?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This paper presents a cartridge filter incorporating a nonwoven fabric impregnated with Prussian blue copper analog (Cu-CF) to effectively concentrate and quantify radiocesium dissolved in seawater. The recovery ratio of the Cu-CF was >95% in laboratory experiments at a flow rate of 0.5 L min−1 through the filter and >93% in field experiments. Test measurements of 137Cs concentrations in seawater using Cu-CF agreed with the results obtained by using a conventional coprecipitation method that employed ammonium phosphomolybdate (within the counting error of the detector). The proposed method can shorten the preconcentration and pretreatment times for radiocesium, to just 40 min in 20 L of seawater, which is much faster than the ∼1 week required by traditional ammonium phosphomolybdate methods.
1. Introduction
The Fukushima Daiichi Nuclear Power Plant accident, which occurred as a result of the 2011 Tohoku earthquake and tsunami, caused release of a large quantity of radioactive substances into the atmosphere [Citation1] and radioactively contaminated water to leak into the environment. These radioactive substances dispersed over a wide area of land and sea centered on Fukushima Prefecture, Japan. Contamination of seawater, marine sediment, and seafood by radioactive substances has been confirmed; fisheries have been greatly affected [Citation2–4]. Taking into account this situation, there is a strong need for a means of understanding the concentrations and distributions of radioactive substances in seawater.
The method most frequently used by the Ministry of the Environment for measuring radiocesium concentration in seawater after the accident had a minimum determination limit of 1 Bq L−1 by direct measurement using 2-L Marinelli container; in the three years since the accident, measured values at many survey locations have become lower than this determination limit [Citation5]. There are several examples of monitoring of low concentrations of radiocesium in seawater, namely, those of Tokyo Electric Power Company (TEPCO) [Citation6], the Nuclear Regulation Authority [Citation7], and Aoyama et al. [Citation8]. TEPCO surveyed six locations in the vicinity of the Fukushima Daiichi Nuclear Power Plant and reported 137Cs concentrations of 3–260 mBq L−1 (as of 14 November 2014) [Citation6]. The Nuclear Regulation Authority survey found 137Cs concentrations of 0.62–5.3 mBq L−1 (as of 30 August 2014) over a wide area spanning 42 coastal and offshore locations in Miyagi, Fukushima, Ibaraki, and Chiba Prefectures [Citation7].
All of the above-mentioned studies used a coprecipitation method, which employed ammonium phosphomolybdate (AMP) [Citation9], to quantify Cs concentrations. The aforementioned AMP coprecipitation method has generally been used to measure radiocesium concentrations in seawater. This method adsorbs and concentrates Cs on AMP; a germanium semiconductor detector (referred to hereafter as a Ge detector) is then employed to quantify radioactive Cs concentrations. This method, however, is laborious: a large quantity (10–100 L) of seawater must be transported to the laboratory followed by approximately 1 week of pretreatment (filtration and concentration). Therefore, a method to readily concentrate seawater and rapidly measure radioactive Cs is needed.
One approach to tackle these issues is a method that uses a Prussian blue copper analog (PB copper analog) such as copper hexacyanoferrate (Cu2[Fe(CN)6]) or potassium copper hexacyanoferrate (K2Cu3[Fe(CN)6]2). The PB copper analog adopts a cubic crystalline structure (Fm-3m), captures alkali metals in its lattice spaces, and readily adsorbs radiocesium [Citation10,Citation11]. The analogs exist in forms such as cubic crystalline Cu2[Fe(CN)6] incorporating no alkali metals [Citation12,Citation13] and triclinic (P-1) crystalline K2Cu[Fe(CN)6] devoid of ferrocyanide defects [Citation14]. Of particular note is the fact that cesium is adsorbed internally through ion exchange with potassium in K2Cu3[Fe(CN)6]2. Ion selectivity is extremely high, with a log Kd of >5 L kg−1 even for a 400 mg L−1 aqueous potassium solution; use of solutions up to a maximum pH of 10.9 has been reported to be possible [Citation14].
Some reported studies have obtained seawater measurements for radiocesium concentrations with PB copper analogs [Citation10,Citation15]. For example, Folsom et al. [Citation10] packed a cartridge with K2Cu3[Fe(CN)6]2-impregnated resin, passed seawater through the cartridge at a flow rate of less than 1 L min−1, and thereby concentrated radiocesium. This radiocesium was dissolved using 3 mol L−1 sodium hydroxide; then, after filtration, nitric acid was added. The resulting PB copper analog was recovered as a floc, and the supernatant liquid was collected and analyzed. In addition, Roos et al. [Citation15] wound Cu2[Fe(CN)6]-impregnated cotton into the form of a cartridge filter (referred to herein as a PB copper analog–impregnated, cotton-wound cartridge filter); they used this cartridge filter to monitor seawater. In that study, 800–5000 L of seawater was passed through a prefilter (pore diameter: 1 μm) to remove any particulate material and then through the PB copper analog–impregnated, cotton-wound cartridge filter at a rate of 4–6 L min−1, thereby concentrating radiocesium. The PB copper analog–impregnated, cotton-wound cartridge filter that had concentrated the radiocesium was combusted at 420 °C for 48 h, the combustion ash was collected, and the Cs that had been dissolved was quantified with a Ge detector. The radiocesium recovery ratio was reported to be 10%–20% lower than that obtained with the AMP method. This lower recovery is attributed to radiocesium being lost through ash scattering during the combustion of the PB copper analog–impregnated, cotton-wound cartridge filter.
As detailed above, methods that employ PB copper analogs as adsorbents to concentrate radiocesium in seawater have been developed and applied, but these methods are still hindered by the following issues: (1) after the radiocesium has been concentrated, pretreatment takes approximately 3 days; (2) in the research of Roos et al., some radiocesium was lost during the combustion process undertaken prior to analysis; and (3) the method adopted by Folsom et al. does not assess the recovery ratio of radiocesium, and the recovery ratio (between 50% and 90%) for the first cartridge in the method of Roos et al. is both low and uns.
Previously, we developed rapid measurement methods for radiocesium concentrations in freshwater that employ a suspended solid recovery cartridge [Citation16] and nonwoven cartridge filters impregnated with PB [Citation17] or zinc-substituted PB [Citation18] (referred to herein as Zn-CF). Zn-CF can concentrate 20 L of water at a flow rate of 2.5 L min−1, and a single cartridge can recover more than 97% of dissolved radiocesium [Citation18]. Zn-CF has been demonstrated to be highly effective for measuring radiocesium in freshwater, but the recovery rate of radiocesium in seawater by Zn-CF is low. For this reason, we developed a cartridge filter incorporating a nonwoven fabric impregnated with a PB copper analog to effectively concentrate and quantify cesium dissolved in seawattableer.
For the this study, we developed a rapid concentration and measurement method for radiocesium concentration in seawater that employs a copper analog, K2Cu3[Fe(CN)6]2. PB has various analogs in which some of its Fe atoms are substituted with other metals, such as Co, Ni, Zn, and Cu. These analogs are known to be good Cs adsorbents [Citation19]. Among them, we chose the copper analog for seawater measurement applications, because other analogs have disadvantages: PB is less robust than the PB copper analog in alkaline solution [Citation20], the zinc analog shows less selectivity against coexistent K+ in solution, and the cobalt and nickel analogs are costly.
In our method, we impregnated a nonwoven fabric with potassium copper hexacyanoferrate (K2Cu3[Fe(CN)6]2) and incorporated the fabric into a cartridge of a size that can be directly measured by a Ge detector. This new cartridge can rapidly concentrate radiocesium (e.g., from 20 L of seawater in ca. 40 min) with neither pretreatment after concentration (because the cartridge can be analyzed directly) nor loss of radiocesium. Using this method, the time for filtration and pretreatment can be greatly reduced; furthermore, rapid preconcentration can be done in the field.
2. Materials and methods
We assessed the performance of the nonwoven fabric cartridge filter impregnated with potassium copper hexacyanoferrate (hereafter referred to as the Cu-CF) for radiocesium concentration measurement in the laboratory and in the field in order to validate the method's application to seawater measurement. For the laboratory tests, we assayed seawater samples with known radiocesium concentrations. For the field application tests, seawater samples were collected offshore of Fukushima Prefecture; radiocesium in these samples was measured with both our method and the traditional coprecipitation method employing AMP, for comparison.
2.1. Monitoring device
Nonwoven fabric manufactured by means of a wet papermaking process (Japan Vilene Co., Ltd., Japan) was impregnated with potassium copper hexacyanoferrate (K2Cu3[Fe(CN)6]2 hydrate solution, Kanto Chemical Co., Inc., Japan) by means of the following process. The fabric (volumetric density 0.03 g cm−3, thickness 0.01 cm) was infiltrated with potassium copper hexacyanoferrate hydrate solution at a controlled concentration. The solution was prepared by dispersing the copper solution in water to create dispersion. The prepared dispersion was then placed in a tank, and the untreated fabric was dipped into it. The areal density of the impregnated potassium copper hexacyanoferrate hydrate in the nonwoven fabric was 4 g m−3. The impregnated fabric was then dried at 130 °C, cut into a rectangle measuring 3.8 × 480 cm, and rolled up into a cartridge to collect dissolved radiocesium (). This cartridge filter is called the Cu-CF. A similar cartridge filter that employed nonwoven fabric impregnated with PB (i.e., a PB-C) and with Zn (i.e., a Zn-CF) have been developed as well; their performance in absorbing dissolved radiocesium in water has been reported elsewhere [Citation18,Citation21]
Figure 1. Conceptual drawing of the apparatus of monitoring of radiocesium using a cartridge filter incorporating a nonwoven fabric impregnated with Prussian blue copper analog (modified from Yasutaka et al. [Citation18]).
![Figure 1. Conceptual drawing of the apparatus of monitoring of radiocesium using a cartridge filter incorporating a nonwoven fabric impregnated with Prussian blue copper analog (modified from Yasutaka et al. [Citation18]).](/cms/asset/f03c75c5-a885-40f7-98a1-83d4d4349a71/tnst_a_1135302_f0001_oc.jpg)
The monitoring apparatus () comprised a feed-water tank, a peristaltic pump (Model-410, Solinst 100 Canada, Ltd., Canada), a cartridge housing (polysulfonic housing PSF-500P, Advantec Co., Ltd., Japan), a monitoring cartridge (Cu-CF or Zn-CF), a flow meter (DigiFlow 6710M, MRT Co., Ltd., or OF10ZZWN, Aichi Tokei Denki Co., Ltd., Japan), and a drainage tank. These components were connected in series by plastic tubes, and the flow rate was controlled by the pump. In most experiments, only one cartridge housing was used. Before each experiment, residual sediment in the apparatus was washed out thoroughly with tap water. The pH of the water was measured with a pH meter (D-50 series, Horiba Co., Ltd., Japan, and Water Quality Meter WQC-24, DKK-TOA Corporation, Japan), the electrical conductivity (EC) was measured with a conductivity meter (ES-51, Horiba Co., Ltd., and Water Quality Meter WQC-24, DKK-TOA), and salinity was measured by multiple water quality meters (WQC-24, DKK-TOA Corporation). The concentration of K+ was measured by inductively coupled plasma atomic emission spectrophotometry (ThermoFisher Scientific iCAP6300 DuoView, emission wavelength 766.490 nm), NH4+ in samples was determined by indophenol blue absorptiometry (U-1900 spectrophotometer, Hitachi Ltd.), and 133Cs was measured by inductively coupled plasma mass spectrometry (Agilent 7500cx, Agilent Technologies, USA).
2.2. Improved AMP method
Several samples in field experiments were treated by not only the Cu-CF method but also an improved AMP method developed by one of the authors [Citation22,Citation23]. This improvement of the AMP method indicated that the weight yield of AMP/Cs compound was 98.6% for 10 L samples. Furthermore, the activities of the AMP/Cs compound were measured at the Ogoya Underground Facility of the Low Level Radioactivity Laboratory of Kanazawa University using high-efficiency, well-type, ultralow background Ge detectors. The detection limit of 137Cs measured at this facility was 0.18 mBq for a counting time of 10,000 min. Therefore, we can effectively repurpose former nuclear weapon testing facilities for oceanography measurements using this improved AMP method and measure the release concentrations of 134Cs and 137Cs caused by the Fukushima Daiichi Nuclear Power Plant accident in 2 L samples of seawater, for which the radiocesium activity was <1 Bq m−3.
Because some of the reagents used in this study contain trace levels of radioactivity, skewing small-volume measurements, it is important to know the specific activity of analytes such as 137Cs in these reagents. The 137Cs activity in CsCl was measured to be 0.03 mBq g−1 by using extremely low background γ-spectrometry; we neglect this amount of 137Cs because we use only 0.26 g 137Cs as a carrier. In contrast, the 137Cs activity in AMP was measured to be 0.024 mBq g−1, and since we use 4–6 g of AMP to extract radiocesium, we subtracted this amount of 137Cs activity from our sample measurements. There was no serious contamination of 137Cs from any other reagents used in this study, and we did not observe any 134Cs contamination from the reagents.
2.3. Radiocesium concentration measurements
A Ge semiconductor detector (SEG-EMS GEM20-70, Seiko EG&G) was used to analyze 137Cs. A weekly background measurement (24 h) and a daily energy calibration were carried out to ensure precision of the apparatus. A 2-L Marinelli standard gamma-ray source (MX033MR) was used for energy calibration; adjustments were made based on the peak tops (60Co: 1332.5 keV, 137Cs: 662 keV). Furthermore, the 2-L Marinelli standard gamma-ray source was measured on a weekly basis, and the decay was corrected to the base date and verified. The lower detection limit was set at the generally accepted value of three times the value of the counting error and was set for each sample by using the Cu-CF method.
2.4. Laboratory application tests
2.4.1. Sample water preparation
Seawater samples collected from Tokyo Bay (Tokyo Metropolis; Sample A), Matsukawa Lagoon (Fukushima Prefecture; Sample B), and Soma Port (Fukushima Prefecture; Sample C) were used for laboratory application tests (see ). The ECs of the water samples taken from Matsukawa Lagoon and Soma Port were 40.5–42.1 mS cm−1. Although the EC was not measured for water sample A, it was inferred from the potassium concentration that the water was seawater.
Table 1. Sampling point and chemical characteristics of seawater samples.
After filtration of the seawater samples through a 0.45-μm-pore-size membrane filter, the conditions for measuring dissolved 137Cs concentrations were modified for this experiment. The dissolved 137Cs was extracted from leaves (picked in Fukushima Prefecture after the nuclear accident) by soaking the leaves in water for 24–168 h and then filtering the water through 0.45-μm-pore-size membrane filters. The extraction and filtered liquid was diluted with seawater to ca. 10 Bq L−1.
2.4.2. Experimental conditions
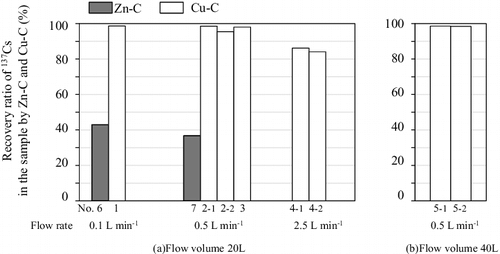
Application tests were undertaken using seven sets of experimental conditions (), where coexisting ion concentrations, flow volumes, and flow rates were varied. Flow volumes were set at either 20 or 40 L, and flow rates at 0.1, 0.5, or 2.5 L min−1. Cu-CF was generally used as the dissolved radiocesium recovery cartridge (test nos. 1–5). Zn-CF was used in a few tests for comparative purposes (nos. 6 and 7) because of its low recovery ratio. 137Cs recovery ratios for dissolved radiocesium were calculated by collecting the water that had passed through the dissolved cesium recovery cartridge, measuring the 137Cs concentration, and comparing the difference between the 137Cs concentration of the sample being tested with the 137Cs concentration (ca. 10 Bq L−1).
Table 2. Experimental conditions of application evaluation test of Cu–C and Zn–C.
2.5. Field experiments
Two types of field application tests were conducted. In the first test, the Cu-CF method and the AMP method were compared; in the second test, two dissolved material recovery Cu-CFs were configured in series, and the recovery ratios of the first and the second cartridges were compared. More specifically, the recovery ratios were calculated as the amount of 137Cs (Bq) in the first Cu-CF divided by the sum of the amounts of 137Cs in the first and second Cu-CFs.
2.5.1. Seawater samples
The seawater used in the first field application test was sampled from Tomioka Port (Tomioka, Fukushima Prefecture) on 30 September 2014. A 20-L sample was used for the Cu-CF method, and a 10-L sample was employed for the AMP method. The EC of the samples was 48.3 mS cm−1 and the pH was 7.7.
Seawater samples for the second field application test were taken from seven locations at Fukushima Prefecture's Matsukawa Lagoon on 23–24 September 2014; the seawater pH was 8.06–8.28 and that of brackish water was 7.50–7.84. The ECs of seawater and brackish water were 45.1–48.0 and 15.0–37.4 mS cm−1, respectively, and the salinities of seawater and brackish water were 27.4–29.0 and 8.2–22.2 PSU, respectively.
2.5.2. Experimental conditions
Pretreatment for the AMP method and details of the analytical method employed were described in Section 2.2. The test with the Cu-CF method used 20 L of water at a flow rate of 0.5 L min−1. One suspended solid cartridge and two Cu-CFs were connected in series in each experiment; two repetitions were conducted in the first test, and a single test was conducted for each of the 11 samples in the second test.
2.5.3. Analysis methods of Cu-CF
The Cu-CFs through which water had flowed were placed in plastic containers as prescribed by the method of Yasutaka et al. [Citation18] and were analyzed directly with a Ge detector. Using a nondestructive detection measurement to calculate the actual 137Cs radioactivity (Bq) or concentration (Bq L−1), the detected 137Cs radioactivity or concentration was divided by nondestructive detection efficiency (NDDE).
Yasutaka et al. [Citation18] calculated the NDDE for Zn-CF in detail and obtained a value of 0.67 for a 20-L sample flowing at a rate of 2.5 L min−1. Furthermore, this correction coefficient was related to the distribution of radiocesium in the cartridge: there was a negative correlation between the radiocesium content in the outer one-third of the total nonwoven fabric length (the nonwoven fabrics located on the outer side of the cartridge) and the correction coefficient because of the increasing distance between 137Cs and the Ge detector.
The relationship between NDDE and the proportion of 137Cs in the outer one-third of the cartridge was calculated in our previous research [Citation18] and is described in EquationEquation (1)(1)
(1)
(1)
(1)
Here, NDDE is the nondestructive detection efficiency, and PCs is the proportion of 137Cs in the outer one-third of the Zn-CF or Cu-CFs. This equation holds for PCs values in the range 45%–95%.
The proportion of 137Cs in the outer one-third of a Cu-CF, at a flow rate of 0.5 L min−1 and flow volume of 20 L, was found to be 67%. The corresponding NDDE was calculated to be 0.68. For a flow rate of 0.5 L min−1 and flow volume of 40 L, the proportion of 137Cs in the outer one-third was found to be 58%, and the NDDE was calculated to be 0.69. Therefore, for this study, we used 0.69 as an NDDE value.
3. Results and discussion
3.1. Laboratory application tests
shows 137Cs recovery ratios for tests with flow rates of 0.1, 0.5, and 2.5 L min−1 at a flow volume of 20 L through the Cu-CF (nos. 1–4) and Zn-CF (nos. 6 and 7) cartridges, and at a flow volume of 40 L through a Cu-CF cartridge (no. 5). Recovery ratios for Zn-CF were less than 43% at the 0.1 and 0.5 L min−1 flow rates. We concluded that this low recovery ratio was observed with Zn-CF because the adsorption rate would have decreased owing to the coexistence of other ions such as potassium.
In contrast, recovery ratios for flow rates of 0.1 and 0.5 L min−1 were 99% and 95%–99% for all samples with Cu-CF cartridges (nos. 1–3 and 5), respectively. The recovery ratio was 84%–86% for a flow rate of 2.5 L min−1 (no. 4) This phenomenon, whereby the Cs recovery ratio falls with rising flow rates, was caused by the decreasing contact time of water with Cu-CF and also was observed in our previous study [Citation18], in which radioactive Cs was concentrated by passing water through Zn-CF; thus, the current findings are consistent with those of past research.
For 137Cs recovery ratios using Cu-CF cartridges at a flow rate of 0.5 L min−1 (nos. 3 and 5), the recovery ratios at flow volumes of 20 and 40 L were the same, 98%.
3.2. Field experiments
shows 137Cs concentrations measured in the first field test using both the Cu-CF method and the AMP method. The differences between each method lie within the range of measurement error; thus, the Cu-CF method has similar measurement accuracy to the traditional AMP method.
Table 3. Concentration of 137Cs measured by an AMP method and Cu-C.
shows the 137Cs concentrations of the first and second cartridges and the recovery ratio of the first cartridge using the Cu-CF method in the second field application. For the first cartridge, recovery ratios were high (>92% to >96%) in seawater (salinity of 27.4–29.0 PSU) and in brackish water (salinity of 8.2–22.2 PSU) at flow volumes of 33–42 L. This finding indicates that this method can maintain high radiocesium recovery ratios for waters with a wide range of salinities, ranging from seawater to brackish water.
Table 4. 137Cs concentrations of the first and second cartridges and the recovery ratio of the first cartridge using the Cu–C method in the second field application.
3.3. Preconcentration and pretreatment considerations
Our proposed method can shorten the preconcentration and pretreatment times required for collecting and measuring dissolved radiocesium in seawater compared with the times required by the AMP method and other previously reported methods using PB copper analog [Citation10,Citation15]. The preconcentration time for our Cu-CF method is only 40 min per 20 L of seawater. This preconcentration time is shorter than that required for the AMP method (>1 day). Our developed method can also shorten the pretreatment time required prior to analysis. The methods using PB copper analog reported by Folsom et al. [Citation10] and by Roos et al. [Citation15] require about 3 days for pretreatment. In contrast, the pretreatment time required by our Cu-CF method is only 40 min.
Another advantage of our developed method is its high recovery of radiocesium during the preconcentration and pretreatment processes. In the research of Roos et al. [Citation15], some radiocesium was lost during the combustion process undertaken prior to analysis. In contrast, we have adopted a nondestructive direct measurement method, which does not require combustion and thus prevents the potential loss of radiocesium.
The recovery ratio of radiocesium using our preconcentration method is >95% in laboratory tests (see ) and >93% in field tests (see ). These recovery ratios are lower than that obtained with the AMP method, but higher and more consistent than those reported by previous methods using the PB copper analog.
4. Conclusions
The findings of this research are summarized as follows.
In laboratory experiments involving 20 L of seawater spiked with dissolved radiocesium, the Cu-CF method resulted in radiocesium recovery ratios of 99% at a flow rate of 0.1 L min−1, 95%–99% at 0.5 L min−1, and 85% at 2.5 L min−1.
Field tests with seawater produced results for the Cu-CF method and the AMP method that were consistent with each other.
For two Cu-CF s connected in series, the radiocesium recovery ratio of the first cartridge was >93%–96% at a flow rate of 0.5 L min−1 among 11 samples of waters ranging from brackish to seawater (flow volume: 20–40 L).
This method can be used to concentrate 20–40 L of brackish water or seawater in 40–80 min at a flow rate of 0.5 L min−1. Furthermore, analysis of Cu-CFs using our nondestructive direct measurement method and a correction coefficient does not require any postconcentration pretreatment; thus, it is possible to significantly shorten the time required from pretreatment through measurement.
Acknowledgements
The authors thank the Japan Environment Science Co., Ltd. (Japan) and Tokyo Power Technology Ltd. (Japan) for analyzing the radioactivity in our samples. This study was financially supported by the Japan Science and Technology Agency through the Development of Systems and Technology for Advanced Measurement and Analysis program, JSPS KAKENHI [grant number 26241023] .
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chino M, Nakayama H, Nagai H, et al. Preliminary estimation of release amounts of 131i and 137cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. J Nucl Sci Technol. 2011;48:1129–1134.
- Nemoto Y, Shimamura S, Igarashi S. Influence of radioactive substances on the marine organisms and fishing areas off Fukushima prefecture [ in Japanese]. Nippon Suisan Gakkaishi. 2012;78:514–519.
- Yokota M, Watanabe T, Nomura H, et al. Radioactive substances detected from fisheries organisms caught in the fresh water area and the pacific ocean on eastern Japan – survey results from September 2011 to March 2013. Rep Mar Ecol Res Inst. 2014;19:17–42.
- Oikawa S, Watabe T, Takata H, et al. Long term temporal changes of 90sr and 137cs in seawater, bottom sediment and marine organism samples – from the Chernobyl accident to immediately after the Fukushima accident. Bunseki Kagaku. 2013;62:455–474.
- Ministry of the environment. Radioactive material monitoring surveys of the water environment. 2014 [ cited 2014 Nov 18]. Available from: http://www.env.go.jp/en/water/rmms/surveys.html.
- Tokyo Electric Power Corporation. Distribution map of seawater radioactivity around tepco Fukushima Dai-ichi npp. 2014 [ cited 2015 Feb 15]. Available from: http://radioactivity.nsr.go.jp/en/contents/10000/9126/24/ 349_6_20141114.pdf.
- Nuclear Regulation Authority. Readings of sea area monitoring at offshore of Miyagi, Fukushima, Ibaraki and Chiba prefecture (seawater). 2014 [ cited 2015 Feb 15]. Available from: http://radioactivity.nsr.go.jp/en/contents/10000/9092/24/ 440_20141110.pdf.
- Aoyama M, Tsumune D, Hamajima Y. Distribution of 137cs and 134cs in the north pacific ocean: Impacts of the TEPCO Fukushima-Daiichi NPP accident. J Radioanal Nucl Chem. 2013;296:535–539.
- Aoyama M, Hirose K, Miyao T, et al. Low level 137cs measurements in deep seawater samples. Appl Radiat Isotopes. 2000;53:159–162.
- Folsom TR, Hansen N, Tatum TJ, et al. Recent improvements in methods for concentrating and analyzing radiocesium in sea water. J Radiat Res. 1975;16:19–27.
- Watari K, Imai K, Izawa M. Isolation of 137Cs with copper ferrocyanide-anion exchange resin. J Nucl Sci Technol. 1967;4:190–194.
- Rigamonti R. Structure of Cupriferrocyanides I. Copper Ferrocyanide and Potassium Copper Ferrocyanide. Gazz. Chim. Ital. 1937;67:137.
- Ayrault S, Jimenez B, Garnier E, et al. Sorption mechanisms of cesium on CuII2FeII(Cn)6 and CuII3[FeIII(Cn)6]2 hexacyanoferrates and their relation to the crystalline structure. J Solid State Chem. 1998;141:475–485.
- Loos-Neskovic C, Ayrault S, Badillo V, et al. Structure of copper-potassium hexacyanoferrate (II) and sorption mechanisms of cesium. J Solid State Chem. 2004;177:1817–1828.
- Roos P, Holm E, Persson RBR. Comparison of AMP precipitate method and impregnated Cu2[Fe(Cn)6] filters for the determination of radiocesium concentrations in natural waters. Nucl Instrum Meth A. 1994;339:282–286.
- Tsuji H, Kondo Y, Suzuki Y, et al. Development of a method for rapid and simultaneous monitoring of particulate and dissolved radiocesium in water with nonwoven fabric cartridge filters. J Radioanal Nucl Chem. 2014;299:139–147.
- Yasutaka T, Kawamoto T, Kawabe Y, et al. Rapid measurement of radiocesium in water using a Prussian blue impregnated nonwoven fabric. J Nucl Sci Technol. 2013;50:674–681.
- Yasutaka T, Tsuji H, Kondo Y, et al. Rapid quantification of radiocesium dissolved in water by using nonwoven fabric cartridge filters impregnated with potassium zinc ferrocyanide. J Nucl Sci Technol. 2015;52:792–800.
- Haas PA. A review of information on ferrocyanide solids for removal of cesium from solutions. Sep Sci Technol. 1993;28:2479–2506.
- Parajuli D, Takahashi A, Noguchi H, et al. Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem Eng J. 2016;283:1322–1328.
- Yasutaka T, Tsuji H, Kondo Y, et al. Development of rapid monitoring for dissolved radioactive cesium with a cartridge type of Prussian blue-impregnated nonwoven fabric. Bunseki Kagaku. 2013;62:499–506.
- Aoyama M, Hirose K. Radiometric determination of anthropogenic radionuclides in seawater. Radioact Environ. 2008;11:137–162.
- Hirose K, Aoyama M, Igarashi Y, et al. Improvement of 137Cs analysis in small volume seawater samples using the Ogoya underground facility. J Radioanal Nucl Chem. 2008;276:795–798.