?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The accident at unit 3 of the Fukushima Dai-ichi nuclear power plant was analyzed with THALES2 for the progression of severe accident coupled with Kiche for the iodine chemistry in aqueous phase. The analysis indicated that, compared with the analysis without the aqueous phase iodine chemistry, a significantly larger amount of iodine was released from the water pool of the suppression chamber (S/C) with forms of molecular iodine and organic iodine due to the repeated operation of the containment venting system. It was also implied in the sensitivity analysis that the late phase release of the volatile iodine species was largely influenced by the pH of the S/C water and gas–liquid mass transfer coefficient under a gas–liquid two-phase flow condition.
1. Introduction
Iodine is one of the most important fission products (FPs) in a severe accident due mainly to its highly volatile nature from the degraded core and large radiological consequences to the public. A large amount of iodine was released into the environment during the accident at the Fukushima Dai-ichi nuclear power plant (NPP) induced by the Tohoku district – off the Pacific Ocean Earthquake and the subsequent tsunami on 11 March 2011.
In order to assess the radiological consequences to the public in a rational way, radiation monitoring information of the environment was needed specifically in the initial phase of the Fukushima Dai-ichi NPP accident, during which the major release of iodine could have occurred. However, it was not available since monitoring systems did not function due to the loss of power supply. The measured data of environmental sampling became available after 14 March 2011, showing that a comparable amount of gaseous iodine was sampled with that of particulate iodine [Citation1,2]. It should be noted that, however, the results of this sampling could not be directly applied to the release characteristics at the Fukushima Dai-ichi NPP since these components of iodine had different advective and diffusive natures within a transportation pathway. Thus, analysis for transportation in the environment is required in order to estimate the on-site release characteristics.
Experimental and analytical studies have been actively performed for the iodine behaviors in a severe accident after the accidents at Three Mile Island and Chernobyl NPPs [Citation3]. These studies indicated that iodine was transformed into a gaseous form including molecular iodine (I2) and organic iodine in the severe accident, especially through the aqueous phase chemistry in a radiation field in the containment vessel. The results of the environmental sampling implied a possibility of such iodine chemistry to occur in the course of the Fukushima Dai-ichi NPP accident.
In most of the analyses for the source term of the Fukushima Dai-ichi NPP accident [Citation4–6], integrated severe accident analysis codes such as THALES2 [Citation7], MELCOR [Citation8] and MAAP [Citation9] were applied. Those analyses were not capable of quantitatively reproducing the results of the above-described environmental sampling because a chemical form of iodine was assumed to be cesium iodide (CsI), which behaved in an aerosol form under a typical severe accident condition in the containment.
Thus, the iodine release associated with the progression of the Fukushima Dai-ichi NPP accident was analyzed in the present study, taking into account the aqueous phase iodine chemistry. In particular, a focus was made on unit 3 of the Fukushima Dai-ichi NPP where venting of the containment vessel atmosphere was repeatedly operated through the suppression chamber (S/C). This was because it was anticipated that, when the venting gas passed through the S/C water pool, I2 and organic iodine generated in the water pool were transferred into the venting gas, resulting in the release of these iodine species out of the containment vessel.
2. Brief description of analysis
Japan Atomic Energy Agency (JAEA) has developed THALES2 to analyze the progression and the source term of severe accidents for level 2 probabilistic risk assessment of light water reactors. The iodine was assumed to have a chemical form of CsI and the aqueous phase iodine chemistry was not taken into consideration in THALES2. In the present analysis, the iodine chemistry simulation code, Kiche [Citation10], based on kinetics of chemical reactions and mass transfer, was coupled with THALES2. The Kiche code has also been developed at JAEA and validated with several iodine release experiments [Citation11–13].
Most of the phenomena in the course of a severe accident, including thermal-hydraulics, core melt progression and FP transportation, were mainly analyzed by THALES2. The analysis by Kiche took a role for the iodine chemistry in the aqueous phase and related phenomena such as gas–liquid mass transfer and adsorption/desorption of I2 onto/from the wall submerged in the aqueous phase. An interface program was developed and applied for the exchange of outputs between both the codes [Citation14].
The aqueous phase reactions considered in Kiche included water radiolysis and inorganic and organic reactions associated with the production of I2 and organic iodine [Citation10]. When CsI enters into the aqueous phase, it becomes dissociated and iodine takes a form of iodide ion (I-). The dose rate in the S/C water, which could affect the water radiolysis and iodine chemistry, was calculated from the amount of FPs retained in the S/C water, assuming that all the emitted gamma and beta rays were absorbed in the aqueous phase. It was estimated to approximately 3 kGy/h at maximum after the core melt started. The iodide ion is oxidized to I2 under a certain condition and a part of I2 could be transformed to organic iodine when organic impurities dissolve in the water pool. Typical organic impurities are caused by the elution of organic solvents from the containment paint. Since both I2 and a part of organic iodine have high volatility, those are transported from the aqueous phase to the gas phase in accordance with gas–liquid mass transfer rates and partitioning limitation.
The gas–liquid mass transfer rates for I2 and organic iodine were calculated by the following equation in THALES2:
(1)
(1) where ϕgas, i is the mass flow rate for iodine species i (mol/s), Cgas, i is the concentration of iodine species i in gas phase (mol/m3), Caq, i is the concentration of iodine species i in aqueous phase (mol/m3), kmt is the overall gas–liquid mass transfer coefficient (m/s), Afs is the free surface area of water pool (m2) and Hi is the partition coefficient of iodine species i (−).
The overall gas–liquid mass transfer coefficient was presumed to include effects of the diffusive resistance through boundary layers of gas and liquid sides, and the increase of gas–liquid interfacial area depending on a gas–liquid two-phase flow condition. In the present analysis, the overall gas–liquid mass transfer coefficient was given as an input.
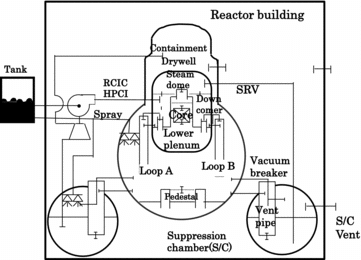
The volume discretization for the present analysis is shown in . The reactor cooling system was divided into seven volumes consisting of reactor core, upper plenum, steam dome, downcomer, lower plenum and recirculation loops A and B. The containment vessel was discretized with drywell (D/W), S/C, pedestal and vent pipes connecting D/W and S/C. The environment was modeled by a hypothetical volume connected to the S/C or the reactor building representing venting through S/C or leaks.
3. Accident sequence and conditions of analysis
3.1. Major event sequence of unit 3
The major sequence of occurrences for the accident at unit 3 of the Fukushima Dai-ichi NPP applied to the present analysis is shown in . The reactor pressure vessel (RPV) was depressurized by the actuation of the safety relief valves after 6.5 hours from the stop of the high-pressure core injection (HPCI) system. Then, the depressurization of the containment vessel was repeatedly made by opening venting line through S/C to promote the alternative water injection into the RPV. The timings of the operation for those equipment and systems were basically given from the publicly available information [Citation15]. Regarding the rate of the alternative water injection by fire engine pumps, it was assumed that a quarter of nominal values [Citation15] reached the RPV.
Table 1. Sequence of major events.
3.2. Base conditions for iodine chemistry
The aqueous phase iodine chemistry and the gas–liquid partitioning of iodine species may depend on various factors such as pH, temperature and radiation intensity of aqueous solution, chemical form of radionuclides dissolved in the aqueous phase, gas–liquid mass transfer and dissolution of organic impurities, and so on. It is considered that pH is one of the most influential factors on the iodine chemistry.
The chemical forms of iodine and cesium were assumed in THALES2 to be CsI and cesium hydroxide (CsOH), the latter being a strong base. In the base case analysis with the initial value of pH at 7.0, pH of the S/C water was internally calculated by Kiche. The dissolution of CsOH and the formation of organic acids and carbon dioxide (CO2) were supposed to dominate the pH variation of the S/C water in the Kiche calculation. The organic acids were generated by the radiolytic decomposition of the organic impurities in the S/C water. The influence of CO2 generated by the oxidation of boron carbide (B4C) control material and the molten core/concrete interaction on the pH variation were not taken into account in the present analysis.
The organic impurities also had an impact on the formation of organic iodine in the S/C water. The initial concentration of the organic impurities in the S/C water was set at 10−4 mol/L in the base case analysis. This value was based on the experiments associated with the dissolution of organic solvent from painted walls [Citation11,Citation16]. It is noted that organic impurities in the sea water injected into the RPV was not taken into account in the present study due to a lack of information.
The gas–liquid mass transfer coefficient for the iodine species was determined on the basis of related experiments and our preliminary analysis by THALES2 without coupling of Kiche for unit 3 of the Fukushima Dai-ichi NPP. The related experiments included No. 41 of International Standard Problem (ISP-41) [Citation12,13] of the Organization for Economic Co-operation and Development/Nuclear Energy Agency (OECD/NEA), Iod-23 experiment [Citation17] and SISYPHE experiment [Citation18]. These experiments indicated that the gas–liquid mass transfer coefficient was of the order of 10−5 m/s for a stratified geometry where a gas flow swept over a circulated water pool, simulating the dissolution of volatile iodine species. The preliminary analysis with THALES2 showed that a gas–liquid two-phase flow was formed in the S/C water. The ratio of the total gas–liquid interfacial area to the free surface area was estimated at the order of 100 with the diameter of bubbles at around 10 mm. Although large uncertainties were expected to exist in the gas–liquid mass transfer coefficient and the gas–liquid interfacial area, the overall gas–liquid mass transfer coefficient, kmt, of 10−3 m/s was applied in the base case analysis.
4. Results of analysis and discussions
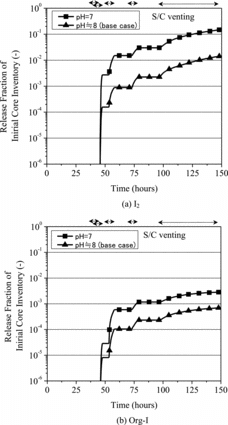
The result of the base case analysis is shown in for the release of CsI, I2 and organic iodine represented by methyl iodide (CH3I) (hereinafter, referred to as Org-I) out of the containment vessel. The vertical axis expresses a fractional amount of the initial core inventory. It is noted that the release fraction of CsI in the preliminary analysis by THALES2 without Kiche was equivalent to that obtained in the base case analysis. The small release fraction of CsI resulted from the effective removal by the pool scrubbing in the S/C water, indicating that the S/C water became a potential source for the late phase release of iodine species.
Figure 2. The release fraction of iodine species out of containment vessel in the base case analysis.
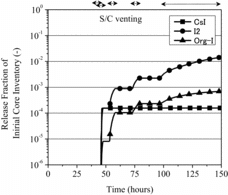
Due to the aqueous phase iodine chemistry, a part of I- derived from CsI was predicted to be transformed to I2 and Org-I. In case no operation of the containment venting was assumed, those iodine species volatilized from the S/C water could have remained in the gas phase of the containment vessel with the equilibrium concentration at the maximum. On the other hand, as clearly found in , I2 and Org-I were intermittently released out of the containment vessel upon the operation of the containment venting, resulting in the increase of iodine source term.
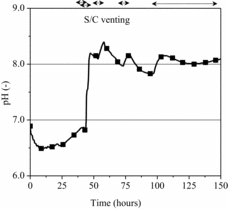
The result of pH variation in the S/C water is plotted in . As previously mentioned, the pH variation is dominated by the dissolution of CsOH and the formation of organic acids and CO2 in the Kiche calculation. The temperature of the aqueous solution, and the dilution and inspissation of the solution by water injection and vaporization are additional influential factors on the pH variation.
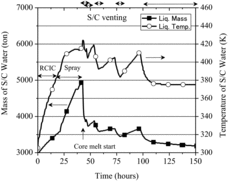
The predicted time histories of mass and temperature of the S/C water are shown in . It was mentioned that, before the core degradation was predicted to start, the pH variation was made by the increase in temperature of the S/C water and the addition of water into the S/C by the operation of engineered safety systems such as RCIC, HPCI and S/C spray systems. The pH increase in around 25 hours was supposed to result from the dilution of the S/C water by the water injection through spray operation.
The intermittent operation of the S/C venting caused the depressurization of the containment vessel. This depressurization resulted in the reduction in mass of the S/C water due to the flushing and the decrease in temperature of the S/C water from approximately 420 to 380 K corresponding roughly to the saturation temperature. Due to the change in the thermal-hydraulic situation in addition to the dissolution of CsOH and the formation of organic acids and CO2, pH of the S/C water was kept at around 8.0 after the core degradation started.
The base case analysis indicated that the operation of the S/C venting results in the significant late phase release of gaseous iodine from the S/C water even in the case of a basic condition in the S/C water, under which the volatile iodine was suppressed to form. The chemical forms of iodine and cesium were anticipated to affect the pH of the S/C water. The in-pile experiment, FPT-3, in the Phebus-FP project and the subsequent analysis with SOPHAEROS code showed that other chemical forms of iodine and cesium rather than CsI and CsOH were possible to be generated under a condition simulating boiling water reactor (BWR) with boron carbide (B4C) as a control material [Citation19,20]. Those chemical forms included hydrogen iodide (HI), cesium borate (CsBO2) and cesium molybdate (Cs2MoO4). It was anticipated that HI was an influential chemical form on the variation of pH since it was a strong acid. According to the SOPHAEROS analysis, 5% of the iodine initial inventory was transformed into HI and dissolved into the S/C water, and pH of the S/C water was estimated to fall by approximately 1.0. Therefore, a sensitivity analysis was carried out by fixing the pH of the S/C water at 7.0 in order to examine the influence of pH on the release of the volatile iodine species. Additional influential factors on the pH variation, which were not taken into account in the present analysis, include the formation of nitric acid (HNO3) and hydrochloric acid (HCl) by the humid air radiolysis and the radiation degradation of cable sheaths, respectively.
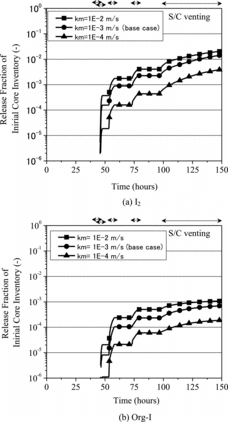
Another uncertain factor in the present analysis was gas–liquid mass transfer rate of the volatile iodine species. This could be affected by the vigorous agitation of gas and aqueous phases, and the variation of the gas–liquid interfacial area under gas–liquid two-phase flow conditions established during the activation of the containment venting. Thus, additional sensitivity analyses were performed with the overall gas–liquid mass transfer coefficients at 10−4 and 10−2 m/s.
The results of the sensitivity analyses are depicted in for the influence of pH in the S/C water and in for the influence of the overall gas–liquid mass transfer coefficient. The sensitivity analysis for pH of the S/C water indicated that the fractional release of I2 and Org-I increased by approximately one order compared with the base case analysis. This increase was supposed to result from the promotion of the oxidation of I- to I2 and the subsequent formation of Org-I under a condition that the concentration of hydroxide ion (OH-) was lower. It was also clarified that the overall gas–liquid mass transfer coefficient had a large impact on the release of I2 and Org-I. However, the amount and ratio of these iodine species released could be influenced by the balance between the gas–liquid mass transfer rate and kinetics of chemical reaction in the S/C water associated with the formation of I2 and Org-I.
5. Conclusion
The accident at unit 3 of the Fukushima Dai-ichi NPP was analyzed with the coupling approach of the integrated severe accident analysis code THALES2 and the iodine chemistry simulation code Kiche. The main target of the present analysis was to examine the influence of the containment venting on the late phase release of the volatile iodine species such as I2 and Org-I. The major course of the accident including thermal-hydraulics, core melt progression and transportation of radioactive materials was predicted by THALES2. The iodine chemistry in the S/C water under a radiation field was calculated by Kiche based on the reaction kinetics.
It was found in the present analysis that, compared with the analysis without the iodine chemistry in the S/C water, a significant amount of I2 and Org-I was released from the S/C water due to the repeated activation of the containment venting system. In addition, the amount of CsI released in a particulate form was predicted to be much lower than I2 and Org-I. These results were qualitatively consistent with the environmental sampling data at JAEA [Citation1,2], in which the amount of gaseous iodine largely exceeded that of iodine with particulate form.
The present analysis also indicated, through the sensitivity analyses, that it could be important to take into consideration the chemical forms of major FPs such as iodine and cesium in the evaluation of the pH variation of the aqueous phase in order to improve the predictive capability for the late phase release of the volatile iodine species during severe accidents. Additionally, it was shown that technical knowledge is desirable to be gained for the characteristics of gas–liquid mass transfer under a gas–liquid two-phase flow condition.
References
- Furuta S, Sumiya S, Watanabe H, Nakano M, Imaizumi K, Takeyasu M, Nakada A, Fujita H, Mizutani T, Morisawa M, Kokubun Y, Kono T, Nagaoka M, Yokoyama H, Hokama T, Isozaki T, Nemoto M, Hiyama Y, Onuma T, Kato C, Kurachi T. [Results of the environmental radiation monitoring following the accident at the Fukushima Daiichi nuclear power plant – interim report (Ambient radiation dose rate, radioactivity concentration in the air and radioactivity concentration in the fallout)]. Japanese. Ibaraki-ken (Japan): Japan Atomic Energy Agency; 2011. (JAEA-Review 2011-035).
- Ohkura T, Oishi T, Taki M, Shibanuma Y, Kikuchi M, Akino H, Kikuta Y, Kawasaki M, Saegusa J, Tsutsumi M, Ogose H, Tanuma S, Sawahata T. Emergency monitoring of environmental radiation and atmospheric radionuclides at nuclear science research institute, JAEA following the accident of Fukushima Daiichi nuclear power plant. Ibaraki-ken (Japan): Japan Atomic Energy Agency; 2012. (JAEA-DATA/Code 2012-010).
- Clement B, Cantrel L, Ducros G, Funke F, Herranz L, Rydl A, Weber G, Wren C. State of the art report on iodine chemistry, working group on analysis and management of accidents (WGAMA). Issy-les-Moulineaux (France): Organization for Economic Co-operation and Development; 2007. (NEA/CSNI/R (2007)1).
- Ishikawa J. Analysis for accident progression with THALES2 code. In: Technical workshop on Fukushima Daiichi NPP accident, Nuclear Regulation Authority; Jul 23–24; Tokyo (Japan): Nuclear and Industrial Safety Agency; 2012.
- Hoshi H, Ogino M, Kawabe R, Fukasawa M. Computational analysis on accident progression of Fukushima Dai-ichi NPS. Proceedings of Tokyo PSAM 2013–1061; Apr 14–18; Tokyo (Japan): Atomic Energy Society of Japan; 2013.
- Mizokami S. Detailed analysis of the accident progression of units 1 to 3 by using MAAP Code. In: Technical workshop on the accident of TEPCO's Fukushima Daiichi NPS; 23–24 July; Tokyo (Japan): Nuclear and Industrial Safety Agency; 2012.
- Kajimoto M, Kuramatsu K, Watanabe N, Funasako M, Noguchi T. Development of THALES-2, A computer code for coupled thermal–hydraulics and radionuclides transport analyses for severe accident at LWRs and its application to analysis of radionuclides revaporization phenomena. In: Proceedings of International Topical Meeting on Safety of Thermal Reactors; Portland, OR. La Grange Park(IL): American Nuclear Society; 1991.
- Gauntt RO, Cole RK, Erickson CM, Gido RG, Gasser RD, Rodriguez SB, Young MF. MELCOR computer code manuals: primer and user's guide version 1.8.5. NUREG/CR-6119. Vol. 1:2. Albuquerque (NM): Sandia National Laboratory; 2005.
- Fauske & Associates, LLC. MAAP4: modular accident analysis program for LWR power plants user's manual. EPRI Project RP3131-02, 3. Burr Ridge (IL: EPRI); 1994.
- Moriyama K, Maruyama Y, Nakamura H. A simulation tool for kinetics of iodine chemistry in the containment of light water reactors under severe accident conditions. Ibaraki-ken(Japan): Japan Atomic Energy Agency; 2010. (JAEA-Data/Code 2010-034).
- Moriyama K, Tashiro S, Chiba N, Maruyama Y, Nakamura H, Watanabe A. Experiment on the gaseous iodine release from irradiated cesium iodide solutions (contract research). Ibaraki-ken(Japan): Japan Atomic Energy Agency; 2011. (JAEA-Research 2011-016).
- Ball J, Wren JC, Rydl A, Poletiko C, Billarand Y, Ewig F, Funke F, Zeh P, Hidaka A, Gauntt R, Young M, Cripps R, Herrero B. ISP 41 containment iodine computer code exercise based on a radioiodine test facility (RTF) experiment. Issy-les-Moulineaux (France): Organization for Economic Co-operation and Development; 2000. (OECD/NEA/CSNI/R(2000)6/VOL1-2).
- Ball J, Marchand C, Allelein H, Cantrel L, Cripps R, Glowa G, Herranz L, Rincon A, Royen J, Rydl A, Schindler P, Weber G, Wren J. ISP-41–follow-up exercise: Phase 2 iodine code comparison exercise against CAIMAN and RTF experiments. Issy-les-Moulineaux (France): Organization for Economic Co-operation and Development; 2004. (OECD/NEA/CSNI/R(2004)16).
- Ishikawa J, Moriyama K. [Investigation for evaluating containment source term of BWR4/Mark-I plant considering iodine chemistry in suppression pool]. Japanese. Ibaraki-ken(Japan): Japan Atomic Energy Agency; 2010. (JAEA-Research 2010-51).
- OECD/NEA. Information portal for the Fukushima Daiichi accident analysis and decommissioning activities [Internet]. Ibaraki-ken(Japan): Japan Atomic Energy Agency; 2012. Available from: href="https://fdada.info/accident/database-for-accident-analysis">https://fdada.info/accident/database-for-accident-analysis/measured
- Wren JC, Ball JM, Glowa GA. The interaction of iodine with organic material in containment. Nucl Technol 1999;125(3):337–362.
- Fischer K, Weber G, Funke F, Langrock G. Experimental determination and analysis of iodine mass transfer coefficients from THAI test Iod-23. 5th European Review meeting on Severe Accident Research (ERMSAR-2012); 2012 Mar 21–23; Cologne (Germany).
- Cantrel L, March P. Mass transfer modeling with and without evaporation for iodne chemistry in the case of a severe accident. Nucl Technol. 2006;154:170–185.
- Girault N, Fiche C, Bujan A, Dienstbier J. Towards a better understanding of iodine chemistry in RCS of nuclear reactors. Nucl Eng Des. 2009;239(6):1162–1170.
- Alpy N, Missane MP, Drosik I, Fiche C, Kaya M. Fission product transport modelling in the ASTEC integral code: the status of the SOPHAEROS module. Proceedings of the Eighth International Conference on CANDU fuel, Canadian Nuclear Society; 2003 Sep 21–24; Honey Harbour (Canada). ISBN 0 919784-77-1.