquid extraction of metal ions by macrocyclic polyethers. Talanta. 2006;69:527–531.
- Armstrong DW, Ward TJ, Czech A, Czech BP, Bartsch RA. Synthesis, rapid resolution, and determination of absolute configuration of racemic 2,2′-binaphthyldiyl crown ethers and analogs via β-cyclodextrin complexation. J Org Chem. 1985;50:5556–5559.
- Helgeson RC, Weisman GR, Toner JL, Tarnowski TL, Chao Y, Mayer JM, Cram DJ. Effects on cation binding of convergent ligand sites appended to macrocyclic polyethers. J Am Chem Soc. 1979;101:4928–4941.
- Rika H, Yasuhiko I. Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J Fluor Chem. 2000;105:221–227.
- Dzyuba SV, Bartsch RA. Efficient synthesis of 1-alkyl(aralkyl)-3-methyl(ethyl)-imidazolium halides: precursors for room-temperature ionic liquids. J Heterocycl Chem. 2001;38:265–268.
- Nishizawa K, Watanabe H, Ishino S, Shinagawa, M. Lithium isotope separation by cryptand (2B, 2, 1) polymer. J Nucl Sci Technol. 1984;21:133–138.
- Marcus Y, Asher LE. Extraction of alkali halides from their aqueous solutions by crown ethers. J Phys Chem. 1978;82:1246–1254.
- Pearson, RG. Hard and soft acids and bases (HSAB). I. Fundamental principles. J Chem Educ. 1968;45:581–587.
- Pearson, RG. Hard and soft acids and bases (HSAB). II. Underlying theories. Fundamental principles. J Chem Educ. 1968;45:643–647.
- Bigeleisen J, Mayer MG. Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys. 1974;15:261–267.
- Urey HC. The thermodynumic properties of isotopic substances. J Chem Soc. 1947;18:562–581.
- Luo HM, Dai S, Bonnasen PV. Solvent extraction of Sr2+ and Cs+ based on room-temperature ionic liquids containing monoaza-substituted crown ethers. J Anal Chem. 2004;76:2773–2779.
- Kojiro S, Masahiro G. Solvent extraction and stripping of silver ions in room-temperature ionic liquids containing calixarenes. J Anal Chem. 2004;76:5039–5044.