?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
For effective recovery of radioactive elements by adsorbents using polymer-immobilized silica (SiO2-P) supports, the microstructure of SiO2-P particles impregnated with octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide as extractants and their change with the crosslinking degree of polymer (CDP) were investigated using scanning transmission X-ray microscopy (STXM) and extended X-ray absorption fine structures (EXAFS) analyses; further, their relation with adsorption/elution behavior was discussed. The results of STXM analysis suggested that the polymer is distributed within several hundred nanometers of the pore surface, and its distributions are spread by the increase of CDP. In addition, the capture of impurity molecules such as H2O by polymers with high CDP was indicated. EXAFS analyses showed that the local structure around adsorbed europium ions is similar irrespective of the CDP. The adsorption/elution tests demonstrated that a higher CDP inhibited the elution of adsorbed europium ions from the adsorbent.
1. Introduction
Spent nuclear fuels generated from nuclear power plants contain several long-lived radioactive nuclides as well as U and Pu that can be reused. Adequately processing the spent fuel is important for the effective utilization of energy and environmental load reduction. Some reprocessing plants have already been commercialized for recovering U and Pu from spent nuclear fuel, and numerous studies on partitioning and transmutation of long-lived radioactive nuclides have been conducted globally [Citation1]. In these studies, several researchers have reported the recovery process of long-lived radioactive nuclides by silica-based adsorbents [Citation2–Citation13]. Wei et al. developed silica-based adsorbents by impregnating an organic extractant into a styrenedivinylbenzene (SDB) copolymer, which is immobilized in porous silica particles [Citation2]. They impregnated octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide (CMPO), di(2-ethylhexyl)phosphoric acid, and bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) into the polymer-immobilized silica (SiO2-P) particles and investigated the adsorption and elution behavior of trivalent actinides (MA(III)) and some fission products (FPs) using simulated high-level liquid waste (HLLW) [Citation2,Citation3]. In some studies [Citation4–Citation8], SiO2-P particles impregnated with N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide (TODGA) and 2, 6-bis(5, 6-dialkyl-1, 2, 4-triazine-3-yl)-pyridine (R-BTP) were prepared and applied for group separation MA(III) and trivalent lanthanide (Ln(III)) and for selective recovery of MA(III), respectively, from simulated HLLW. Ito et al. impregnated two types of molecules simultaneously into SiO2-P particles and studied the application of these adsorbents on the recovery of Cs or platinum group metals (PGM) (N,N′-dimethyl-N,N′-di-n-octyl-thiodiglycolamide and tri-n-octylamine for Cs recovery [Citation9], and 1,3-[(2,4-Diethylheptylethoxy)oxy]-2,4-crown-6-calix[4]arene (Calix[4]) and 1-dodecanol for PGM recovery [Citation10]). Koma et al. assessed the adsorption/elution ability, durability, and after-treatment of several SiO2-P adsorbents with different extractants, and the combination of TODGA/SiO2-P and R-BTP/SiO2-P adsorbents was evaluated as the primary candidate for MA(III) recovery from HLLW [Citation11]. Based on this assessment, MA(III) recovery trials from genuine HLLW were conducted using columns packed with TODGA/SiO2-P and R-BTP/SiO2-P adsorbents, and the recovery yields of MA(III) and decontamination factors (DF) of other FPs were obtained [Citation12,Citation13]. These previous studies showed that long-lived radioactive nuclides in HLLW could be separated and recovered quantitatively by SiO2-P adsorbents impregnated with suitable extractants; however, improvements and optimizations of adsorbents are required for higher recovery yields and DF with less eluent. Some improvements in TODGA/SiO2-P adsorbents were attempted and evaluated by batch-wise adsorption/elution and column separation experiments [Citation14]. Although these improvements indicated the existence of the optimum crosslinking degree of polymer (CDP) in SiO2-P particles and the optimum amount of extractants impregnated into them, there is no information about the state of the extractants and polymer present in the silica particles, which will be important for optimizing the adsorbents more efficiently.
In this study, we prepared several CMPO/SiO2-P adsorbents with different CDP and obtained some data such as uniformity of polymer and CMPO and CMPO/polymer/SiO2 interaction in a CMPO/SiO2-P particle by scanning transmission X-ray microscopy (STXM) with a soft X-ray region. In addition, the adsorption and elution behavior of Ln(III) was investigated using these adsorbents and extended X-ray absorption fine structures (EXAFS) of the adsorbents adsorbing Ln(III) were measured. From these results, the effect of microstructure in the adsorbent on the adsorption/elution behavior of Ln(III) was discussed.
2. Experimental
2.1. Materials
Spherical silica particles with an average diameter of 50 μm and a mean pore size of 600 (from Asahi Chemical Industry) or 50 nm (from Fuji Silysia Chemical Ltd.) were used. The synthesis of an inert copolymer with styrene and divinylbenzene and its embedding into the pores of the silica particles were conducted based on the flow sheet reported by Wei et al. [Citation2]. The CDP, which can be defined by the following equation, was parametrically changed to 5%–15% by controlling the ratio of divinylbenzene to styrene when conducting the polymerization.
(1)
(1) where WDVB is divinylbenzene weight in the monomer solution (g) and WSty is styrene weight in the monomer solution (g).
The extractant, CMPO, was used as a commercially available product without further purification. The impregnation of CMPO into the SiO2-P particles was also conducted as described by the previous report [Citation2], and its amount was adjusted to 15 wt% of adsorbents. Figure S1 shows the scanning electron microscope (SEM) image of the prepared CMPO/SiO2-P adsorbent.
2.2. STXM analyses
For STXM analyses, samples with a 10 μm width, 10 μm height, and 300 nm thickness (for C–K edge measurements) or with a 10 μm width, 10 μm height, and 1 μm thickness (for O–K edge measurements) were cut from the adsorbents by a focused ion beam (JEM-9310FIB, JEOL, Japan) located at the Instrument Center in the Institute for Molecular Science. The sliced samples were picked up on a Si3N4 membrane and supplied for the analyses. CMPO samples were also prepared by dropping a small amount of CMPO onto the membrane. The STXM analyses for C–K and O–K edges were performed at room temperature on a STXM system (Bruker) installed in BL-4U at ultraviolet synchrotron orbital radiation facility (UVSOR) [Citation15]. All STXM data processing was conducted using the interactive data language (IDL) package aXis 2000 [Citation16].
2.3. Adsorption/elution tests
The Eu(III) solution for the batch and column tests was prepared by dissolving Eu(III) nitrate hexahydrate [Eu(NO3)3·6H2O] of analytical grade in HNO3 solution of appropriate concentration.
In the batch tests for Eu(III) adsorption, approximately 0.2 g of CMPO/SiO2-P adsorbent and 4 cm3 of 10 mmol/dm3 Eu(III) solution were taken in a corked test tube and shaken mechanically at a temperature of 298 K in a water bath for a predetermined time. After the batch experiment, the solution was separated by filtration. The Eu(III) concentrations before and after the adsorption experiment were measured by colorimetric assay method using an ultraviolet and visible spectrophotometer (JASCO Inc.: V-630). The sample solution was diluted with 0.1 M formic acid (pH = 3) so that the Eu (III) concentration was 1–5 ppm. And then, 1000 ppm 2,7-Bis(2-arsonophenylazo)-1,8-dihydroxynaphthalene-3,6-disulfonic acid solution was injected into the diluted sample solution (volume ratio of 1:10), and the absorbance was measured at 655 nm.
The distribution coefficients (Kd) were calculated by using the following relation:
(2)
(2) where C0 and Cs denote Eu(III) concentration in aqueous solution before and after adsorption, and WA and Vs indicate weight of dry CMPO/SiO2-P adsorbent and volume of aqueous phase, respectively. Some adsorbents after the batch tests in 3 mol/dm3 HNO3 solution were supplied for EXAFS analyses to investigate the effect of CDP on the structure around the adsorbed Eu atoms.
Column tests were conducted using a pressure-resistant Pyrex-glass column with a 10 mm inner diameter and 150 mm length. Figure S2 shows the schematic diagram of the equipment for column elution experiments. The prepared CMPO/SiO2-P adsorbents were packed into the column in a slurry state at 0.1 MPa. The bed volume of the adsorbents in the column was approximately 7.9 cm3. The column temperature was kept constant at 298 K with water jackets. Prior to the introduction of feed solution containing 10 mmol/dm3 Eu(III) in 3.0 mol/dm3 HNO3 solution, the adsorbents were conditioned by passing 100 cm3 of 3.0 mol/dm3 HNO3 solution through the column. A 9 cm3 portion of the feed solution was fed to the column at a constant linear flow rate of 6.4 cm/min. Then, 36 cm3 of 3 mol/dm3 HNO3 as rinse solution and 90 cm3 of ion-exchange water as eluent were passed through the column, successively. The effluents from the column were collected using an auto-fractional collector in 3 cm3 aliquots. The Eu(III) concentration in each fraction was analyzed using an ultraviolet and visible spectrophotometer.
2.4. EXAFS analyses
The adsorbents obtained after the batch tests for Eu(III) adsorption were placed in a hole of 3 mm depth in a plastic plate with polyimide film windows. The CH2Cl2 solvent containing Eu and CMPO, which was prepared by mixing 3.0 mol/dm3 HNO3 solution with 10 mmol/dm3 Eu(III) and CH2Cl2 with 0.2 mol/dm3 CMPO at 298 K, was also supplied for EXAFS spectra measurements. The beam size at the sample was 1 mm in height and 3 mm in width. Europium LIII-edge (6.977 keV) EXAFS spectra in transmission mode have been measured with the step scan method by X-ray from a double Si (111) crystal monochromator at High Energy Accelerator Research Organization (KEK), Photon Factory, BL27B. The X-ray of higher order reflection was removed by 80% detune. EXAFS data were analyzed using the WinXAS ver.3.2 [Citation17,Citation18]. Structural parameters were derived by curve fitting analysis and phase shift, and backscattering amplitude was used by calculation using FEFF7 [Citation19,Citation20].
3. Results and discussion
3.1. STXM analyses
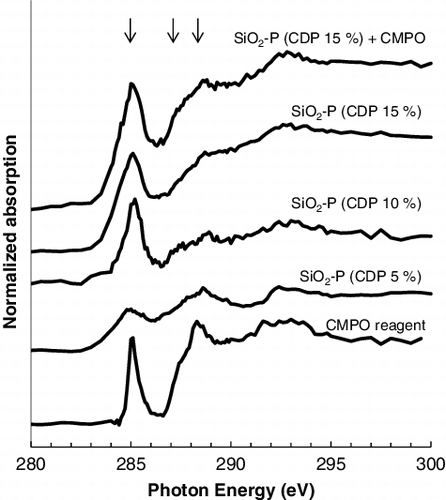
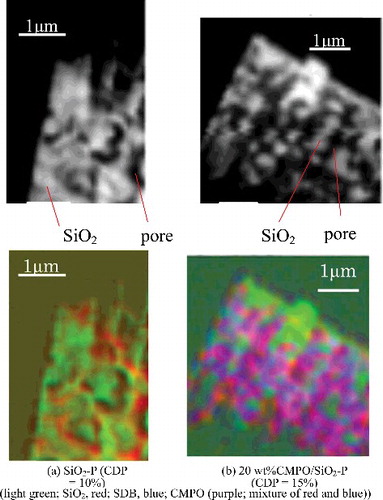
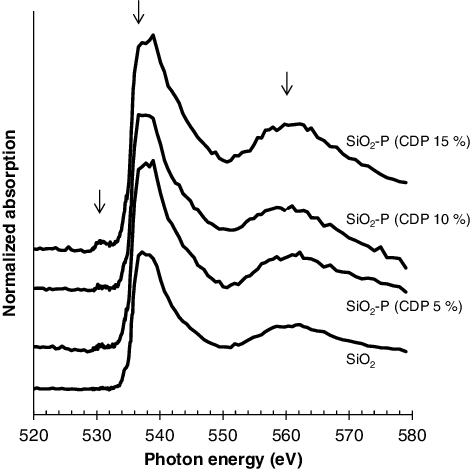
shows the change of C - near edge X-ray absorption fine structure (NEXAFS) spectra with CDP, which were obtained near the pore surface on the cross section of the sliced samples with a mean pore size of 600 nm. The C-NEXAFS spectrum obtained from the CMPO sample is also shown in the figure. The intensities of the peaks at 285 eV, which are assigned to aromatic C [Citation21] in SDB copolymer, increased with CDP. The impregnation of CMPO raised the intensity of the peaks at 287.5 and 288.5 eV, which are assigned to aliphatic C and carbamoyl C, respectively [Citation21], as well as that of the peaks at 285 eV, but there was no significant energy shift of these peaks from those observed on CMPO reagent. This result implies that CMPO takes a similar structure in a SiO2-P particle to when it is in an independent state. shows the colorized composition maps on the cross section of the sliced samples with 10% of CDP and with 15% of CDP and impregnated CMPO, which were obtained by fitting C-NEXAFS spectra with those of SDB polymer and CMPO in . The SDB polymer was distributed within several hundred nanometers of the pore surface, and its distributions were spread by the increase of CDP. The impregnated CMPO existed uniformly on the SDB polymer embedded into the pores of the silica particle. This indicates that the adsorption by CMPO extractant will proceed through the entire area of the adsorbent. shows the change of O-NEXAFS spectra with CDP, which were obtained near the pore surface on the cross section of the sliced samples with a mean pore size of 50 nm. In addition to the large asymmetric peak near 538 eV and the broad peak near 562 eV, which is assigned to O 1s core excitations of Si–O–Si bridges in the silica particle [Citation22], a small pre-edge peak appeared at 532 eV with embedding the SDB polymer in the silica. The intensity of the pre-edge peak increased with CDP, which indicates the capture of a type of impurity molecules such as H2O [Citation23,Citation24] by SDB polymer. A high CDP will bring a narrowing of the pore space in the adsorbent as shown in and make it easier to keep eluents (mainly H2O) in the pore once they have penetrated. Such a restriction of H2O molecules in higher CDP condition has also been reported by the structural analyses on the water in ion-exchange resins with different CDP [Citation25].
3.2. Adsorption/elution tests
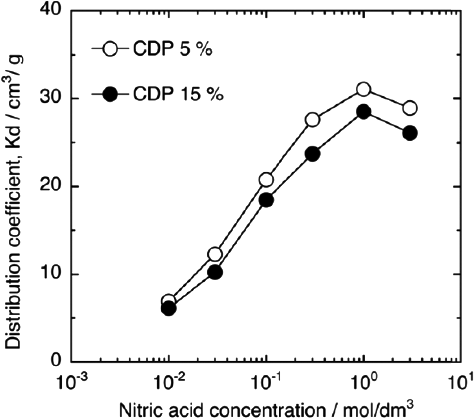
The distribution coefficients of Eu(III) in various HNO3 concentrations onto CMPO/SiO2-P adsorbents with 5% and 15% CDP are summarized in . The distribution coefficient of Eu(III) increased with HNO3 concentration and reached a maximum at approximately 1 mol/dm3 HNO3 solution irrespective of CDP. As shown in the previous reports [Citation3,Citation26], CMPO can extract trivalent metal ions and HNO3 from HNO3 solution according to the following reactions:
(3)
(3)
(4)
(4)
(5)
(5)
With an increase in HNO3 concentration, the formation of the nitrato-complex will proceed as the reaction (Equation3(3)
(3) ), resulting in an increase of the distribution coefficients. At more than 1 mol/dm3 HNO3 concentration, however, the distribution coefficient should reduce because the adsorption of Eu(III) will be inhibited by HNO3, which occupies the adsorption site of CMPO/SiO2-P adsorbent by reactions (Equation4
(4)
(4) ) and (Equation5
(5)
(5) ).
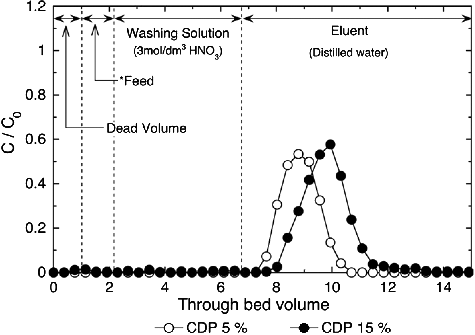
shows results of the column tests using CMPO/SiO2-P adsorbents with 5% and 15% CDP. Almost all Eu(III) in the feed solution was adsorbed onto CMPO/SiO2-P adsorbents and was eluted out by distilled water as eluent, but the elution of Eu(III) was started and ended earlier in the test using the adsorbents with 5% CDP than those with 15% CDP. The volumes of distilled water required for eluting 99% of Eu(III) were estimated to be about 3.25 (25.5 ml) and 4.08 BV (32 ml) in the test using the adsorbents with 5% CDP and 15% CDP, respectively. This means that it is more difficult to elute adsorbed metal ions from the adsorbent with higher CDP, which was also observed in the column tests using TODGA/SiO2-P adsorbents [Citation14]. Such a suppression of prompt elution from the adsorbents with high CDP is because of the narrowing of the pore space and the decrease in eluent diffusion in the adsorbent.
3.3. EXAFS analyses
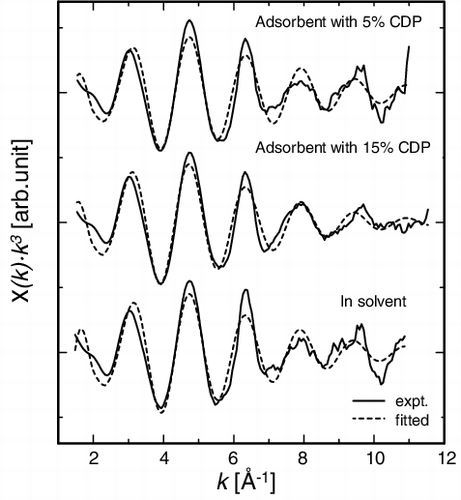
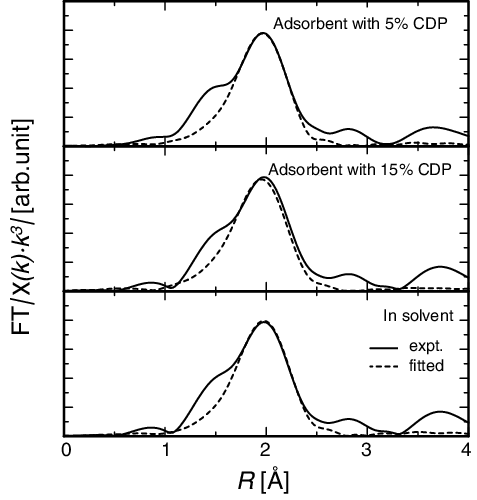
and show the EXAFS oscillations and structure functions of adsorbents with 5% and 15% CDP and that in solvent. shows the local structural parameters of the first neighboring O around Eu in CMPO/SiO2-P adsorbents with 5% and 15% CDP. The number of the nearest O atoms and the interatomic distance between Eu and O atoms in the adsorbents were similar to those in Eu/CMPO/CH2Cl2 solvent, and there was little effect of the CDP on them. This suggests that a similar complex will be formed inside the adsorbents irrespective of the CDP. Nakamura and Miyake have reported that the number of co-extracted CMPO was 2.5 by solvent extraction, which is corresponding to 8–9 coordination structure model [Citation27]. Tkac et al. suggested the ninefold coordination model for solvent extraction system with CMPO by using fourier transform infrared spectroscopy (FT-IR) technique, which well agrees with our fitting data [Citation28]. Wang et al. have reported that in the neutral 3:1 type complexes Eu(NO3)3·3CMPO, which will be generated in this study by the reaction (Equation3(3)
(3) ), the CMPO ligands and the NO3− anions serve as monodentate ligands with their phosphoric O atoms and bidentate ligands, respectively, which results in ninefold-coordinated complexes [Citation29]. Recent EXAFS studies also support our discussion. For example, Yaita et al. suggested the 2 CMPOs coordinated model at holmium complex in ethanol, which is corresponding to 8 coordination [Citation30]. Dietz and Jensen have reported the strontium complexation in a polymer-supported crown ether by EXAFS analysis, and exactly analogous behavior in solvent extraction system [Citation31]. These facts agree fairly well with the number of the nearest O atoms obtained in our EXAFS analyses.
Table 1. Local structural parameters of the first neighboring O around Eu in CMPO/SiO2-P adsorbents with 5% and 15% CDP.
4. Conclusion
The effect of microstructure in CMPO/SiO2-P adsorbents with different CDP on the adsorption/elution behavior of Eu(III) was investigated using STXM and EXAFS analyses. The results of STXM analyses indicated that the SDB polymer was distributed within several hundred nanometers from the pore surface in the silica particle and its distributions were spread by an increase in CDP. The impregnated CMPO existed uniformly on the SDB polymer and took a similar structure as in its independent state. These results suggest that the adsorption by CMPO extractant will proceed through the entire area of the adsorbent. The STXM analyses also implied the capture of eluents such as H2O by SDB polymer with high CDP. The adsorption/elution tests showed that higher CDP slowed the elution of adsorbed europium ions from the adsorbent. The EXAFS analyses for the europium ions in the adsorbents suggested that the local structure around adsorbed europium ions will be similar regardless of CDP.
Supplementary_Data.docx
Download MS Word (186.2 KB)Acknowledgments
The STXM measurements and sample preparation were conducted at the UVSOR Synchrotron Facility and the Instrument Center, respectively, in the Institute for Molecular Science (IMS), supported by Nanotechnology Platform Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The EXAFS measurements were carried out under the proposal of 2014G606 of the Photon Factory, KEK. The authors wish to thank Dr Ohigashi and Dr Nakao for the helpful supports to have STXM analyses and sample preparation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Taylor R, editor. Reprocessing and recycling of spent nuclear fuel. Cambridge (UK): Woodhead Publishing; 2015.
- Wei YZ, Kumagai M, Takashima Y, et al. Studies on the separation of minor actinide from high-level wastes by extraction chromatography using novel silica-based extraction resins. Nucl Technol. 2000;132:413–423.
- Wei YZ, Zhang AY, Kumagai M, et al. Development of the MAREC process for HLLW partitioning using a novel silica-based CMPO extraction resin. J Nucl Sci Technol. 2004;41(3):315–322.
- Hoshi H, Wei YZ, Kumagai M, et al. Group separation of trivalent minor actinides and lanthanides by TODGA extraction chromatography for radioactive waste management. J Alloys Compd. 2004;374:451–455.
- Wei YZ, Hoshi H, Kumagai M, et al. Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinylpyridine extraction chromatography for radioactive waste management. J Alloys Compd. 2004;374:447–450.
- Hoshi H, Wei YZ, Kumagai M, et al. Separation of trivalent actinides from lanthanides by using R-BTP resins and stability of R-BTP resin. J Alloys Compd. 2006;408–412:1274–1277.
- Matsumura T, Matsumura K, Morita Y, et al. Separation of trivalent minor actinides from fission products using single R-BTP column extraction chromatography. J Nucl Sci Technol. 2011;48(6):855–858.
- Liu R, Wang X, Wei YZ, et al. Evaluation study on a macroporous silica-based isohexyl-BTP adsorbent for minor actinides separation from nitric acid medium. Radiochim Acta. 2014;102(1–2):93–100.
- Ito T, Xu Y, Kim SY, et al. Adsorption behavior and radiation effects of a silica-based (Calix(4)+Dodecanol)/SiO2-P adsorbent for selective separation of Cs(I) from high level liquid waste. Sep Sci Technol. 2016;51(1):22–31.
- Ito T, Kim SY, Xu Y, et al. Study on separation of platinum group metals from high level liquid waste using macroporous (MOTDGA-TOA)/SiO2-P silica-based absorbent. Proceedings of the Global 2013; 2013 Sep 29–Oct 3; Salt Lake City (USA); La Grange Park (IL): American Nuclear Society, 2014.
- Koma Y, Sano Y, Nomura K, et al. Development of the extraction chromatography system for separation of americium and curium. In: Actinide and fission product partitioning and transmutation, Issy-les-Moulineaux (France): OECD Nuclear Energy Agency; 2012.
- Sano Y, Surugaya N, Yamamoto M. Selective recovery of minor trivalent actinides from high level liquid waste by R-BTP/SiO2-P adsorbents. IOP Conf Ser: Mater Sci Eng. 2010;9:012064.
- Watanabe S, Senzaki T, Shibata A, et al. MA recovery experiments from genuine HLLW by extraction chromatography [CD-ROM]. Proceedings of the Global 2011, 2011 Dec 11–16; Chiba (Japan); Tokyo (Japan): Atomic Energy Society of Japan; 2011.
- Watanabe S, Arai T, Ogawa T, et al. Optimizing composition of TODGA/SiO2-P adsorbent for extraction chromatography process. Procedia Chem. 2012;7:411–417.
- Ohigashi T, Arai H, Kondo N, et al. Construction of the scanning transmission X-ray microscope beamline at UVSOR. J Phys: Conf Ser. 2013;463:012006.
- Hitchcock Group [Internet]. Hamilton (ON): Hitchcock AP; [cited 2017June 23]. Available from: http://unicorn.mcmaster.ca/aXis2000.html.
- Ressler T. WinXAS: a new software package not only for the analysis of energy-dispersive XAS data. Journal de Physique IV. 1997;7:C2–269-C2-270.
- Ressler T. WinXAS: a program for X-ray absorption spectroscopy data analysis under MS-Windows. J Synchrotron Radiat. 1998;5:118–122.
- Rehr JJ, Albers RC, Zabinsky SI. High-order multiple-scattering calculations of x-ray-absorption fine structure. Phys Rev Lett. 1992;69:3397–3400.
- Zabinsky SI, Rehr JJ, Ankudinov A, et al. Multiple-scattering calculations of x-ray-absorption spectra. Phys Rev B. 1995;52:2995–3009.
- Mitsunobu S, Zhu M, Takeichi Y, et al. Nanoscale identification of extracellular organic substances at the microbe–mineral interface by scanning transmission X-ray microscopy. Chem Lett. 2015;44:91–93.
- Davoli I, Paris E, Stizza S, et al. Structure of densified vitreous silica: silicon and oxygen XANES spectra and multiple scattering calculations. Phys Chem Miner. 1992;19:171–175.
- Wilson KR, Rude BS, Catalano T, et al. X-ray spectroscopy of liquid water microjets. J Phys Chem B. 2001;105:3346–3349.
- Schwartz CP, Uejio JS, Duffin AM, et al. Soft X-ray absorption spectra of aqueous salt solutions with highly charged cations in liquid microjets. Chem Phys Lett. 2010;493:94–96.
- Yamanaka K, Yano D, Nagasawa A. Structural analyses on the water in non-ambient fields using magnetic resonance methods III. Report of cooperative research center, Saitama University. Vol. 2. Saitama (Japan): Cooperative research center of Saitama University; 2001. p. 33–33. Japanese.
- Chaiko DJ, Fredrickson DR, Reichley-yinger L, et al. Thermodynamic modeling of chemical equilibria in metal extraction. Sep Sci Technol. 1988;23:1435–1451.
- Nakamura T, Miyake C. Extraction of lanthanide (III) from nitric acid solution by octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide. J Alloys Compd. 1993;192:138–140.
- Tkac P, Vandegrift GF, Lumetta GJ, et al. Study of the interaction between HDEHP and CMPO and its effect on the extraction of selected lanthanides. Ind Eng Chem Res. 2012;51:10433–10444.
- Wang CZ, Shi WQ, Lan JH, et al. Complexation behavior of Eu(III) and Am(III) with CMPO and Ph2CMPO ligands: insights from density functional theory. Inorg Chem. 2013;52:10904–10911.
- Yaita T, Narita H, Suzuki S, et al. Structural study of holmium (III) and uranium (VI) organic ligand complexes by extended X-ray absorption fine structure spectroscopy. J Alloys Compd. 1998;271–273:184–188.
- Dietz ML, Jensen MP. EXAFS investigation of strontium complexation by a polymer-supported crown ether. Talanta. 2004;62:109–113.