?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The process of nanoparticle formation by radiochemical synthesis in a heterogeneous system has been investigated considering the effects of the metal ion location in the reaction medium. PtCu nanoparticles supported on carbon and γ-Fe2O3 were synthesized using a high-energy electron beam. The metal ions in the precursor were categorized as those dissolved in solution, adsorbed on support, and precipitated. The ratio of metal ions in the solution was varied prior to the electron beam irradiation and its effects on the synthesized particle structures were examined. The nanoparticles were characterized by inductively coupled plasma-atomic emission spectrometry, transmission electron microscopy, X-ray diffraction, and X-ray absorption spectroscopy. A PtCu alloy and CuO were immobilized on the support in all the samples. The PtCu alloy nanoparticle composition depended on the Cu ion content in the solution. The nanoparticle formation mechanism could be explained using the obtained results. Metal ions present in the solution resulted in formation of the alloy. The adsorbed ions also contributed to the alloy formation by desorbing from the support when irradiated. On the other hand, alloy formation with Pt from the precipitated Cu ions was found to be difficult.
1. Introduction
The properties of nanoparticles strongly depend on their structure. Pt-based nanoparticles supported on carbon or ceramics have been reported to exhibit high catalytic activities. The introduction of a support prevents agglomeration of the reduced metal clusters, thereby retaining the small size of the synthesized metal nanoparticles. We previously attempted to modify a Pt catalyst by partially replacing Pt with other metals using a unique radiation-induced synthesis method. We synthesized bimetallic nanoparticles of various structures including PtRu alloy, PtCo alloy-Co3O4, and Pt-SnO2 supported on carbon and ceramics using electron beam irradiation [Citation1–3]. We also reported the use of γ-Fe2O3-supported PtCu alloy–CuO nanoparticles as catalysts for preferential CO oxidation [Citation4,Citation5], where PtCu alloy and CuO acted as the main catalyst and the promoter, respectively [Citation6]. Moriya et al. successfully synthesized PtCu nanoparticles with different amount of CuO promoter and reported that the catalytic activity depended on the amount of CuO promoter used [Citation7]. However, the formation process of this radiation-induced synthesis method has not been examined, and control of the nanoparticle structure has not been achieved yet.
The purpose of this study is to examine the formation mechanism of supported nanoparticles obtained using the radiation-induced synthesis method. In order to synthesize catalysts with an intended structure, it is essential to reveal the synthesis mechanism of the nanoparticles in a system containing the support. However, determining the mechanism in a heterogenetic system is difficult. Belloni estimated the fundamental reactions in a radiolytic process in a homogeneous solution system using the pulse radiolysis method [Citation8]; however, this method cannot be applied to systems containing a support.
We synthesized PtCu nanoparticles supported on carbon and γ-Fe2O3. The synthesis mechanism is discussed by focusing on the relationship between the location of the metal ions in the reaction medium and the PtCu alloy composition. We classified metal ions in the precursor by their locations as those existing in solution, adsorbed on support, and precipitated. The reduction process of the metal ions is discussed separately with respect to their presence in the reaction medium. This report explains the formation process of PtCu nanoparticles in a system containing a support.
2. Experimental
2.1. Material synthesis
The detailed synthesis procedure using the electron beam irradiation method has been previously reported [Citation9] and hence, described only briefly here. Ultrapure water (18.2 MΩ cm) and 1 vol.% of 2-propanol (Wako Chemicals, Japan.) were used as a solvent and a scavenger for hydroxyl radicals, respectively. H2PtCl6•6H2O (99.9%, Wako Chemicals, Japan.) and CuSO4•5H2O (99.9%, Wako Chemicals, Japan.) were used as metal precursors, and Pt and Cu solutions (each of 0.5 mM) were prepared. Carbon black powder (Vulcan XC-72R, Cabot Corp.) with an average particle size of 30 nm or γ-Fe2O3 (Nanophase Tech. Corp.) with an average particle size of 26 nm, each of 58.2 mg, were used as the support. The total weight of the metal ions was fixed at 10% of the weight of the catalyst nanoparticles. The precursor solutions, 50 mL each, were added to a 100 mL glass vial and were well mixed and sonicated. The mixture was bubbled with Ar gas in order to remove the dissolved oxygen in the solution. In order to vary the concentration of metal ions in the solution, PtCu nanoparticles were synthesized at various pH conditions and with different supports.
Each vial containing the precursor solution and support was irradiated with a 4.8 MeV electron beam for several seconds at room temperature to a surface dose of 20 kGy in a commercial facility (Electron Beam Irradiation Service Ltd., Izumi-otsu, Japan). The irradiated samples were subsequently washed with ultrapure water and dried at 60 °C overnight to obtain samples in the powder form.
The elementary process of the electron beam irradiation reduction was reported previously in the case of a solution system [Citation8]. The electron beam irradiation induces radiolysis of water and 2-propanol in the aqueous phase. These processes can be expressed as follows:
(1)
(1)
(2)
(2)
(3)
(3)
The hydrated electrons and the secondary radicals reduce the metal ions easily [Citation8]. The following equations are written taking Pt ions for example:
(4)
(4)
(5)
(5)
The surface dose applied in this study is calculated to the amount that the obtained reducing species is high enough to reduce all the Pt4+ and Cu2+ in the above equations.
2.2. Characterization of materials
2.2.1. Before irradiation
The metal ion ratio in solutions was analyzed before irradiating with the electron beam. The precursors were centrifuged at 30,000 g for 10 minutes to separate the metal ions in solution and those precipitated or adsorbed on the support. The supernatant was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES; SHIMADZU, ICPE-9000). The samples were dissolved in the HCl–HNO3 mixture and sprayed into the plasma torch through a nebulizer.
2.2.2. After irradiation
The structure of the electron beam irradiated powder was determined as follows. The chemical composition of the nanoparticles was analyzed using ICP-AES. The samples were dissolved in the HCl–HNO3 mixture and sprayed into the plasma torch through a nebulizer. The morphology of the composite particles was investigated using transmission electron microscopy (TEM; carbon-supported samples with Hitachi H-8100, 200 kV and γ-Fe2O3-supported samples with FEI Technai 20, 200 kV). For TEM measurements, the samples were suspended in ultrapure water and a few drops of the suspension were cast on carbon-coated copper grids followed by drying in air at 60 °C. Crystallographic analysis was carried out using an X-ray diffractometer (XRD; RIGAKU, RINT2100-Ultima, 40 kV, 30 mA) with Cu–Kα radiation in a continuous scan mode. The phases present in the sample were identified by comparing XRD patterns with the corresponding Joint Committee on Powder Diffraction Standards (JCPDS) database files. The crystal lattice parameter was calculated from the peak position using the Bragg's equation. The (111) crystal plane of a face-centered cubic (fcc) was used for the lattice constant calculations. The chemical states of Pt and Cu in the particles were examined by assessing the X-ray adsorption near edge structure (XANES) around the Pt–LIII and Cu–K edges at the BL01B1 beamline of SPring-8 (Hyogo, Japan; grant number 2014A1798, 2015A1693) and the BL9C beamline of the Photon Factory at the High Energy Accelerator Research Organization (Tsukuba, Japan; grant number 2012G078, 2014G633) at room temperature. The chemical state of Pt and Cu is obtained by fitting the absorption spectra of the samples with a linear combination of the reference spectra.
3. Results
3.1. Metal ion ratio in solution
The amount of metal ions in solution was measured. The ratio of Pt and Cu ions in solution to the total amount is plotted against the pH of the precursor in . Pt and Cu ions behave differently in the precursors containing the carbon support. At lower pH, Pt ions are not found in the solution, but ∼50% Cu ions remain in the solution. On the other hand, at higher pH, almost no Pt and Cu ions are present in the solution. In case of samples containing the γ-Fe2O3 support, both the Pt and Cu ion contents in the solution decrease with increasing pH.
3.2. Nanoparticle characterization
The chemical compositions of the PtCu nanoparticles measured by ICP-AES are shown in . Pt and Cu are found in the samples. The atomic ratios between Pt and Cu are a little different from the targeted ratio of 50:50. The analysis of the supernatant liquid revealed that the reduced amount of Cu is relatively smaller than that of Pt. These results reflect the fact that Pt is a noble metal and is easily reduced. Metal loadings of about 8 wt.% for the carbon-supported samples and 10 wt.% for the γ-Fe2O3-supported samples are obtained.
Table 1. Average size and atomic ratios of PtCu nanoparticles measured using TEM, ICP-AES, and XRD. The sample IDs note the pH of the reaction medium and the support used.
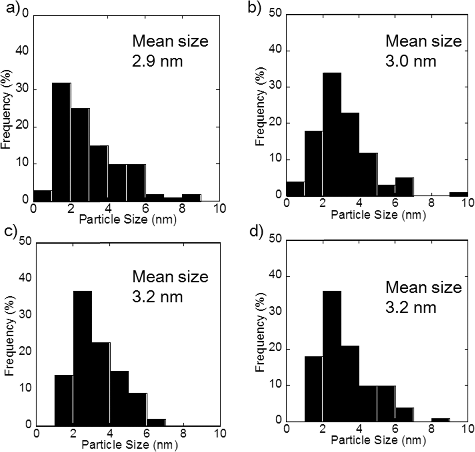
show TEM micrographs of PtCu nanoparticles supported on carbon and γ-Fe2O3, respectively. The larger particles with weaker contrast correspond to carbon and γ-Fe2O3 supports, and the smaller particles with stronger contrast correspond to metallic nanoparticles. The metallic nanoparticles, the PtCu alloy nanoparticles as confirmed by the XRD results discussed later, are sparsely distributed. From the TEM micrographs, the nanoparticles present on the support are found to be of a few nanometers in diameter. The average diameter measured by examining a hundred PtCu nanoparticles in each sample is shown in . The average size of the PtCu nanoparticles increases with an increase in the pH for carbon-supported samples. There is only a little pH dependence for γ-Fe2O3 samples. The size distribution is shown in and . Similar size dependence on the precursor pH was also reported in the case of PtRu nanoparticles supported on carbon [Citation10].
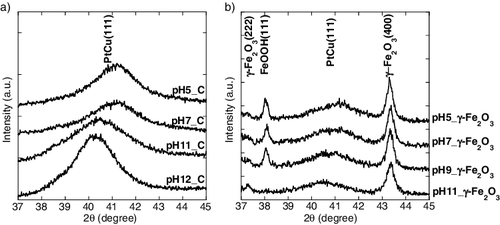
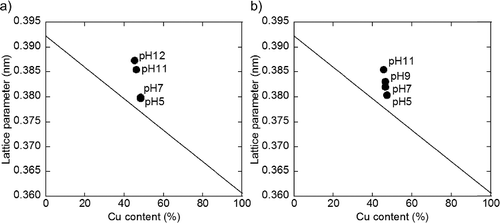
XRD patterns of the samples are shown in . The broad peaks around 40°–41° originate from the (111) plane of the PtCu fcc structure. For all the samples, diffraction peaks corresponding to PtCu (111) planes are observed between 40.3° and 41.1°, at a higher angle than that of the pure Pt (39.75°), indicating that a part of Cu is incorporated into the Pt lattice. In , the lattice parameter measured by XRD is plotted against Cu content observed by ICP-AES analysis. Samples prepared at lower pH values exhibit peaks at higher angles, indicating an increase in Cu composition in the PtCu alloy. The composition of the PtCu alloy determined using a similar method as in the previous reports [Citation11,Citation12] is listed in . The compositions determined using XRD reflect the crystalline species which satisfy the Bragg's equation. This means that the surface of the nanoparticle and poor crystalline species are excluded compared to the results obtained by ICP-AES analysis. On comparing the compositions obtained from ICP-AES and XRD, the composition of the core and shell of PtCu nanoparticle can be discussed. Since the composition calculated from XRD pattern shows a high Pt percentage, a part of Cu is likely to exist in the amorphous phase or on the surface of the PtCu alloy, which is also suggested in the previous report [Citation5,Citation7]. For the γ-Fe2O3-supported samples, three sharp peaks are exhibited at 2θ values of 37.2°, 38.1°, and 43.2°, which could be attributed to γ-Fe2O3 and lepidocrocite (FeOOH), derived from γ-Fe2O3.
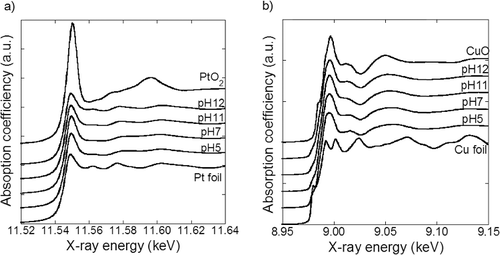
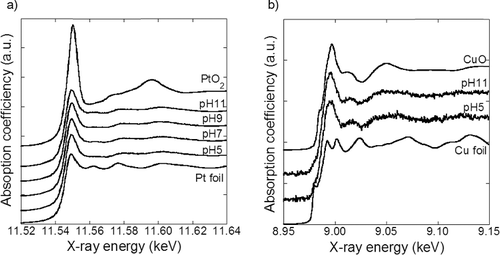
The chemical states of the synthesized samples were investigated using XANES analysis. The Pt–LIII and Cu–K edge XANES spectra of the PtCu nanoparticles along with those of the reference materials are shown in and . The absorption spectra of the samples are well described by a linear combination of the reference spectra and the fitted results are shown in . The results obtained for Pt and Cu are different; most of the Pt exist as Pt metal and most of the Cu exist as CuO. This result is in agreement with that reported in a previous study [Citation7]. The metallic Pt and Cu are expected to exist as PtCu alloy. Pt oxide is expected to exist on the surface layer of the metallic Pt nanoparticles as reported earlier [Citation13]. By contrast, the significant amount of CuO obtained cannot be explained only by the Cu existence in the surface layer. Considering the XRD results, which indicate only partial Cu incorporation in PtCu alloy, Cu species that exist separately from the PtCu alloy are expected to be in the CuO phase.
A schematic diagram of the synthesized nanoparticle structure determined by structure analyses is shown in . The nanoparticles are composed of PtCu alloy, CuO, and the support. PtCu and CuO particles with an average size of 3–5 nm are immobilized on the support nanoparticles. The PtCu composition varies depending on the type of the support used and the pH of the precursor. Supported PtCu nanoparticles with a similar structure were previously synthesized [Citation7]. The chemical states of Pt and Cu examined using the ICP-AES and XRD results are listed in . One hundred weight percentage corresponds to the weight of the sample powder, which contains Pt, Cu, and support. Since Pt is more noble than Cu, all Pt is assumed to exist in PtCu alloy. The Pt composition determined by ICP-AES is listed as ‘Pt in alloy.’ The Cu composition determined by ICP-AES is divided into ‘Cu in alloy’ and ‘CuO’ referring to the XRD result. The difference between the amount of metal ions put in the precursor and those found in the samples is expressed as ‘metal ions not reduced.’ In the case of samples with the γ-Fe2O3 support, the metal composition exceeded their input amount. The reason for this is discussed using EquationEquations (6(6)
(6) ) and (Equation7
(7)
(7) ), which show the formula used to calculate the compositions of Pt and Cu from the ICP-AES results, respectively. The terms in the formula are the weight of the species, and the input weight of γ-Fe2O3 was substituted in w_γ-Fe2O3. We have revealed, however, that a part of γ-Fe2O3 is dissolved by addition of H2PtCl6•6H2O, which lowers the pH of the solution. Therefore, the calculated Pt and Cu composition increases due to the decrease of the dominator and as a result, exceeded their input amount. For this reason, ‘the metal ions not reduced’ is not calculated for the γ-Fe2O3 support samples.
(6)
(6)
(7)
(7)
Figure 1. Metal ion ratio in solution as a function of pH of precursor containing (a) carbon support and (b) γ-Fe2O3 support (circle denotes Pt ion and square denotes Cu ion).
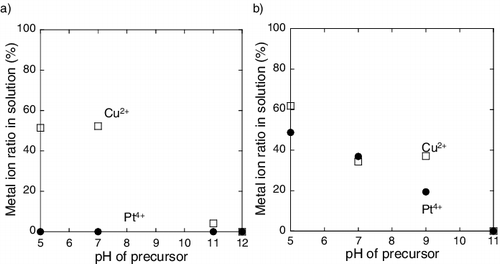
Figure 2. TEM micrographsaphs of PtCu nanoparticles supported on carbon at (a) pH 5, (b) pH 7, (c) pH 11, and (d) pH 12.
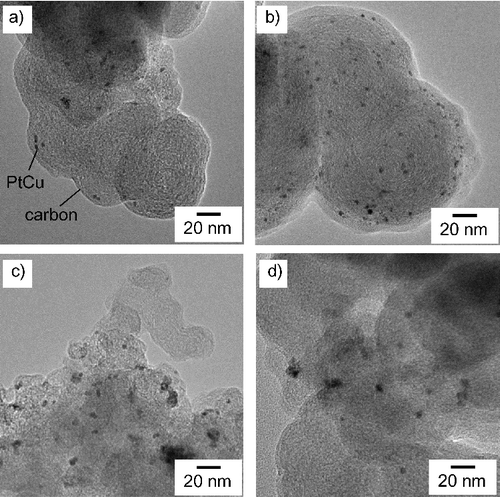
Figure 3. TEM micrographs of PtCu nanoparticles supported on γ-Fe2O3 at (a) pH 5, (b) pH 7, (c) pH 9, and (d) pH 11.
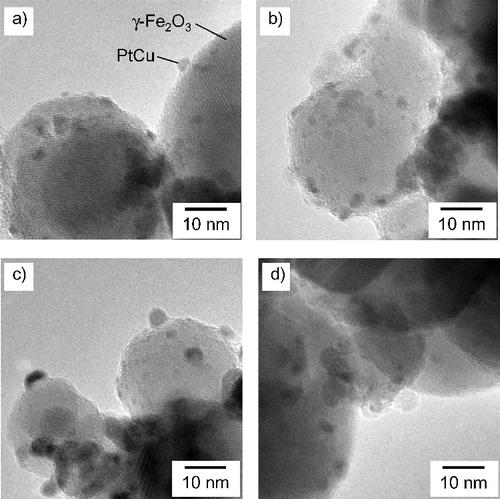
Table 2. Composition of PtCu nanoparticles determined from results (wt.%).
3.3. Relation between the metal ion content in the solution and the composition in the alloy
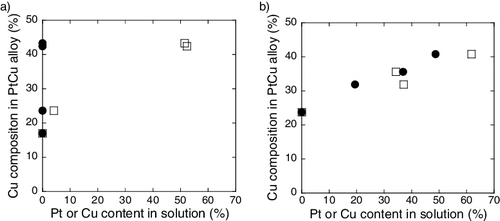
Cu composition in the PtCu alloy, which is revealed by XRD analysis, is plotted against the Pt and Cu ion content in the solution and is shown in . For samples containing the carbon support, Cu composition in PtCu alloy is associated with the Cu content in solution, but not with Pt content in solution. PtCu alloy with a higher Cu composition is obtained with increasing Cu content in the solution. However, PtCu alloy is obtained regardless of the presence of Pt metal in the solution. For samples containing the γ-Fe2O3 support, PtCu alloy with a higher Cu composition is obtained from precursors with a higher Pt and Cu ion contents in the solution. This is an important fact on investigating the formation process of PtCu nanoparticle, which is discussed later.
4. Discussion
4.1. Elementary process of the electron beam irradiation reduction
The elementary process of the electron beam irradiation reduction was reported previously as shown in Equations (1)–(5). Belloni [Citation8] reported that when the initial reduction reactions are followed by a mixed coalescence, an alloy structure is obtained. The radiolytic reduction process of metal ions in solution is expected to follow Belloni's theory. In this study, we introduced support nanoparticles, which enables us to classify metal ions into those precipitated or adsorbed on the support and those dissolved in the solution.
4.2. Effect of pH
The pH of the reaction medium could affect the following: yield of the radiochemical species, redox potentials, complexing state of the metal ions, and surface charges of the support and the metal particles. The yields of the radiochemical species have been reported to be a constant in the pH range of 3–11 [Citation14] and are expected to be a constant in our case. Moreover, although the redox potentials change with pH, the difference in the redox potentials of Pt and Cu does not change and the formation process would not differ significantly by changing the redox potentials. Thus, the first two factors cannot affect the formation process. However, the complexing states and the surface charges vary with changes in the pH of the reaction medium. A major change would be that the Cu ions could form hydroxides and precipitate at higher pH. Moreover, pH of the precursor affects the surface charge of the support and the PtCu particles. Owing to these factors, pH of the precursor affects the metal ion contents in solution.
4.3. PtCu nanoparticle formation process
The formation process of the synthesized PtCu nanoparticles could be explained based on the results obtained. Cu content in the PtCu alloy increases with an increase in the Cu ion content in solution as shown in . This result suggests that Cu ions in solution could involve in the alloy formation relatively easier as compared to those precipitated and adsorbed on the support.
An alloy structure is obtained when the initial reduction reactions of metal ions are followed by a mixed coalescence [Citation8]. At this point, the contribution of the precipitated and adsorbed metal species towards alloy formation is expected to be difficult. However, the alloy structure is formed even though no Pt ions are present in the solution. Thus, it is presumed that the metal ions adsorbed on the support surface gain energy from the irradiated electron beam and desorb from the surface as previously suggested by Lawless et al. in the reduction process using gamma rays [Citation15]. The desorbed metal ions contribute to the alloy formation along with the ions in the solution. This theory is consistent with the results obtained from samples containing the γ-Fe2O3 support, and this could probably be the mechanism of alloy formation in heterogeneous systems.
The formation process of CuO is speculated. Hori et al. reported the formation of Cu0 using gamma-ray irradiation [Citation16]. Our study is likely to follow a similar formation process. The Cu ions reduced by an electron beam form Cu0, which is unstable in the colloidal state. Therefore, Cu0 is easily oxidized by the oxygen dissolved in solution or oxygen in the atmosphere during the drying process.
(8)
(8)
5. Conclusions
PtCu alloy and CuO nanoparticles supported on carbon and γ-Fe2O3 were synthesized using a radiochemical method. The alloy composition and the amount of CuO obtained depended on the Cu ion ratio dissolved in the solution. The formation process of the nanoparticles is discussed. We found that the amount of metal ions in solution is a critical factor in determining the structure of the bimetallic nanoparticles.
Acknowledgments
The authors thank Japan Electron Beam Irradiation Service (EBIS, Japan) for allowing the use of the electron accelerator. We thank the Institute for NanoScience Design, Osaka University and Dr Satoshi Ichikawa for the TEM measurements.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan under Grant-in-Aid for Scientific Research [grant number A, 15H02342]; Japan Society for the Promotion of Science under Grant-in-Aid for JSPS Fellows [grant number 201500803].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kageyama S, Seino S, Nakagawa T, et al. Formation of PtRu alloy nanoparticle catalyst by radiolytic process assisted by addition of DL-tartaric acid and its enhanced methanol oxidation activity. J Nanopart Res. 2011;13:5275–5287.
- Ohkubo Y, Hamaguchi Y, Seino S, et al. Preparation of carbon-supported PtCo nanoparticle catalysts for the oxygen reduction reaction in polymer electrolyte fuel cells by an electron-beam irradiation reduction method. J Mater Sci. 2013;48:5047–5054.
- Okazaki T, Seino S, Nakagawa T, et al. Radiochemical synthesis of a carbon-supported Pt-SnO2 bicomponent nanostructure exhibiting enhanced catalysis of ethanol oxidation. Radiat Phys Chem. 2014;108:1–6.
- Yamamoto TA, Nakagawa T, Seino S, et al. Bimetallic nanoparticles of PtM (M= Au, Cu, Ni) supported on iron oxide: radiolytic synthesis and CO oxidation catalysis. Appl Catal A Gen. 2010;387:195–202.
- Kugai J, Kitagawa R, Seino S, et al. γ-Fe2O3-supported Pt-Cu nanoparticles synthesized by radiolytic process for catalytic CO preferential oxidation. Appl Catal A Gen. 2011;406:43–50.
- Ferrandon M, Ferrand B, Bjornbom E, et al. Copper oxide–platinum/alumina catalysts for volatile organic compound and carbon monoxide oxidation: synergetic effect of cerium and lanthanum. J Catal. 2001;202:354–366.
- Moriya T, Kugai J, Seino S, et al. CuO role in γ-Fe2O3-supported Pt-Cu bimetallic nanoparticles synthesized by radiation-induced reduction as catalysts for preferential CO oxidation. J Nanopart Res. 2013;15:1451.
- Belloni J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: application to catalysis. Catal Today. 2006;113:141–156.
- Seino S, Kinoshita T, Nakagawa T, et al. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J Nanopart Res. 2007;10:1071–1076.
- Ohkubo Y, Kageyama S, Seino S, et al. Radiolytic synthesis of carbon-supported PtRu nanoparticles using high-energy electron beam: effect of pH control on the PtRu mixing state and the methanol oxidation activity. J Nanopart Res. 2013;15:1597.
- Vegard L. The constitution of the mixed crystals and the filling of space of the atoms. Z Phys. 1920;5:17–26.
- Schneider A, Esch U. Das system Kupfer-Platin [The system copper-platinum]. Z Elektrochem. 1944;50:11–12.
- Tanaka T, Yamamoto T, Kohno Y, et al. Application of XANES spectra to supported catalysts. Japan J Appl Phys. 1999;38:30–35.
- Woods R, Pikaev A. Applied radiation chemistry: radiation processing. New York (NY): Wiley-Interscience; 1993. Chapter 6, Selected topics in radiation chemistry, Water and aqueous solutions; p. 168–171.
- Lawless D, Kapoor S, Kennepohl P, et al. Reduction and aggregation of silver ions at the surface of colloidal silica. J Phys Chem. 1994;98:9619–9625.
- Hori T, Nagata K, Iwase A, et al. Synthesis of Cu nanoparticles using gamma-ray irradiation reduction method. Japan J Appl Phys. 2014;53: 05FC05.
Appendix
Figure A1. Particle size distribution of PtCu nanoparticles supported on carbon at (a) pH 5, (b) pH 7, (c) pH 11, and (d) pH 12.
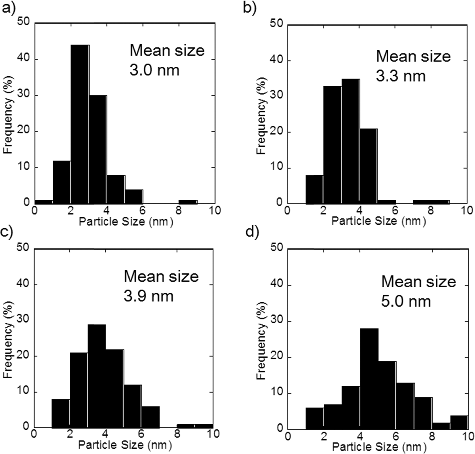
Table B1. Chemical states of Pt and Cu calculated from linear combination fit of XANES spectra.