?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Liquid chromatography operated in a breakthrough mode was employed to study calcium isotope fractionation in the aqueous hydrobromic acid medium. Highly porous silica beads, the inner pores of which were embedded with a benzo-18-crown-6 ether resin, were used as column packing material. Enrichment of heavier isotopes of calcium was observed in the frontal part of respective calcium chromatograms. The values of the isotope fractionation coefficient were on the order of 10−3. The observed isotope fractionation coefficient was dependent on the concentration of hydrobromic acid in the calcium feed solution; a higher HBr concentration resulted in a smaller fractionation coefficient value. The present calcium isotope effects were most probably mass-dependent, indicating that they mostly came from isotope effects based on molecular vibration. Molecular orbital calculations supported the present experimental results in a qualitative fashion. Chromatography operated in aqueous HBr media is a better system of Ca isotope separation than that operated in aqueous HCl media.
1. Introduction
Calcium (Ca) isotopes have applications in various research fields ranging from astrophysics to medicine. 48Ca, a stable isotope of Ca with double magic numbers, is an important nuclide to synthesize super-heavy elements and is expected to be used in the research of the neutrino-less double beta decay [Citation1]. 47Ca, a radioisotope of Ca, which is produced from 48Ca by neutron bombardment, may be important in certain medical and biological research because of its short half-life of 4.5 days and convenient β and γ spectra [Citation2]. In these applications, the key isotope of Ca is 48Ca. Due to its low natural abundance of 0.19%, however, it needs to be concentrated prior to applications.
Several methods for Ca isotope separation and enrichment have been reported. Among them, chromatographic processes based on chemical exchanges are considered to be desirable for large-scale production of enriched isotopes due to operational simplicity and easy accumulation of small single-stage isotope fractions. Ion exchange resins including chelating resins [Citation3,4] and polymer-bound cryptands [Citation5–7] have been reported as chromatographic column packing materials.
A chromatographic Ca isotope separation process with a crown ether resin was shown to be an improvement over those with cryptands [Citation7,8]. In passing, isotope fractionation/isotope separation by chromatography with crown ether resin is reported for lithium [Citation9–11], zinc [Citation12–15] and strontium (Sr) [Citation16], other than Ca. Recently, accumulation of Ca isotope fractions was developed for liquid chromatography using a benzo-18-crown-6-ether crown ether (B18C6) resin as column packing material, and it was indicated that liquid chromatography with the B18C6 resin may be a promising process for large-scale production of enriched Ca isotopes [Citation17,18]. The B18C6 resin, embedded in the inner pores of highly porous silica beads (diameter: 40–60 μm), is a copolymer of phenol and monobenzo-18-crown-6 [Citation17]. In our previous papers [Citation19,20], we examined the Ca isotope effects in breakthrough-mode liquid chromatography with the B18C6 resin in aqueous and methanol hydrochloric acid (HCl) media. We found that (1) the lighter isotopes of Ca are preferentially fractionated to the B18C6 resin phase rather than the solution phase; (2) a higher concentration of HCl resulted in smaller Ca isotope effects; (3) observed isotope effects are mass-dependent; and (4) the use of methanol as solvent has little advantage over the aqueous system as far as the magnitude of Ca isotope effects is concerned, but a substantial improvement was observed concerning the adsorption capacity of the resin for Ca2+ ions.
In the present paper, we investigated Ca isotope fractionation in liquid chromatography with the B18C6 resin in aqueous hydrobromic acid (HBr) medium (HBr system). We expected HBr solution to be a better medium than the HCl solution, because the bromide (Br−) ion is a softer base than the chloride (Cl−) ion. In addition, we conducted molecular orbital (MO) calculations for elucidation of Ca isotope effects observed in those experiments. Such chromatographic experiments and MO calculations and their results are described in the following sections.
2. Experimental
2.1. Crown ether resin and other materials
The B18C6 resin used as the column packing material in this study was synthesized at the Research Laboratory for Nuclear Reactors, Tokyo Institute of Technology, and was kindly donated to us. All reagents were of analytical reagent grade and used without further purification.
2.2. Ca isotope fractionation experiments
Chromatography was operated in the breakthrough mode using five Pyrex glass columns (100 cm length and 0.8 cm diameter) packed with the resin and connected in series with polytetrafluoroethylene tubes (1 mm diameter). The resin column was first washed with aqueous HBr solution with the same HBr concentration as that of Ca feed solution. The aqueous HBr solution containing calcium bromide (CaBr2) of the natural abundance of the Ca isotopes (feed solution) was introduced continuously from the top of the first column at a constant flow rate controlled with a Nihon Seimitsu Kagaku NP-KX-105U high-pressure pump, and it flowed through all the columns. The effluent from the bottom of the final (fifth) column was collected into small fractions and analyzed for Ca contents and Ca isotopic compositions. The experimental temperature was kept constant at 40, 25 or 5 °C by circulating thermostatted water through jackets surrounding the columns.
The experimental conditions are summarized in .
Table 1. Experimental conditions and results other than isotopic dataa.
2.3. Analyses
The Ca2+ ion concentrations in feed solutions and effluent fractions were measured after appropriate dilution by inductively coupled plasma atomic emission spectrometry (ICP-AES) with a SII SPS3520UV-DD ICP-atomic emission spectrometer. For the Ca isotope analysis, the chemical form of Ca was converted from CaBr2 to calcium iodide (CaI2) through anion exchange using the Muromac 1 × 8 resin (200–400 mesh, OH form) followed by the addition of hydroiodic acid [Citation18,Citation21]. Ca isotopic ratios (42Ca/40Ca, 43Ca/40Ca, 44Ca/40Ca and 48Ca/40Ca) of the feed solutions and the selected fractions of the effluent in the frontal part of the respective Ca adsorption zones were determined by thermal ionization mass spectrometry with a Finnigan MAT261 mass spectrometer [Citation18,Citation21]. A droplet of a CaI2 solution containing ca. 10 μg of Ca was placed on a vaporization filament in the thermo-ionization cartridge of the mass spectrometer. In a vacuum, the heating current for the vaporization filament and the ionization current for the ionization filament were gradually increased, and the measurement of the Ca isotopic ratios was initiated when the former and the latter currents became ca. 0.4 and 3 A, respectively. The relative standard deviations were typically 0.08%–0.21%, 0.13%–0.32%, 0.09%–0.18% and 0.13%–0.32% for the Ca isotopic pairs: xCa/40Ca, x = 42, 43, 44 and 48, respectively.
2.4. MO calculations
MO calculations of the reduced partition function ratios (RPFRs) of Ca species expected to be involved in the experiments were carried out to elucidate the Ca isotope effects observed in the present study in a qualitative fashion.
The Ca isotope exchange reaction that gives rise to the present Ca isotope effects may be expressed, for 42Ca/40Ca, 43Ca/40Ca, 44Ca/40Ca and 48Ca/40Ca isotopic pairs, as
(1)
(1) where Ca2+(sol) and Ca2+(crown) denote the Ca2+ ions in the aqueous solution and crown ether phases, respectively. The equilibrium constant Kx/40 (x = 42, 43, 44 or 48) of Equation (1) is given as the ratio of two RPFRs for the x/40 isotopic pair [Citation22]:
(2)
(2) where (s/s’)fx/40(sol) is the RPFR of the xCa/40Ca isotopic pair in the aqueous solution phase and (s/s’)fx/40(crown) is that in the crown ether phase.
The general expression for the RPFR is, under Born–Oppenheimer and harmonic oscillator approximation and the approximation that the rotations are classical, given as follows[Citation22]:
(3)
(3) where ui = hcωi/(kT) and u′i = hcω′i/(kT); p is the degree of freedom of molecular vibration; h is the Planck constant; c is the velocity of light; ωi and ω′i are the wave numbers of the ith molecular vibration of the heavier and the lighter isotopic species, respectively; k is the Boltzmann constant; and T is the absolute temperature [Citation22]. Equation (3) shows that the RPFR for the given species and for the given isotopic pair can be theoretically estimated by knowing all the isotopic vibrational frequencies of the species.
As model Ca species in aqueous solution, we considered a Ca2+ ion with the solvation number of six or seven, surrounded by water (H2O) molecules and additionally a Br− ion, like [Ca2+(H2O)6], [Ca2+(H2O)6](H2O), [Ca2+(H2O)7], [Ca2+(H2O)5Br−], [Ca2+(H2O)6Br−] and [Ca2+(H2O)7](Br−)(H2O). In those expressions, H2O molecules and a Br− ion in the brackets denote that they are in the primary solvation sphere of the Ca2+ ion, directly interacting with the Ca2+ ion, and the H2O molecule and the Br− ion outside the brackets are located in the secondary solvation sphere. As model Ca species in the crown ether phase, we considered clusters consisting of a B18C6 molecule, a Ca2+ ion and two Br− ions with or without up to two HBr molecules ([B18C6+Ca2++2Br−], [B18C6+Ca2++2Br−+HBr] and [B18C6+Ca2+ +2Br−+2HBr]) and some others in which H2O molecules were also included. Inclusion of HBr molecules in the crown ether phase was inferred from the results of our previous paper [Citation19] and from the results of Sr isotope fractionation in a solvent extraction system reported by Shibahara et al. [Citation23]. The existence of organic networks of the resin in the crown ether phase was ignored. The existence of silica beads was also ignored.
Calculations were made at the B3LYP/6-311G(d) level of theory using the Gaussian 09 program package (Gaussian Inc.) [Citation24]. The combination of the B3LYP method and the 6-311G(d) basis set seems one of the most standard ones in MO calculations nowadays. The Gauss View (Gaussian Inc.) and the Free Wheel programs (Butch Software Studio) were used for the graphics. All the geometry optimizations were conducted without symmetry constraints; for each of the structures considered, the bond lengths, bond angles and dihedral angles were varied independently to achieve the minimum-energy geometry, at which the vibrational analysis was carried out. The Ca isotopic RPFRs and equilibrium constants for the Ca isotope exchange reactions were then calculated by using the frequencies scaled by 0.966 given in [Citation25].
3. Results and discussion
3.1. Chromatograms and calcium ion adsorption characteristics of B18C6 resin
Seven chromatographic experiments in the breakthrough mode were carried out. The experimental conditions and results other than isotopic data are summarized in together with the one in the HCl system from our previous paper [Citation19] for comparison. The quantity V1/2 is the effluent volume at which the Ca2+ ion concentration becomes equal to a half of its value in the feed solution, c0, after the breakthrough point. The total Ca2+ ion adsorption capacity, Q, of the resin column in each experiment was calculated as follows:
(4)
(4) where V0 is the value of V1/2 in the case of no Ca2+ ion adsorption. The parameter, Q/Vr, is the adsorption capacity per unit volume of the resin column, where Vr is the resin column volume.
The chromatograms including the one from the previous paper [Citation19] are depicted in . The findings obtained from and may be summarized as follows:
Figure 1. Chromatograms of Runs CB-1 (▪), CB-2 (•), CB-3 (○), CB-4 (Δ), CB-5 (×), CB-6 (+), CB-7 (□) and Ca18-9 (▴).
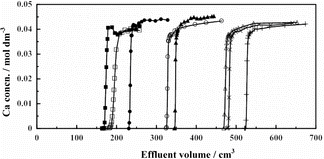
The quantity Q/Vr, and V1/2 and Q, too, increases with increasing HBr concentration in the feed solution for a constant Ca2+ ion concentration in the feed solution and for a constant resin column height at a constant temperature (Runs CB-1 (▪), CB-2 (•), CB-3 (○) and CB-4 (Δ) in ). This is reasonable since Ca2+ ions are considered to be adsorbed on the B18C6 resin as a neutral species, Ca2+ + 2Br−. The resin does not function as Ca2+ ion adsorbent at HBr concentration of zero (Run CB-1).
The quantity Q/Vr, and V1/2 and Q, too, slightly increases with decreasing temperature for a constant Ca2+ ion and HBr concentrations in the feed solution (Runs CB-4 (Δ), CB-5 (×) and CB-6 (+) in ). The adsorption of Ca2+ ion from aqueous HBr solution onto the resin is thus expected to be slightly exothermic.
Runs CB-4 (Δ in ) and Ca18-9 (▴) [Citation19] were carried out under similar experimental conditions except for the kind of acid, HBr or HCl. A comparison of the results of these two experiments reveals that the value of Q/Vr is much large in the HBr system than in the HCl system. This may be explained by the hard and soft acids and bases (HSAB) rule. The Br− ion is a softer base than the Cl− ion, which means the former ion interacts with the Ca2+ ion, a hard ion, less strongly than the latter ion. This property of Br− as a soft base interferes the interaction of Ca2+ with the crown ether less substantially than Cl−, which leads to a larger adsorption capacity of the B18C6 resin for the Ca2+ ion in the HBr system than in the HCl system. HBr is thus a preferable acid than HCl as far as the adsorption capacity of the B18C6 resin for the Ca2+ ion is concerned.
Use of sodium bromide instead of HBr substantially reduced the adsorption capacity of the resin for the Ca2+ ion (Runs CB-2 (•) and CB-7 (□) in ), which indicates that the presence of sodium (Na+) ions largely interferes the adsorption of Ca2+ ion by the resin. This is understandable since the Na+ ion with the diameter of 232 pm better fits the 260–320 pm hole (ring) formed by the six oxygen atoms of the B18C6 molecule than the Ca2+ ion with the diameter of 228 pm [Citation26].
3.2. Ca isotopic data
Runs CB-2, CB-3, CB-4 and CB-5 were analyzed for their Ca isotopes. The results are shown in –. In each of those figures, the chromatogram is drawn with a solid line using the scale on the right-hand-side vertical axis and the isotopic data are given with ○, •, Δ and ▴ marks using the scale on the left-hand-side vertical axis. The isotopic data are plotted as the ratio of the xCa/40Ca (x = 42 (▴), 43 (Δ), 44 (•) or 48 (○)) isotopic ratio of a fraction of the effluent, xrfraction, to the corresponding ratio in the feed solution, xrfeed. As seen in those figures, the xrfraction/xrfeed value of an isotopic pair, in general, decreases monotonously with the increasing effluent volume. It is generally the largest in the breakthrough-point fraction and asymptotically and promptly approaches unity with the increasing effluent volume. The xrfraction/xrfeed values larger than unity mean the heavier isotopes prefer remaining in the solution phase while the lighter ones are preferentially fractionated into the crown ether phase. In all of the experiments for which isotopic measurements were done, lighter isotopes of Ca are preferentially fractionated in the crown ether phase. The degree of the deviation of the xrfraction/xrfeed value from unity is larger for a larger value of x in a given experiment. That is, a larger mass difference between the isotopic pair results in a larger Ca isotope effect. These Ca isotope characteristics in the present HBr system are qualitatively consistent with those in the HCl system in our previous paper [Citation19].
Figure 2. Chromatogram and isotope accumulation curves of Run CB-2: 48Ca/40Ca (○), 44Ca/40Ca (•), 43Ca/40Ca (Δ) and 42Ca/40Ca (▴).
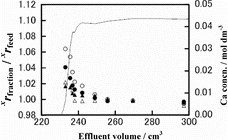
Figure 3. Chromatogram and isotope accumulation curves of Run CB-3: 48Ca/40Ca (○), 44Ca/40Ca (•), 43Ca/40Ca (Δ) and 42Ca/40Ca (▴).
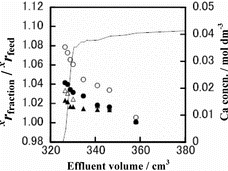
Figure 4. Chromatogram and isotope accumulation curves of Run CB-4: 48Ca/40Ca (○), 44Ca/40Ca (•), 43Ca/40Ca (Δ) and 42Ca/40Ca (▴).
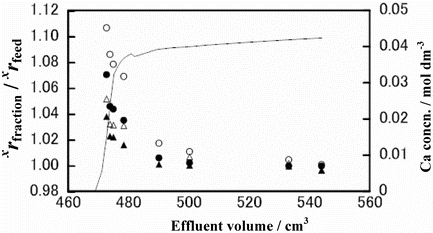
Figure 5. Chromatogram and isotope accumulation curves of Run CB-5: 48Ca/40Ca (○), 44Ca/40Ca (•), 43Ca/40Ca (Δ) and 42Ca/40Ca (▴).
To evaluate the magnitudes of the Ca isotope effects observed in the present study, the Ca isotopic fractionation coefficient, ϵ = S − 1, was calculated. The single-stage separation factor for the Ca isotopes, S, is defined as
(5)
(5) where (xCa/40Ca)solution and (xCa/40Ca)crown denote the xCa/40Ca (x = 42, 43, 44 or 48) isotopic ratios in the solution phase and in the crown ether phase, respectively, and ϵ can be calculated using the chromatographic experimental data as follows[Citation27]:
(6)
(6)
In Equation (6), assuming the two isotope systems, Ri and qi are the atomic fraction of the isotope of interest and the amount of Ca in the ith fraction of the effluent, respectively, R0 is the atomic fraction of the isotope of interest in the feed solution, Q is the amount of Ca taken up by the resin, and the summation is taken over all the fractions where isotope fractionation is observed. The values of ϵ are listed in together with the ϵ data for Run Ca18-9 from the previous paper [Citation19] for comparison.
Table 2. Fractionation coefficients obtained in this work.
The findings in and may be summarized as follows:
The heavier isotopes of Ca are preferentially fractionated into the solution phase, while the lighter ones into the crown ether phase. The direction of this Ca isotope fractionation is common to all the chromatographic systems so far reported [Citation3–8,Citation17–20].
As a whole, the values of ϵ on the order of 10−3 were obtained with the B18C6 resin; they are on the same order as those of the previous studies with the same resin [Citation17–20], and larger than those of the studies with ion exchange resins [Citation3,4] and with the B15C5 resin (benzo-15-crown-5 ether resin synthesized in the inner pores of highly porous silica beads [Citation11]) [Citation19].
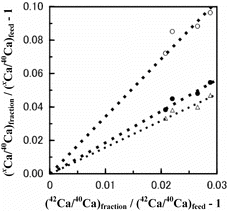
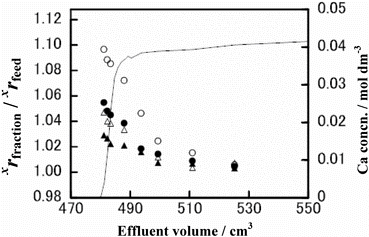
The ϵ value seems, admittedly roughly, nearly proportional to the isotopic mass difference, ΔM. In other words, the ϵ/ΔM value is nearly constant in a given run. This indicates that the present Ca isotope effects are mostly mass-dependent ones and are little affected by the nuclear size and shape effects (the nuclear field shift effects) [Citation28]. The data supporting the present mass-dependent Ca isotope effects can be provided by the so-called three isotope plots. An example of such plots (Run CB-5) is shown in where the value of the xrfraction/xrfeed ratio (x = 43, 44, 48) minus unity is plotted against the value of the 42rfraction/42rfeed ratio minus unity. The slope of the plots is calculated by the least-squares method to be 1.59, 1.89 and 3.45 for x = 43, 44 or 48, respectively. The corresponding theoretical values, estimated on the basis that the observed isotope effects arise solely from molecular vibration [Citation22], are 1.47, 1.91 and 3.50. The smallness of the deviation of the experimental slope from the theoretical one supports that the isotope effects in the present systems are normal mass-dependent ones. We note, however, that in some experiments of our present and previous papers [Citation19,20], the ϵ/ΔM value is found to be larger for the 43Ca/40Ca isotopic pair than for the other pairs beyond experimental uncertainties. For instance, the ϵ/ΔM values for the xCa/40Ca (x = 42, 43, 44 and 48) of Run CB-4 are 3.0, 8.0, 4.5 and 5.3 × 10−4. This may be a revelation of the nuclear field shift effects [Citation28].
A higher HBr concentration in the feed solution yields a smaller ϵ value (Runs CB-2, CB-3 and CB-4) at a given temperature. This trend is the same as that observed for the HCl system in the previous paper [Citation19]. A similar acid concentration dependence of the ϵ value was also observed in chromatographic Sr isotope fractionation experiments with the B15C5 resin in another previous paper of ours [Citation16].
The temperature dependence of the ϵ value seems normal (Runs CB-4 and CB-5); a higher temperature yielded a smaller ϵ value [Citation22].
Compared under similar experimental conditions, except for the kind of acid (Runs CB-2 and Ca18-9 [Citation19]), the present aqueous HBr medium yielded nearly equivalent results to those of the previous aqueous HCl medium as far as the Ca isotope effects are concerned. However, as mentioned in item (3) in Section 3.1 the adsorption capacity of the B18C6 resin for the Ca2+ ion is much larger with HBr than with HCl compared at the similar acid concentration. These results mean that chromatography operated in the aqueous HBr medium is of much higher performance than that in the aqueous HCl medium as a Ca isotope separation method.
3.3. MO Calculations
The major purpose of the present MO calculations is to obtain reasonable elucidation on the findings (1) and (4) in Section 3.2. For all the Ca species considered, no imaginary frequency was obtained, which means that the optimized structures are all at the global or local minimum of the potential energy surface.
To determine the hydration number of the aqueous Ca2+ ion, we first compared the electronic energies corrected for the zero point energy of the pair of the Ca species such as [Ca2+(H2O)6](H2O)2 and [Ca2+(H2O)7](H2O), [Ca2+(H2O)6](H2O)3 and [Ca2+(H2O)7](H2O)2, and so forth, at their optimized structures. As a result, we found that [Ca2+(H2O)6] and [Ca2+(H2O)7] are equally stable within computational uncertainties. We thus regarded both [Ca2+(H2O)6] and [Ca2+(H2O)7] as the simply hydrated Ca species in aqueous HBr solution. The conclusion that the hydration number of the Ca2+ ion is 6 or 7 is consistent with the range of 5–13 reported as hydration number of aqueous Ca2+ ion [Citation29], although the most probable number seems 8 [Citation30]. The present estimation slightly contradicts with our previous paper [Citation19] that reported that [Ca2+(H2O)6] was more stable than [Ca2+(H2O)7] at the B3LYP/6-31G(d) level of theory.
In Ca2+ ion-bearing aqueous HBr solution, part of Ca2+ ions is expected to form complex species with Br− ions:
(7)
(7) where (aq) indicates that these species are hydrated. Our MO calculations show that the structure of CaBr+(aq) is [Ca2+(H2O)6Br−], an inner-sphere complex in which the Br− ion directly interacts with the Ca2+ ion. Unfortunately, no paper that reports values of the stability constant of Equation (7) can be found. Instead, a value of the stability constant of Equation (8), i.e., the formation of the chloro-compex of the Ca2+ ion, is reported to be 1.2 at the ionic strength of 0.7 mol dm−3 at 25 °C [Citation31]:
(8)
(8)
Under the similar conditions, the value of the stability constant of Equation (7) is expected to be smaller than 1.2. If we apply this value to the present system with HBr concentrations of 9 mol dm−3 and Ca2+ ion concentration of 0.05 mol dm−3, the percentage of the complex species is calculated to be about 92%. Of course, this value cannot be directly applicable to the present chromatographic experiments. It must be true, however, that the existence of the Ca2+ ions in the complex form with the Br− ions in the present experiments cannot be negligible and its proportion is higher at a higher HBr concentration.
The optimized structures of [B18C6+ Ca2++2Br−], [B18C6+Ca2++2Br−+HBr] and [B18C6+Ca2++2Br−+2HBr], the model Ca species expected for the crown ether phase, are shown in (a)–(c), respectively. In each of these structures, the six oxygen atoms of the B18C6 molecule and the Ca2+ ion are nearly coplanar; the size of the Ca2+ ion fits that of the hole (ring) formed by the six oxygen atoms [Citation26]. In [B18C6+Ca2++2Br−], one of the two Br− ions is bonded to the Ca2+ ion from one side of the plane and another Br− ion from the other side of the plane. In [B18C6+Ca2++2Br−+HBr], the HBr molecule interacts with a Br− ion through its hydrogen atom. In [B18C6+Ca2++2Br−+2HBr], each of the two HBr molecules interacts with each of the two Br− ions. Although no experimental data exist, it seems reasonable to consider that the proportion of [B18C6+Ca2++2Br−+HBr] and [B18C6+ Ca2++2Br−+2HBr] increases and that of [B18C6+ Ca2++2Br−] decreases with increasing HBr concentration.
Figure 7. The optimized structures of (a) [B18C6+Ca2++2Br−], (b) [B18C6+Ca2++2Br−+HBr] and (c) [B18C6+Ca2++2Br−+2HBr].
![Figure 7. The optimized structures of (a) [B18C6+Ca2++2Br−], (b) [B18C6+Ca2++2Br−+HBr] and (c) [B18C6+Ca2++2Br−+2HBr].](/cms/asset/31c43a60-c495-40ce-b0c9-42bdc3b35c51/tnst_a_969348_f0007_oc.jpg)
The values of RPFRs for the 48Ca/40Ca isotopic pair of some of the considered Ca species at 40 °C are summarized in . Our present estimate of the RPFRs for the aqueous Ca2+ ion is reasonable compared with those of previous works [Citation19,Citation32]. First of all, we notice that the RPFR values for the species in the solution phase are larger than those for the species in the crown ether phase. This means that the sum of the forces acting on the Ca2+ ion in the solution phase is larger than those in the crown ether phase, and as a consequence, the lighter Ca isotopes are preferentially fractionated into the crown ether phase. The MO results are thus consistent with the results of the present chromatographic Ca isotope fractionation experiments (Equation (1)).
Table 3. The values of the reduced partition function ratios for the 48Ca/40Ca isotopic pair at 40 °C.
The RPFR value of [Ca2+(H2O)6] (and that of [Ca2+(H2O)7] too) is larger than that of [Ca2+(H2O)6Br−]; the difference is in the order of 10−3. This indicates that the RPFR value of the solution phase decreases with increasing HBr concentration since the proportion of [Ca2+(H2O)6Br−] is expected to be higher at a higher HBr concentration. The decreasing order of the RPFR value of the Ca species in the crown ether phase is as follows: [B18C6+Ca2++2Br−] > [B18C6+Ca2++2Br−+HBr] > [B18C6+Ca2++2Br−+2HBr]; the stepwise inclusion of HBr molecules gradually reduces the RPFR value. The difference between the neighboring values is in the order of 10−4. This indicates that, like the RPFR value of the solution phase, the RPFR value of the crown ether phase also decreases with increasing HBr concentration. However, the degree of decrease is expected to be larger in the solution phase (in the order of 10−3) than in the crown ether phase (in the order of 10−4); the dependence of the RPFR value on the HBr concentration is more drastic in the solution phase than in the crown ether phase. Thus, the present MO calculations indicate that the ϵ value decreases with increasing HBr concentration, which is in qualitative agreement with the experiments (Equation (4)).
4. Conclusion
The following statements summarize our present study:
Heavier isotopes of Ca were enriched in the frontal part of the respective chromatograms obtained in the breakthrough-mode chromatographic experiments in which the benzo-18-crown-6 ether resin functioned as the absorbent of Ca2+ ions. This direction of the Ca isotope fractionation was consistent with the results of MO calculations on the RPFRs of the Ca species assumed to exist in the solution and crown ether phases of the present chromatographic experiments. The assumed Ca species were a simply hydrated Ca2+ ion and an aqueous Ca2+ ion directly interacting with a Br− ion for the solution phase, and those for the crown ether phase were a Ca2+ ion interacting with a benzo-18-crown-6 ether molecule and two Br− ions accompanied by zero, one or two HBr molecules.
The observed Ca isotope effects were probably mass-dependent; the values of the fractionation coefficients per unit mass difference between two Ca isotopes were nearly constant, albeit sometimes with large experimental uncertainties, and the values of the slopes of the three isotope plot were close to the theoretical values that were inferred from the isotope effects based solely on the molecular vibration.
A higher HBr concentration in the feed solution resulted in a smaller value of the Ca isotopic fractionation coefficient. A support for this was also provided by the results of the MO calculations in a qualitative fashion.
On comparison under similar experimental conditions, the present HBr system gave Ca isotope effects, the magnitude of which is nearly equal to that of the HCl system [Citation19]. However, the former had much larger adsorption capacity for Ca2+ ions than the latter. These results showed that chromatography operated in aqueous HBr medium is a better system of Ca isotope separation than that operated in aqueous HCl medium.
Acknowledgements
The authors acknowledge Dr M. Nomura, Tokyo Institute of Technology, for his assistance in mass spectrometric measurements of Ca isotopic ratios.
References
- Kishimoto T, Ogawa I, Umehara S, Hirano Y. Double beta decay and CANDLES test. Genshikaku Kenkyu 2007;51:36–48. Japanese.
- Reeve J, Arlot M, Bernat M, Charhon S, Edouard C, Slovik D, Vismans FJFE, Meunier PJ. Calcium-47 kinetic measurements of bone turnover compared to bone histomorphometry in osteoporosis: the influence of human parathyroid fragment (hPTH 1-34) therapy. Metab Bone Dis Rel Res. 1981;3:23–30.
- Oi T, Morioka N, Ogino H, Kakihana H, Hosoe M. Fractionation of calcium isotopes in cation-exchange chromatography. Sep Sci Technol. 1993;28:1971–1983.
- Jepson BE, Shochey GC. Calcium hydroxide isotope effects in calcium isotope enrichment by ion exchange. Sep Sci Technol. 1984;19:173–181.
- Heumann KG, Schiefer H. Calcium isotope separation on an exchange resin having cryptand anchor groups.Angew Chem Int Ed Engl. 1980;19:406–407.
- Heumann KG, Schiefer HP, Spiess W. Enrichment of heavy calcium isotopes by ion exchange resins with cyclopolyethers as anchor groups. In: Schmidt HL, Förstel H, Heinzinger K, editors. Stable isotopes. Amsterdam: Elsevier; 1982. p. 711–718.
- Jepson BE, Evans WF. Chromatographic separation of calcium isotopes with polymer-bound ligands. Sep Sci Technol. 1990;25:1893–1908.
- Jepson BE. Calcium isotope separation by chemical exchange with polymer-bound crown compound. Bull Res Lab Nucl Reactors. 1:307–312. Special Issue.
- Kim DW, Jeon YS, Eom TY, Suh MY, Lee CH. Lithium isotope separation on a monobenzo-15-crown-5 resin. J Radioanal Nucl Chem. 1991;150:417–426.
- Ban Y, Nomura M, Fujii Y. Chromatographic separation of lithium isotopes with cilica based monobenzo-15-crown-5 resin. J Nucl Sci Technol. 2002;39:279–281.
- Otake K, Suzuki T, Kim HJ, Nomura M, Fujii Y. Novel syntheses of phenol type benzo-15-crown-5 ether resin and its application for lithium isotope separation. J Nucl Sci Technol. 2006;43:419–422.
- Ding X, Kim HJ, Nomura M, Suzuki T, Sugiyama Y, Fujii Y. Zinc isotope separation by phenol formaldehyde type 15-crown-5 resin in organic solvents. J Nucl Sci Technol. 2006;43:411–414.
- Zhang YH, Fukuda Y, Nomura N, Fujii Y, Oi T. Zinc isotope effects observed by liquid chromatography with benzo-15-crown-5 resin. J Nucl Sci Technol. 2006;43:415–418.
- Fukuda Y, Zhang YH, Suzuki T, Fujii Y, Oi T. Zinc isotope accumulation in liquid chromatography with crown ether resin. J Nucl Sci Technol. 2006;43:446–449.
- Ding X, Nomura M, Suzuki T, Sugiyama Y, Kaneshiki T, Fujii Y. Chromatographic zinc isotope separation by phenol formaldehyde benzo crown resin. J Chromatogr A. 2006;1113:182–185.
- Fukuda Y, Zhang YH, Nomura M, Suzuki T, Fujii Y, Oi T. Strontium isotope effects observed in liquid chromatography with crown ether resins. J Nucl Sci Technol. 2010;47:176–183.
- Hayasaka K, Kaneshiki T, Nomura M, Suzuki T, Fujii Y. Calcium ion selectivity and isotope effects studied by using benzo-18-crown-6 resins. Prog Nucl Energy. 2008;50:510–513.
- Fujii Y, Nomura M, Kaneshiki T, Sakuma Y, Suzuki T, Umehara S, Kishimoto T. Mass dependence of calcium isotope fractionations in crown-ether resin chromatography. Isot Environ Health Stud. 2010;46:233–241.
- Nemoto S, Suga K, Fukuda Y, Nomura M, Suzuki T, Oi T. Calcium isotope fractionation in liquid chromatography with crown ether resins. J Nucl Sci Technol. 2012;49:425–437.
- Nemoto S, Nomura M, Oi T. Calcium isotope fractionation in liquid chromatography with crown ether resin in methanol medium. Isot Environ Health Stud. 2013;49:257–268.
- Saito S, Yanase S, Oi T. Calcium isotope effects accompanying electrochemical insertion of calcium into graphite from electrolyte solution. Radioisotopes. 2011;60:265–274.
- Bigeleisen J, Mayer MG. Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys. 1947;15:261–267.
- Shibahara Y, Takaishi H, Nishizawa K, Fujii T. Strontium isotope effects in liquid exchange reaction. J Nucl Sci Technol. 2002;39:451–456.
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai N, Vreven T, Montgomery, JAJr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian 09, Revision B.01, Wallingford (CT): Gaussian, Inc.; 2010.
- http://cccbdb.nist.gov/vibscalejust.asp
- Greenwood NN, Earnshaw A. Chemistry of the elements. Oxford: Pergamon Press; 1984. p. 107. ISBN 0-08-022056-8.
- Kakihana H, Kanzaki T. A simplified and generalized method for analyzing chromatographic isotope separation data. Bull Tokyo Inst Technol. 1969;90:77–89.
- Bigeleisen J. Nuclear size and shape effects in chemical reactions. Isotope chemistry of the heavy elements. J Am Chem Soc. 1996;118:3676–3680.
- Zavitsas AA. Aqueous solutions of calcium ions: hydration numbers and the effect of temperature. J Phys Chem B 2005;109:20636–20640.
- Jalilehvand F, Spångberg, D, Lindqvist-Reis P, Hermansson K, Persson I, Sandström M. Hydration of the calcium ion. An EXAFS, large-angle X-ray scattering, and molecular dynamics simulation study. J Am Chem Soc. 2001;123:431–441.
- Martell A, Sillen LG. Stability constants of metal-ion complexes. London: The Chemical Society; 1964. p. 418.
- Colla CA, Wimpenny J, Yin Q, Rustad JR, Casey WH. Calcium-isotope fractionation between solution and solids with six, seven or eight oxygens bound to Ca(II). Geochim Cosmochim Acta 2013;121:363–373.