?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper presents a new technique for synthesizing silver nanoparticles immobilized on textile fabrics using a radiochemical process. In this process, the irradiation of a high-energy electron beam on an aqueous solution containing silver ions induces a reducing reaction that forms metallic silver nanoparticles. Small Ag particles of about 2–4 nm were observed together with relatively large particles of more than 10 nm. These nanoparticles are firmly immobilized on the surface of a support textile fabric without the need for any binder or surfactant. The amount of silver nanoparticles immobilized was found to depend on the water content of the support textile fabric, suggesting that the silver ions are reduced not only by radiochemical species generated by the radiolysis of water, but also by radiochemical species generated in the irradiated support fabric itself. The silver nanoparticles that were immobilized on the support textile fabric exhibited an excellent antibacterial activity across a wide antibacterial spectrum, even after a durability test involving washing the fabric 100 times.
1. Introduction
Textile fabrics with antibacterial properties are widely used in various products in the fields of medical care, healthcare, food sanitation, everyday clothing, and so on [Citation1]. With conventional techniques, the antibacterial agents are attached to the fiber surface by means of a chemical binder or embedding. In the case of the former, the durability of the antibacterial property is often not dependent on the antibacterial agent, but rather on the performance of the binder. With the embedding process, antibacterial agents are mixed in during the synthesis of fibers; however, as antibacterial agents embedded inside the fiber matrix have no influence on bacteria, an excess amount must be used to obtain sufficient activity and durability. This excess amount of antibacterial agent can sometimes cause a reduction in the tensile properties, softness, abrasion resistance, appearance, and other practical properties of textiles, and can even change its color. The development of new technique for immobilizing antibacterial agents onto textile fabrics therefore presents a significant research challenge.
Of the various kinds of antibacterial agents available, metallic silver nanoparticles have received significant attention because of their excellent antibacterial properties [Citation2–4]. In this paper, we report on the direct immobilization of metallic silver nanoparticles onto textile fabrics using a radiochemical process in which noble metal ions are reduced by the radioactive species generated by water radiolysis to form metallic nanoparticles [Citation5]. This procedure was originally developed for the synthesis of composite nanoparticles, but it was subsequently found that the noble metal nanoparticles generated are firmly immobilized on the surface of support materials to minimize their surface energy [Citation6]. This led to various composite nanoparticles such as Au/iron-oxide and Pt/C being successfully synthesized by this radiochemical process [Citation6,Citation7]. In the present system, however, textile fabrics made of natural or synthetic fibers were used as a support material for stabilizing Ag nanoparticles. The resulting immobilized Ag nanoparticles were characterized by transmission electron microscope (TEM), inductively coupled plasma-atomic emission spectrometry (ICP-AES), and X-ray absorption spectroscopy (XAS), and their antibacterial properties and durability against washing are discussed herein.
2. Experimental procedure
2.1. Materials
The fiber materials used in this study were commercial textile fabrics of cotton, rayon, polyester, polyamide (nylon 66), acryl, polypropylene, and microfiber (polyester, 80%; polyamide, 20%). All fabrics were prepared for use by washing with tap water for over 90 min in a washing machine (PS-60AS, Hitachi Ltd.), and were then completely dried in a drier (DE-N55FX, Hitachi Ltd.). Chemicals reagents in the form of AgNO3, Ag metal, AgCl, Ag2O, AgO, and HNO3 were purchased from Wako Pure Chemical Industries Ltd., and pure water was obtained using a Millipore Direct-Q system.
2.2. Radiochemical synthesis of silver nanoparticles onto textile fabrics
To provide a silver source, aqueous solutions were prepared by dissolving AgNO3 powder in pure water at concentrations ranging from 0.5 to 10 mM. Samples of the textile fabrics (20–30 g) were then immersed in 500 mL of AgNO3 solution for 15 min, during which time a vacuum was applied by a vacuum desiccator to ensure that the AgNO3 solution could spread into the pores between fibers. Following immersion, any excess solution was removed by centrifugal dewatering (PS-60AS, Hitachi Ltd) for 7 min. The resulting AgNO3-soaked textile fabrics were then sealed in a plastic bag to avoid solvent evaporation. They were kept in a light-proof container to avoid photoreduction. The amount of AgNO3 solution contained in each textile fabric sample was determined by the following EquationEquation (1)(1)
(1) :
(1)
(1)
Without removing the AgNO3-soaked textile fabrics from their sealed plastic bags, they were subsequently irradiated with a high-energy electron beam at room temperature. Electron beam irradiation was carried out at the Japan Electron Beam Irradiation Service using a 4.8 MeV beam to deliver a controlled surface dose of 40 kGy. Dose rate was approximately calculated as 3 kGy/sec. Following irradiation, the textile fabrics were washed with pure water for 15 min, dewatered for 7 min, and then dried for 2 h.
2.3. Durability against washing
The durability of each of the Ag nanoparticle-modified textile fibers against washing was evaluated according to the method prescribed in JIS L 0217, which can be described as follows:
| (1) | Washing process | ||||
The washing solution was prepared by dissolving synthetic detergents in tap water (40 mL/30 L) at a controlled temperature of 40 °C. The textile fabric samples were added to this washing solution at a bath ratio of 1/30 (1 kg/30 L), and were then washed for 5 min in a washing machine.
| (2) | Rinsing process | ||||
Following centrifugal dewatering, the textile fabric samples were rinsed with tap water at room temperature for 2 min in a washing machine. This process was carried out twice.
| (3) | Drying process | ||||
The rinsed textile fabric samples were dried at room temperature in the absence of light.
Each textile type was washed 1, 2, 5, 10, 20, 50, or 100 times prior to characterization.
2.4. Material characterization
Observation of Ag nanoparticles immobilized on the textile fabrics was performed by TEM (H-8100, Hitachi Ltd.) using an acceleration voltage of 200 kV. For this, the Ag nanoparticle-modified textile fabric samples were first sonicated in ethanol for more than 30 min to obtain a small fiber fraction, which was then dropped onto a carbon-coated grid.
The amount of Ag nanoparticles immobilized on each textile fabric was analyzed by dissolving them in HNO3 solution (ca. 10%). The amount of Ag ions adsorbed onto the textile fabrics prior to irradiation was also analyzed by measuring the difference in AgNO3 solution concentration before and after their immersion using an ICP-AES (ICPS7500, Shimadzu Corp.).
The chemical state of Ag on the textile fabrics was investigated by comparing the X-ray absorption near edge structure (XANES) spectra around the Ag-K edge of each sample with those of a suitable reference material (Ag metal, AgNO3, AgCl, Ag2O, and AgO). The XANES spectra were measured at the beam lines PF-AR NW10A of the Photon Factory (Tsukuba, Japan) and BL01B1 of the SPring-8 (Hyogo, Japan). All samples were measured using the fluorescence method, wherein the intensities of the incident X-rays were measured in ion chambers filled with Ar gas, and fluorescence X-rays were measured by a solid state detector. The X-ray beam itself was focused using a Si(311) crystal monochromator.
2.5. Antibacterial tests
Antibacterial tests were performed in essentially the same manner outlined in ISO 20743. The detailed procedure is as follows:
Textile fabric samples (0.4 g) were sterilized by autoclaving in darkness, and were then dried on a clean bench for 60 min.
Test bacteria suspensions (0.2 mL, 1–3 × 105 CFU/mL) were dropped onto each sample fabric, which were then sealed in individual glass vials.
Samples were incubated at 37 °C for 0–8 h.
After incubation, 20 mL of soybean-casein digest broth with lecithin & polysorbate (SCDLP) medium was added to each vial and the bacteria were shaken out by vortex mixing.
The washed out suspensions were smeared on agar plates and incubated at 37 °C for 24 h.
The number of viable bacterial colonies on each textile fabric sample was determined by the plate count method.
The antibacterial data are represented here as an antibacterial activity value, S, which was calculated as per the following equation:
(2)
(2) where Ma is the average common logarithm for the number of bacterial colonies obtained from three test samples of control fabric immediately after inoculation, Mb is the average common logarithm for the number of bacterial colonies obtained from three test samples of control fabric after 18 h incubation, Mc is the average common logarithm for the number of bacterial colonies from three antibacterial-treated test samples after 18 h incubation, and M0 is the average common logarithm for the number of bacterial colonies from three antibacterial-treated test samples immediately after inoculation.
3. Results
3.1. Characterization of Ag nanoparticles on textile fabrics
After irradiation, those textile fabrics with a high Ag nanoparticle loading became notably yellow in color, which was ascribed to the surface plasmon resonance of metallic Ag nanoparticles. The strength of this color increased with AgNO3 concentration, and at low Ag nanoparticle loadings, the original color of the textile fabric was retained. Handling of the support textile fabrics did not change by the irradiation.
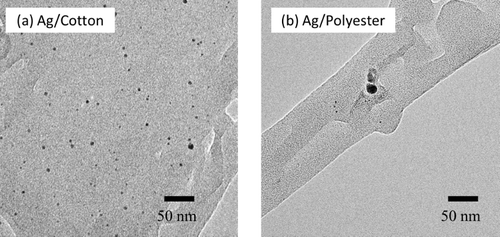
shows a typical TEM image of Ag nanoparticles synthesized from an AgNO3 concentration of 2.0 mM and immobilized on support textile fabrics of cotton and polyester. This reveals small Ag particles of about 2–4 nm together with relatively large particles of more than 10 nm in size. Note that a relatively greater number of Ag nanoparticles were observed with the Ag/cotton sample than with Ag/polyester. Irradiation of AgNO3 solution without support textile fabrics resulted in the formation of agglomerated Ag grains and colloidal Ag solution was not obtained.
Figure 1. TEM images of Ag nanoparticles on support textile fabrics of (a) cotton and (b) polyester. The Ag nanoparticles were synthesized using an Ag concentration of 2.0 mM.
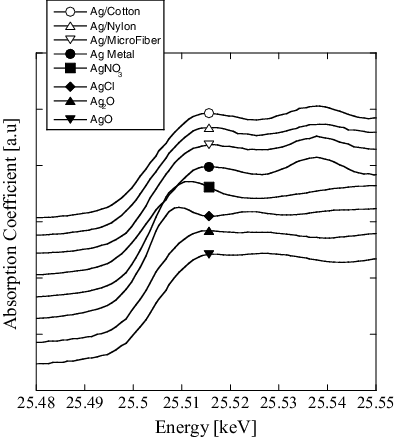
The XANES spectra of Ag immobilized from a 2.0 mM AgNO3 solution onto textile fabrics of cotton, polyamide, and microfiber are given in . This shows that, with all samples, Ag exists on the fibers mainly in a metallic state.
Figure 2. XANES spectra of the Ag immobilized on support fibers and standard samples. The Ag nanoparticles were prepared using an AgNO3 concentration of 2.0 mM.
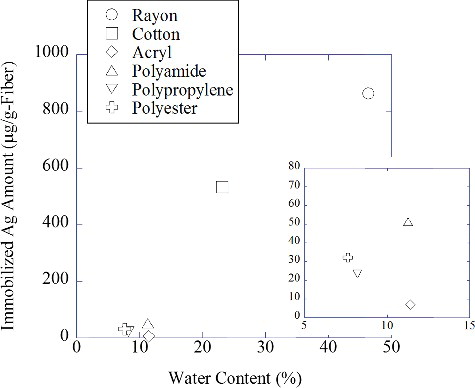
shows the amount of Ag nanoparticles immobilized from a 2.0 mM AgNO3 solution on textile fabrics in relation to the water content of each irradiated fabric (see EquationEquation (1)(1)
(1) ). Each irradiated textile fabric was washed once with tap water to remove any remaining Ag ions or free Ag nanoparticles. It can be clearly seen from this that hydrophilic fibers like cotton and rayon immobilize Ag nanoparticles quite well, whereas hydrophilic fibers such as polyamide, polyester, polyamide, acryl, and polypropylene do not. This indicates that the amount of Ag nanoparticles immobilized on a fiber surface depends on the amount of AgNO3 solution it contains.
Figure 3. Relationship between the amount of immobilized Ag nanoparticles and the water content of textile fabrics.
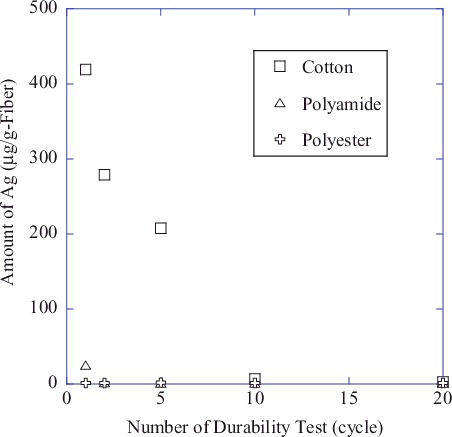
The amount of Ag nanoparticles retained after a given number of washing cycles is plotted in for support textile fabrics of cotton, polyamide, and polyester. This indicates that the amount of Ag nanoparticles immobilized onto textile fabrics decreases significantly to below detection limits (about 1 µg Ag/g fiber) after 20 washes in the case of cotton, and with just three washes with polyamide and polyester. The existence of Ag on the textile fabrics after this durability testing was qualitatively confirmed by XANES measurement, revealing a slight edge jump with the Ag/cotton sample even after 100 washing cycles (data not shown).
3.2. Antibacterial activity of Ag nanoparticles on textile fabrics
To investigate the antibacterial spectrum of Ag nanoparticles, Staphylococcus aureus, Klebsiella pneumonia, MRSA (methicillin-resistant S. aureus), Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Vibrio parahaemolyticus, and Bacillus cereus were used for antibacterial testing of an Ag/microfiber test sample prepared with an Ag ion concentration of 0.5 mM using electron beam irradiation of 40 kGy. It was confirmed that the support microfibers themselves have no antibacterial activity, with showing the antibacterial activity of the Ag/microfiber obtained for each bacterial strain. It can be clearly observed that the Ag nanoparticles offer an excellent antibacterial activity and broad antibacterial spectrum, with the viable bacteria count drastically decreasing to below or around detection limit except in the case of the spore-forming bacterium B. cereus.
Table 1. Antibacterial activity of Ag nanoparticles immobilized on textile microfiber fabric from an Ag ion concentration of 0.5 mM. All antibacterial tests were performed in accordance with ISO 20743.
An excellent antibacterial activity was observed even after durability test of 100 times washing, suggesting that the few Ag nanoparticles remaining on the textile fabrics (less than 1 μg/g fiber) still provide a strong antibacterial effect. This differs from the Ag nanoparticle-immobilized textile fabrics prepared by Lee et al. [Citation8] using a simple padding process, as their antibacterial activity significantly decreased after washing 20 times. Thus, the immobilization of Ag nanoparticles by radiochemical means provides much greater durability against washing.
The antibacterial activity and durability against washing process was examined by looking at the change in the viable bacteria count during antibacterial testing using an Ag/microfiber prepared from a 0.5 mM Ag ion solution, and S. aureus, E. coli O-157, and E. coli O-111 as model bacteria. The antibacterial test condition was compliant with ISO 20743. shows the viable bacteria plotted against the incubation time, revealing the number of bacteria in the control sample (textile fabric without Ag) to increase slightly over time. In contrast, with Ag/microfibers, the viable bacteria count drastically decreased to below detection limits within 120 min in the case of S. aureus and 30–40 min with the two strains of E. coli. Following durability testing, the antibacterial activity was slightly suppressed, which is most likely due to a decrease in the number of Ag nanoparticles on the textile fabric.
Figure 5. Number of viable bacterial colonies versus antibacterial test time for: (a) Staphylococcus aureus, (b) E. coli O-157, and (c) E. coli O-111. An Ag/microfiber synthesized using an Ag ion concentration of 0.5 mM was used as the test fabric.
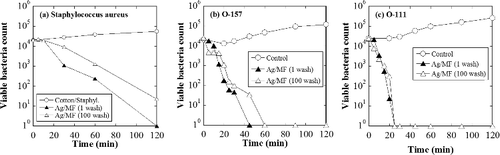
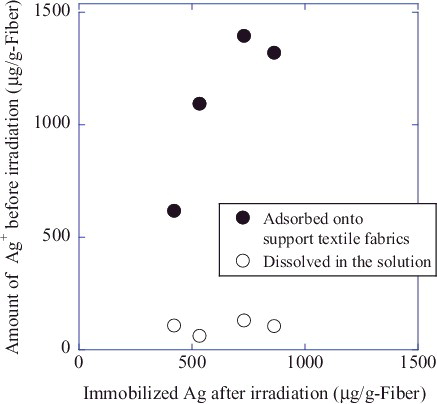
Figure 6. Relationship between the amount of adsorbed source Ag ions and Ag nanoparticles immobilized on cotton textile support fabric.
shows the antibacterial properties of cotton, polyamide, polyester, and microfiber textile support fabrics. All samples were prepared from an Ag concentration of 0.5 mM using electron beam irradiation of 40 kGy, and antibacterial testing was performed following ISO 20743 using S. aureus as a model bacterium. In all cases, the Ag-immobilized textile fabrics demonstrated excellent antibacterial properties, even after 100 washes, which indicates that the present technique is applicable regardless of the kind of fabric used.
Table 2. Antibacterial properties of Ag nanoparticles synthesized from an AgNO3 concentration of 0.5 mM and supported on various textile fabrics. All antibacterial tests were performed in accordance with ISO 20743 using Staphylococcus aureus.
Safety of the Ag nanoparticles were checked by a skin irritation study. Microfiber textile fabrics modified with Ag nanoparticles (Ag concentration: 0.5 mM) were used as test sample, and no dermal irritancy was observed (20 people, skin patch for 48 h).
4. Discussion
4.1. Immobilization of Ag nanoparticles onto textile fabrics
To better understand the process by which Ag nanoparticles are immobilized, the adsorption of source Ag ions onto cotton was investigated by controlling the concentration of AgNO3 from 0.5 to 2.0 mM. It was observed that before irradiation, the source Ag ions existed on the support textile fabric mainly as adsorbents; through comparison of the amount of adsorbed Ag ions and immobilized Ag nanoparticles, it was revealed that Ag nanoparticles are mainly formed from adsorbed Ag ions rather than Ag ions in solution.
The radiochemical processes for the reduction of Ag ions in an aqueous solution system has been previously described as follows [Citation5]:
(3)
(3)
(4)
(4)
(5)
(5)
Note that EquationEquation (3)(3)
(3) represents water radiolysis [Citation8], and that the hydrogen atoms and hydrated electrons generated are strong reductants capable of reducing Ag+ to metallic Ag0. In the presence of organic molecules, any organic radicals generated can also contribute to the reduction process. These in situ-generated reductants transform Ag ions to metallic Ag seeds, with the coalescence of these Ag seeds forming Ag metal nanoparticles [Citation5]. In the synthesis of colloidal Ag nanoparticles, addition of surface-stabilizing agent is necessary to keep their surface stable and avoid agglomeration. In the present system, Ag particles of 2–4 nm were observed along with particles of more than 10 nm in size, even in the absence of a surface-stabilizing agent. The Ag nanoparticles that are generated are therefore considered to be immobilized on the support textile fabric surface as a way of minimizing their surface energy. Contribution of functional groups in each textile fabrics should also be considered for further discussion.
To consider the reduction process of the present experimental system in more detail, the number of hydrated electrons and hydrogen radicals generated in the aqueous solution were calculated using the G-values of each species [Citation9]. To provide a representative example, Ag/cotton synthesized with various AgNO3 concentrations was chosen and the calculated results are presented in along with the amount of source Ag+ and immobilized Ag0. Note that the water content shows little fluctuation from a value of about 25%, which means that the amount of reducing species (hydrated electrons hydrogen radicals) generated in the solution remains consistent at about 5 μmol. With AgNO3 concentrations of 5.0 mM and 10.0 mM, however, the number of reduced Ag atoms is greater than the number of reducing species generated in the solution. This implies that the radiochemical species generated in the irradiated support textile fabrics also reduce adsorbed Ag ions to form metallic Ag nanoparticles.
Table 3. Comparison of supported metallic Ag atoms and reducing species generated by water radiolysis (textile fabric; cotton).
4.2. Antibacterial activity of Ag nanoparticles immobilized on textile fabrics
The antibacterial activity of Ag nanoparticles has been reported in various research papers, with the major pathway believed to be the dissolution of Ag ions that work as a molecular toxicant. Consequently, antibacterial activity tends to depend on the size, shape, and/or type of surface coating of the Ag nanoparticles [Citation2,Citation3]. Yang et al. reported that the threshold lethal dose (i.e., the minimum observed dose causing 100% lethality within 24 h) of 8 or 38 nm polyvinylpyrrolidone (PVP)-coated Ag nanoparticles against E. coli was 1 μM, a value that corresponds to the 20 μg/g fiber of immobilized Ag in the present system if we consider all 0.4 g of Ag nanoparticles to be in contact with a 0.2 mL bacterium solution [Citation10]. However, with the present system, antibacterial activity was still observed for Ag amounts of less than 1 μg/g fiber. It is therefore considered that Ag nanoparticles radiochemically immobilized onto textile fabrics have a greater bare surface due to the fact that no surfactants or polymers are applied in the synthesis process, which would be expected to result in a much higher antibacterial activity. Nevertheless, further study is still needed to ascertain the precise mechanism behind the antibacterial activity of the present system.
5. Conclusion
The radiochemical reduction of Ag ions has been demonstrated to lead to the immobilization of Ag nanoparticles on support textile fabrics, with Ag particles 2–4 nm stabilized without any surface stabilizing agent. XANES analysis revealed that these Ag nanoparticles exist in a metallic state, with the number of immobilized Ag nanoparticles depending on the water content of the textile fabric used and the amount of adsorbed Ag ions. Textile fabrics with immobilized Ag nanoparticles demonstrated high antibacterial activity and durability against washing regardless of the specific fabric used, and thus this radiochemical process is expected to provide a valuable new technique for producing antibacterial textile fabrics.
Acknowledgement
This work was partially supported by the Japan Science and Technology Agency. The authors also wish to thank the staff of Japan Electron Beam Irradiation Service Co. Ltd. for their assistance in the electron beam irradiation experiment. The XAFS experiments were performed at the BL01B1 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (Proposal No. 2011B1343) and at the NW10A of PF-AR with the approval of the High Energy Accelerator Research Organization (Proposal No. 2012G088). The authors particularly acknowledge Mr K. Ujiie, Mr A. Kadobayashi, Mr M. Ide, and Mr H. Ueda (Osaka University, Japan) for their contributions to the initial research idea.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B. 2010;79:5–18.
- Xiu ZM, Zhang QB, Puppala HL, et al. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12:4271–4275.
- Liu J, Sonshine DA, Shervani S, et al. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–6913.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83.
- Belloni J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: application to catalysis. Catal Today. 2006;113:141–156.
- Seino S, Kinoshita T, Nakagawa T, et al. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J Nanoparticle Res. 2007;10:1071–1076.
- Yamamoto TA, Nakagawa T, Seino S, et al. Bimetallic nanoparticles of PtM (M = Au, Cu, Ni) supported on iron oxide: radiolytic synthesis and CO oxidation catalysis. Appl Catal A. 2010;387:195–202.
- Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38:2199–2204.
- Woods R, Pilaev AK. Applied radiation chemistry. New York (NY): Wiley; 1994.
- Yang X, Gondikas AP, Marinakos SM, et al. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in caenorhabditis elegans. Environ Sci Technol. 2012;46:1119–1127.