ABSTRACT
In the operation of the sodium-cooled fast reactor, the leakage and fire accident of liquid sodium is common and it is frequent in sodium-related facilities. This study focuses on the combustion and suppression characteristics of sodium fire in a columnar flow. Liquid sodium (250 °C) is injected into a 7.9 m3 cylindrical chamber at a flow rate of about 1.0 m3/h to create a columnar sodium fire, and 18.4 kg class D extinguishing powder is sprayed after the liquid sodium injection. The temperature in the chamber space and sodium collection plate and the heat release rate from sodium fire are measured and analyzed. Based on the temperature data the sodium fire under suppression could be divided into four phases of dropping sharply, continuously remaining lower, rising and declining mildly, and depressing. The sodium fire in the space could be suppressed and cooled down if the extinguishing agent could spray in the early period of the liquid sodium injection. The extinguishing agent could suppress the combustion and spreading of liquid sodium dropping on the collection plate, limit the pool combustion area and postpone the commencement of sodium pool burning in spite of its later re-ignition happening. This study promises to evaluate the combustion and suppression characteristics of sodium fire in the sodium-related facilities.
1. Introduction
The liquid sodium is commonly used as a coolant in liquid metal fast reactors (LMFRs) for its excellent thermal hydraulic characteristics. However, its high chemical reactivity with the atmospheric oxygen could cause a potential sodium fire hazard. A significant portion of sodium combustion product could be in the form of aerosols posing a health hazard to the exposed personnel, and the resultant high temperature may also cause structural damage to concrete buildings. The leakage and combustion of liquid sodium is chosen as a design basis accident of the LMFRs due to its high frequency and severity of consequences. Therefore, an extensive study of sodium fire has been actively carried out in France, Japan, and Russia. There have been a number of experimental and numerical studies on combustion behavior of liquid sodium by Hilliard, Newman, Yamaguchi, and Takata et al. [Citation1–7]. Recently, Zhang et al. [Citation8–14] reported the combustion characteristics of sodium fire in a columnar flow and droplet. Furthermore, it is important to extinguish or suppress the sodium fire immediately and effectively in case of liquid sodium leakage and combustion. Deukkwang et al. [Citation15] investigated experimentally the suppression of sodium small pool fire with liquid nitrogen in 125- and 250-mL beakers. Mark et al. [Citation16] concluded that suppression of metal fires was particularly difficult and extinguisher agents available in powder form like Class D could suppress metal fires of small scale. Robert [Citation17] reported that Class D fire suppression agents could affect the sodium pool fire with the boundary limited in some way before further additional tests have been carried out. Claire et al. [Citation18] reported that suitable Class D fire extinguishing agent was effective for metal pool fire, but it is easy to reignite when the bed was disturbed. Snehalatha et al. [Citation19] developed the carbon microsphere to extinguish the small scale of sodium pool fire with 2 g sodium. However, the combustion and suppression characteristics of sodium fire in a columnar flow and the pool fire gathered by the liquid sodium on the floor have not been studied in the available literature.
In the present study, in order to clarify the combustion and suppression characteristics of sodium fire in a columnar flow, this paper focuses on the study of the sodium fire by conducting the experiment using class D extinguishing powder and ISO9705 standard fire experiment platform, in which the liquid sodium of 250 °C is injected into a cylindrical chamber to shape a sodium fire in a columnar flow and then the extinguishing powder is sprayed. The combustion and suppression characteristics of the columnar sodium fire are discussed by the analysis of the temperature fields of space and sodium collection plate of the chamber and the heat release rate, etc.
2. Experimental apparatus and procedure
2.1. Experimental apparatus
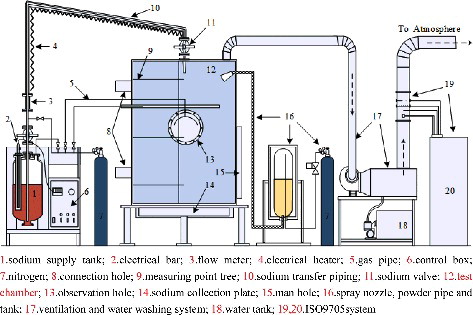
The experimental apparatus consists mainly of the sodium supply tank, the test chamber, data acquisition system, ventilation and water washing system, and extinguishing system as shown in .
| (1) | The sodium supply tank is installed in an incubator packed with thermal insulation materials for reducing the heat loss from the tank to external environment, and is connected with the test chamber by the sodium transfusion pipe. The top of the sodium supply tank is equipped with the sodium transfer piping, electrical bar, nitrogen connection, pressure gage, sodium flow meter, pressure control valve, etc. | ||||
| (2) | The test chamber is a stainless steel cylindrical tank of 2500 mm height and inner diameter of 2000 mm. The cylinder is double-layered with the interlayer packed with thermal insulation materials for accurate detection of the experimental temperature and for the safety of operating personnel. The chamber is equipped with a heavy gauge observation hole and a manhole, the sodium nozzle on the top connected with sodium pipes and the supply tank. | ||||
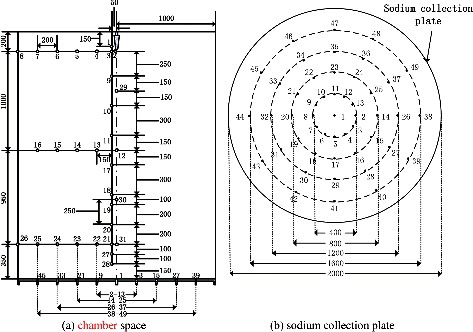
| (3) | The data acquisition system consists of 31 thermocouples (T1–T31) distributed in chamber space and 49 thermocouples (Td1–Td49) distributed in the sodium collection plate, the multi-channel temperature acquisition apparatus, and the ISO9705 fire experiment platform attached on the ventilation pipe. The temperature measuring points are shown in in detail. For the convenient data analysis, the thermocouples distributed in the space and sodium collection plate are distinguished by using the marks ‘T’ and ‘Td,’ respectively. Thirty-one measuring points (T1–T31) in the space are located on the same vertical plane of a right angle whose horizontal plane is just the sodium collection plate, where the intersection of the measuring points tree and sodium collection plate planes is nearly a radius line of measuring points Td1–Td45. The sodium spraying nozzle lies on the centerline of the sodium chamber. | ||||
| (4) | Sodium fire extinguishing system is connected to the chamber with three nozzles equidistributed in the same horizontal section of the chamber (included angle: 120°). Three sets of instruments could work jointly or separately to spray the extinguishing powder into the chamber. Each set of them includes the nitrogen source, gas pipe, powder vessel, powder transfusion pipe, magnetic valve, and the spiral groove nozzle, as shown in . | ||||
| (5) | The ventilation and water washing system is connected with the top of the test chamber via a smoke tube. During the experiment, the aerosols inside the chamber could be removed and cleaned by the fan and water pump of the system, and then it will be discharged into the ISO9705 standard fire experiment platform. | ||||
| (6) | The ISO9705 standard fire experiment platform can measure the heat release rate in the chamber from sodium fire based on the oxygen consumption method [Citation20]. The washed gas from ventilation and water washing system is sampled and analyzed by the gauges, and then released into the atmosphere. | ||||
2.2. Experimental procedure
During the experiment, the solid metal sodium of about 12.68 kg in the sodium supply tank was steadily heated up to melt the solid sodium and to preheat the molten sodium to the initial temperature of 250 °C using electrical bar. Then the liquid sodium at a flow rate of about 1.0 m3/h was released into the chamber through the sodium transfer piping via a nozzle with a diameter of 10 mm by using the nitrogen gas at 0.2 MPa. The beginning of the liquid sodium injection in this paper is set as the initial time (0 s) for graph analysis. The suppression powder was sprayed at the time of 100 s after the liquid sodium injection, and this extinguishing process lasted for approximately 120 s. In the present work, two powder tanks in the sodium fire extinguishing system were originally loaded with the class D powder of 10 kg. After the experiment, the remnant powder was weighted; the powder sprayed ratios were 97.4% and 86.4%, and the total consumed mass was 18.4 kg.
The main ingredients of the class D powder include the sodium chloride (85% in mass fraction), aluminum chloride (5% in mass fraction), and additional agents (10% in mass fraction) for maintaining the fluidity, aridity, and dispersivity of the powder. In the cold test, the systems were tested without the liquid sodium injection and confirmed that the powder could cover the whole sodium collection plate with the uniform thickness.
3. Experimental results and analysis
3.1. Experimental phenomena
In the whole process of the liquid sodium burning, a mass of combustion aerosol products was generated so that the manhole must be kept close until the chamber cooled down completely after the experiment.
The experiment phenomena were observed and recorded through the observation hole. The main stream of the liquid sodium flowed in a columnar form when the injection began, and dozens of droplets splashed with sparkling away from the main steam. Soon the chamber was filled with the dense white smoke and aerosol; therefore, the observation was obstructed except the yellow light observed.
3.2. Space temperature of chamber
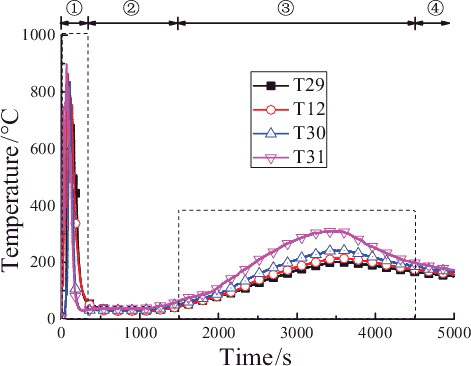
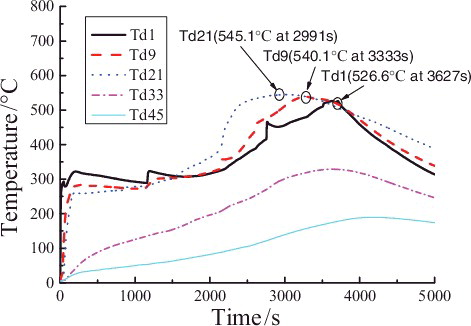
The beginning of the liquid sodium injection is set as the initial time of the temperature distributions in curve graphs. shows the temperature distributions of representative measuring points in the longitudinal centerline (T29, T12, T30, and T31) of the chamber space. It could be obviously observed that the temperature curve could be divided into the following four phases:
 temperature dropping sharply due to the powder spraying and extinguishing of sodium fire,
temperature dropping sharply due to the powder spraying and extinguishing of sodium fire, temperature continuously remaining lower after extinguishing,
temperature continuously remaining lower after extinguishing, temperature rising and declining mildly for reigniting of liquid sodium, and
temperature rising and declining mildly for reigniting of liquid sodium, and temperature depressing for natural extinguishment of the liquid sodium.
temperature depressing for natural extinguishment of the liquid sodium.
In phase ![]() , as shown in , the temperatures in the longitudinal centerline have exceeded 800 °C before the powder spraying. When the powder spraying starts, the temperatures of measuring points closer to the sodium collection plate fall faster than the further ones, and the temperatures fall more mildly with increasing of the height. Especially the temperature curve gradient of T31 in the lower position of the chamber space doubles that of T29 in upper. All the temperatures of the measuring points in the longitudinal centerline decrease to under 100 °C after the powder spraying is finished.
, as shown in , the temperatures in the longitudinal centerline have exceeded 800 °C before the powder spraying. When the powder spraying starts, the temperatures of measuring points closer to the sodium collection plate fall faster than the further ones, and the temperatures fall more mildly with increasing of the height. Especially the temperature curve gradient of T31 in the lower position of the chamber space doubles that of T29 in upper. All the temperatures of the measuring points in the longitudinal centerline decrease to under 100 °C after the powder spraying is finished.
Figure 4. Temperature distributions at different heights of chamber in the longitudinal central line in phase ![]()
As shown in , in phase ![]() the temperatures of measuring points in different heights start obviously to rise after approximately 1500 s, which implies that reignition of sodium happens and the combustion of liquid sodium in the collection plate gets stronger. It is found that the maximum temperature peaks of all measuring points rise simultaneously after nearly 2000 s. T31 closing to the liquid sodium is 310 °C, while the temperature peaks of T30, T12, and T29 reach, respectively, 242, 213, and 200 °C, which shows the temperature peak gradually decreases with the increasing of the height. These peak values are close to the temperature values measured in chamber at stable combustion stage in the experiment without spraying the extinguishing powder [Citation8]. Additionally, the temperature of T31 starts to rise in a relatively high speed after 2000 s with the maximum slope of 0.19 °C/s, and the slopes gradually reduce with the heights increasing. The temperatures rise relatively slowly, last shortly, and decline quickly after the peaks in this phase, which means although there is the reignition of liquid sodium, the ensuing sodium pool fire is still suppressed to a certain extent by using extinguishing powder.
the temperatures of measuring points in different heights start obviously to rise after approximately 1500 s, which implies that reignition of sodium happens and the combustion of liquid sodium in the collection plate gets stronger. It is found that the maximum temperature peaks of all measuring points rise simultaneously after nearly 2000 s. T31 closing to the liquid sodium is 310 °C, while the temperature peaks of T30, T12, and T29 reach, respectively, 242, 213, and 200 °C, which shows the temperature peak gradually decreases with the increasing of the height. These peak values are close to the temperature values measured in chamber at stable combustion stage in the experiment without spraying the extinguishing powder [Citation8]. Additionally, the temperature of T31 starts to rise in a relatively high speed after 2000 s with the maximum slope of 0.19 °C/s, and the slopes gradually reduce with the heights increasing. The temperatures rise relatively slowly, last shortly, and decline quickly after the peaks in this phase, which means although there is the reignition of liquid sodium, the ensuing sodium pool fire is still suppressed to a certain extent by using extinguishing powder.
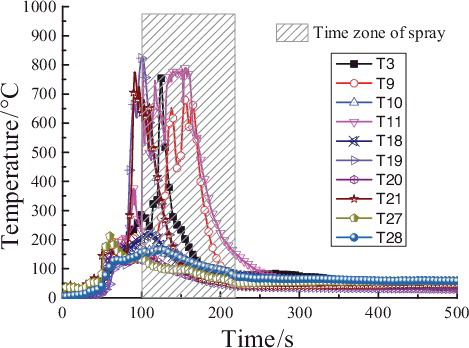
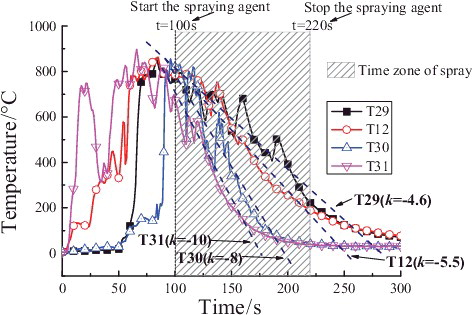
shows the temperature values at the different heights of longitudinal measuring point tree (direction: T1–T28) at a distance of 0.05 m from the central line. It could be clearly observed that all the measuring points temperatures are quite low and the maximum temperature is under 70 °C before 50 s. During 50–80 s, the temperatures of the measuring points begin to climb and the maximum temperature reaches up to 200 °C. After 80 s, the temperatures of T19 and T21 rise rapidly and reach nearly 800 °C before the powder sprays. It could be confirmed that the temperature in the chamber space is quite low and the combustion is not violent in the early time of the liquid sodium injection. When the powder spray begins at 100 s, several temperatures of the measuring points (T3, T9, and T11) have jumped high in a short time, notably up to 700 °C during the powder spray. However, the temperatures at all heights of the chamber space fall down below 100 °C. It could be inferred that the accident of sodium fire in a columnar flow could be suppressed and cooled down if the extinguishing agent could spray in the early period of the liquid sodium injection, and therefore the thermal consequence of the sodium fire could be reduced as early as possible.
shows the temperature values at the different heights of longitudinal measuring point tree (direction: T1–T28) at a distance of 0.05 m from the central line at 50, 90, 160, 200, and 250 s. Compared with , the temperatures of measuring points at different heights keep relatively low at 50 s and 90 s except 0.35 m (T21) or 0.75 m (T19). As the extinguishing powder sprays, the region below 1.0 m is cooled down at 160 s, but the temperatures of the region above 1.0 m rise and reach up to 770 and 587 °C at 1.45 and 2.05 m, respectively. At 200 s, the temperatures of measuring points at different heights have dropped down obviously, and at 250 s dropped below 100 °C at all heights. These results indicate that the suppression effect to sodium fire is more notable in the lower region of the chamber space especially at the powder spraying initial period, which is because the sodium fire source at this time is mainly on the sodium collection plate, it is easy to form the higher concentration extinguishment power in the lower region near the sodium collection plate so that oxygen would be isolated here; later due to the comprehensive mixture of cold nitrogen with hot combustion product aerosol and suppression in the lower region, the sodium fire in the upper region is also suppressed so that at this time the sodium fire in the chamber could be all suppressed.
Figure 6. Temperature distributions at longitudinal heights (direction: T1–T28) in the chamber space.
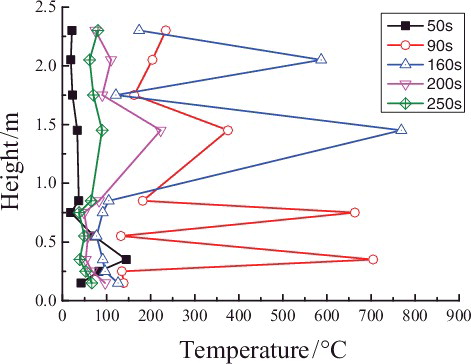
shows the maximum temperature values (TMax) and corresponding time values (tMax T) at different heights of longitudinal measuring point tree (direction: T3–T28) at a distance of 0.05 m from the central line in the reigniting phase ![]() (1500--4500 s). The maximum temperature near the sodium collection plate reaches 350 °C, while the temperatures of measuring points above 0.5 m are about between 200 and 300 °C. It could be observed that the corresponding time values of the maximum temperatures at different heights are all about 3500 s, except that the time for the measuring point closest to the sodium collection plate is earlier, about 1000 s in advance.
(1500--4500 s). The maximum temperature near the sodium collection plate reaches 350 °C, while the temperatures of measuring points above 0.5 m are about between 200 and 300 °C. It could be observed that the corresponding time values of the maximum temperatures at different heights are all about 3500 s, except that the time for the measuring point closest to the sodium collection plate is earlier, about 1000 s in advance.
Figure 7. TMax and corresponding tMax T at different longitudinal heights (direction: T1–T28) in the chamber space.
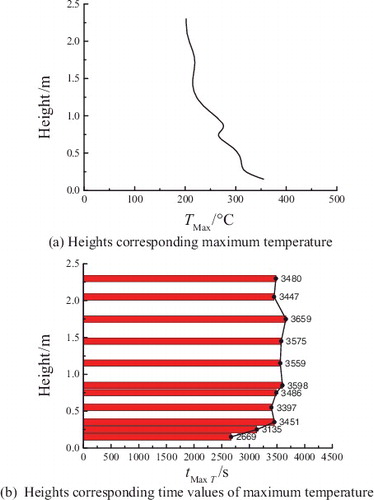
The temperature distributions in the radial direction at the different heights are shown in and . The temperature of T21 close to the central line of chamber space reaches the maximum value of 745.7 °C before the powder spraying, while the peak temperatures of T23, T25, and T26 far from the central line keep quite low and below 50 °C at approximate 30 s after the powder spraying; Until about 1300 s, the temperatures of T21, T23, T25, and T26 start to rise slowly. At around 3441–3839 s, the temperatures rise to the peak values and decrease gradually with the distance from the spraying central line. The temperatures of the measuring points of group T3, T5, T6, and T8 also show similar trends as that of groups T21, T23, T25 and T26.
Figure 8. Temperature distributions of radial measuring points T21, T23, T25, and T26 in the chamber space.
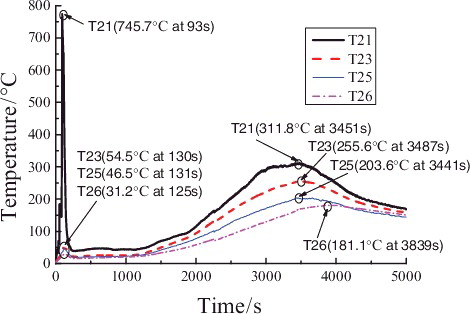
Figure 9. Temperature distributions of radial measuring points T13, T14, T15, and T16 in the chamber space.
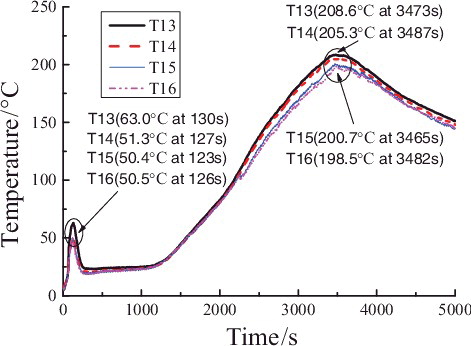
In , the temperature distribution of the measuring points of group T13, T14, T15, and T16 is similar to that of groups T21, T23, T25 and T26; however, at the initial period (phase ![]() ), the maximum temperature of T13 is quite low because it lies further away from the longitudinal spraying central line that of than T21. At the reigniting phase (phase
), the maximum temperature of T13 is quite low because it lies further away from the longitudinal spraying central line that of than T21. At the reigniting phase (phase ![]() ), the overall temperature distributions of the measuring points of groups T13, T14, T15, and T16 are a little lower than that of groups T21, T23, T25, and T26, because the height of measuring points of groups T13, T14, T15, and T16 is further away from the reigniting sodium on the collection plate.
), the overall temperature distributions of the measuring points of groups T13, T14, T15, and T16 are a little lower than that of groups T21, T23, T25, and T26, because the height of measuring points of groups T13, T14, T15, and T16 is further away from the reigniting sodium on the collection plate.
3.3. Surface temperature of sodium collection plate
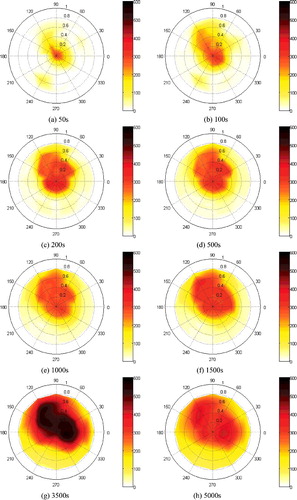
shows the dynamic temperature field on the sodium collection plate. The liquid sodium flows onto the sodium collection plate and burns in a form of sodium pool. However, it could not spread rapidly on the plate due to power spray and the area of liquid sodium pool takes up only approximately one quarter of the sodium collection plate throughout the experiment. Before 1500 s, the maximum temperatures of all the measuring points in the sodium collection plate are not higher than 350 °C due to spraying of extinguishing powder. At 3500 s, after re-ignition of sodium pool (phase ![]() ), the maximum temperature of the part collection plate measuring points could reach 550 °C; Compared with the low space temperature at the same time, it implies the reignition could happen under the powder layer, probably because the powder layer has a thinner thickness (average thickness is about 6.7mm) in this experiment, the powder layer is easy to be destroyed by the splashing from local sodium burning, or after the powder layer is heated up, some cracks will appear on the powder layer or some salient points so that the oxygen could contact the sodium pool through the powder layer. Therefore, the high temperature appears in limited spots, which means the complete reignition does not arise. From the temperature field in (g,h), in the late period of the experiment, the temperature around the center of the plate declines and the high temperature district splits into pieces, which means that the liquid sodium with extinguish powder splits into blocks. These results indicate that the powder agent could suppress the flowing of the liquid sodium in the plate and limit the pool combustion area.
), the maximum temperature of the part collection plate measuring points could reach 550 °C; Compared with the low space temperature at the same time, it implies the reignition could happen under the powder layer, probably because the powder layer has a thinner thickness (average thickness is about 6.7mm) in this experiment, the powder layer is easy to be destroyed by the splashing from local sodium burning, or after the powder layer is heated up, some cracks will appear on the powder layer or some salient points so that the oxygen could contact the sodium pool through the powder layer. Therefore, the high temperature appears in limited spots, which means the complete reignition does not arise. From the temperature field in (g,h), in the late period of the experiment, the temperature around the center of the plate declines and the high temperature district splits into pieces, which means that the liquid sodium with extinguish powder splits into blocks. These results indicate that the powder agent could suppress the flowing of the liquid sodium in the plate and limit the pool combustion area.
The radial temperature change of groups Td1–Td45 in the sodium collection plate has a significant stratification as shown in . In the present experiment, the temperatures of all the measuring points were below 350 °C before 1500 s, and the temperature in the center district is approximately 300 °C, which holds in a large radius. However, compared with the results of the sodium fire experiment without the extinguishing suppression procedure in the literature [Citation8] in which the temperature in the center district of the plate rapidly reaches 500 °C in 500 s, it indicates that the powder agent could suppress the combustion before 1500 s in spite of the reignition happening. The temperatures begin to increase after 1500 s, which indicates that the burning strengthens and the suppression effect weakens. In the phase ![]() , the reignition arise initially from the edge of the liquid sodium pool and then develops to the center district, and the sequence of the temperatures starting to rise is successive. The similar distribution could be also observed in other groups such as Td1–Td49.
, the reignition arise initially from the edge of the liquid sodium pool and then develops to the center district, and the sequence of the temperatures starting to rise is successive. The similar distribution could be also observed in other groups such as Td1–Td49.
3.4. Experimental residues on the sodium collection plate
After 12 hours of the experiment, the manhole was opened; it could be clearly observed that the residual on the sodium collection plate emerges as three districts. The central district consisted of several light yellow blocks covered with a thin layer of white powder. The middle district encircling the central district was light gray, which was caused by the absorption of moisture of the powder agent after the manhole was opened. The white powder agent covered the periphery district extending to the edge of the sodium collection plate. The whole sodium collection plate was covered with the powder agent everywhere, which could cumber the spreading of the liquid sodium to restrict the development of the combustion. The solid silvery white sodium was observed to be enfolded in the yellow blocks when the residue was shoveled away, which indicates that the powder agent and sodium oxides formed on the surface of the liquid sodium pool could isolate the liquid sodium from the oxygen in the chamber space.
3.5. Heat release rate of sodium fire
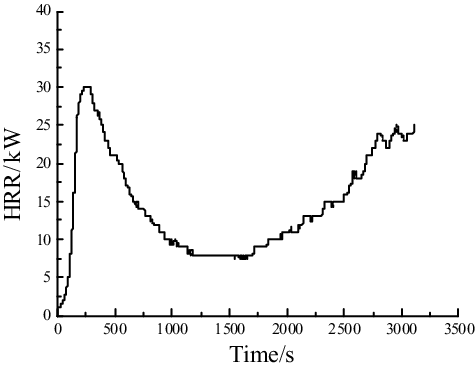
The heat release rate of the combustion for the liquid sodium is measured in the present experiment, as shown in . It is observed that the fundamental value of energy released per unit mass of oxygen consumed in the process of liquid sodium burning is lacked; therefore, the reference value of 13,100 kJ/kg is used from the conventional fire measurement, which could influence the accuracy of the experiment measurement [Citation20]. It could be observed clearly that the heat release rate changes with the burning process in the experiment including the combustion and reignition process of liquid sodium. It indicates that using ISO9705 standard fire experiment platform based on the oxygen consumption method is a feasible way to measure the heat release rate of the sodium fire in a columnar flow or other forms of sodium fire. Further sodium fire experiments would be conducted to add up the experiment results and test the accuracy.
4. Conclusion
A sodium fire in a columnar flow and extinguishing experiment was carried out to focus on the combustion and suppression characteristics. Compared with the results of the sodium fire experiment without the extinguishing suppression procedure, the conclusions are summarized as follows:
| (1) | The sodium fire process in a columnar flow in the chamber under suppression by extinguishing agent could be divided into four phases: temperature dropping sharply during powder spraying, temperature continuously remaining lower after extinguishing, temperature rising and declining mildly due to reigniting sodium pool, and continuously depressing temperature phase for natural extinguishment. | ||||
| (2) | Before the class D extinguishing agent spraying, the maximum temperatures of measuring points reached beyond 700 °C; after the powder agent spraying, the temperatures dropped quickly below 100 °C. The sodium fire in a columnar flow in the chamber space could be suppressed and cooled down if the class D extinguishing agent could spray in the early period of the liquid sodium injection, so that the thermal consequence of the sodium fire could be reduced as early as possible. | ||||
| (3) | The powder agent could suppress the combustion of liquid sodium dropping on the chamber collection plate by postponing the sodium ignition and burning. Moreover, the powder agent could suppress the flowing of the liquid sodium in the plate and limit the combustion area. | ||||
| (4) | Using ISO9705 standard fire experiment platform which works based on the oxygen consumption method, it is feasible to measure the heat release rate of sodium fire in columnar flow or other forms of sodium fire. Further sodium fire experiments would be conducted to add up the experiment results and test the accuracy. | ||||
A number of experiments with different experimental conditions would be carried out continuously, and we will pay more attention to the combustion and suppression characteristics and models of the different sodium fires.
Acknowledgments
The authors thank the National Natural Science Foundation of China [grant numbers 51476040 and 51676051], Project of Nuclear Energy Development, for the financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hilliard RK, Mccormack JD, Postma AK. Aerosol behavior during sodium pool fires in a large vessel–CSTF tests AB1 and AB2. Hanford (WA): Hanford Engineering Development Laboratory; 1979. (Report no. HEDL-TME 79-28 UC-79). p. 79.
- Newman RN. The ignition and burning behavior of sodium metal in air. Prog Nucl Energ. 1983;12:119–147.
- Yamaguchi A, Tajima Y. Numerical investigation of mass and heat transfer in sodium pool combustion. Numer Heat Transfer. 2002;A41:697–709.
- Takata T, Yamaguchi A, Maekawa I. Numerical investigation of multidimensional characteristics in sodium combustion. Nucl Eng Des. 2003;220:37–50.
- Subramani A, Jayanti S, Shet USP et al., Dynamics of liquid sodium pool spreading under sodium fire conditions. Nucl Eng Des. 2009;239:1354–1361.
- Bae JH, Ahn DH, Kim YC et al., An experimental study on the characteristics of sodium fires. J Korean Nucl Soc. 1994;26:471–483.
- Du HO, Wang RD, Hu WJ. [The experimental research on the sodium spray fire]. At Energy Sci Technol. 2011;31:41–47. Chinese.
- Zhang ZG, Peng KW, Huo Y et al., Experimental study on combustion characteristics of sodium fire in a columnar flow. J Nucl Sci Technol. 2014;51(2):166–174.
- Peng KW, Zhang ZG, Guo M et al., Experimental study on sodium column fire of sodium-cooled fast reactor. Proceedings of ICONE21-16089; 2013 July 28–Aug 2; Chengdu (China).
- Wand C, Zhang ZG, Peng KW et al., Simulation Research on Combustion of Liquid Sodium Droplet. Proceedings of the 21st International Conference on Nuclear Engineering (ICONE21), 2013 July 28–Aug 2, Chengdu (China).
- Guo M, Zhang ZG, Tang YX et al., Experimental study on sodium fire combustion in a columnar flow. Proceedings of ICONE22-30692; 2014 July 7–11, Prague (Czech Republic).
- Tang YX, Zhang ZG, Guo M et al., Experimental study on burning process and characteristic of sodium columnar fire. Proceedings of ICONE22-30943; 2014 July 7–11, Prague (Czech Republic).
- Li JK, Zhang ZG, Huo Y et al., Experimental study on sodium fire combustion in a columnar flow. Proceedings of ICONE23-1888; 2015 May 17–21, Chiba (Japan).
- Zhang ZG, Sun SB, Liu CC et al., [Experimental study on oxidation and combustion characteristics of sodium droplets]. At Energy Sci Technol. 2015;49(4):667—673. Chinese.
- Deukkwang A, Peter B S, Daniel P L. Suppression of sodium fires with liquid nitrogen. Fire Saf J. 2013;58:204–207.
- Mark R, William J R, Mannan M S et al., Prevention and suppression of metal packing fires. J Hazard Mater. 2003;104:247–253.
- Robert Z. Metal hydride fires and fire suppression agents. J Loss Prevent Proc. 2008;21:214–221.
- Claire F, Timothy H, Kenneth P M et al., Development of detailed action plans in the event of a sodium hydride spill/fire. Process Saf Prog. 2005;24:86–90.
- Snehalatha V, Ponraju D, Nashine B K et al., Synthesis and Characterization of Carbon Microsphere for extinguishing sodium fire. Carbon Materials 2012 (CCM12). AIP Conf. Proc. 2013;1538:89–93.
- Parker WJ. Calculations of the heat release rate by oxygen consumption for various applications. J Fire Sci. 1984;2(5):380–395.