ABSTRACT
Research on the recycling of useful elements in spent nuclear fuels of light-water cooling reactors is progressing. Palladium (Pd), a fission product in high-level radioactive waste, is one of the elements that can be recovered and recycled. Even if the Pd recycling system is successfully established, 107Pd contamination will be unavoidable. In this report, we review the lifecycle of Pd (applications, environmental levels, and human exposure) with respect to setting clearance levels for 107Pd. The major applications of Pd in Japan are catalysts for automobile exhausts and chemical industries, followed by dental prostheses and electrical devices. The World Health Organization reported a daily uptake of Pd from drinking water and food of 0.03 and <2 μg person−1, respectively. The uptake of a person with a dental prosthesis containing Pd might reach up to 15 μg day−1. The Pd uptake by the public via inhalation of particulate matter from automobile exhaust catalysts is not as large, even in urban areas, although this industrial application is responsible for the largest portion of Pd released into the environment globally. The data presented here will be useful for setting clearance levels for 107Pd.
1. Introduction
Palladium is a platinum-group metal and is used for various purposes as catalysts for automotive emission control and the chemical industry and in dental prostheses, electrical devices, and jewellery [Citation1]. A stable supply of palladium at a reasonable price is essential for many industries worldwide.
Recently, a transmutation technology for reducing the amount of high-level radioactive waste (HLW) in spent nuclear fuels of light-water cooling reactors and recovering useful elements has been developed [Citation2–4]. Palladium, a fission product of HLW, is one of the elements that can be recovered and recycled. One ton of HLW contains approximately one kilogram of palladium, 17% of which is 107Pd, which is a long-lived fission product with a half-life of 6.5 × 106 y [Citation5]. Separation processes such as odd-mass-selective ionization can be used to separate 107Pd from the recovered palladium. Locke et al. [Citation6], for example, confirmed high selectivity and measured odd-mass isotopes of >99.7(3)% of the total ionized product. However, the contamination of recovered Pd by a small amount of 107Pd is unavoidable. Therefore, internal and/or external exposure of the public to 107Pd may occur when recycled Pd is transferred from the radiation-controlled facility to the living environment. Therefore, it is necessary to evaluate its radiation dose and determine its clearance levels to ensure public safety.
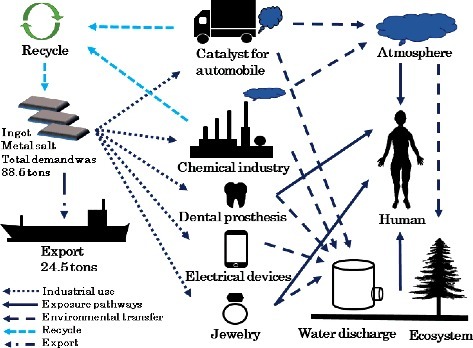
Clearance levels for radionuclides in solid materials disposed by nuclear reactor facilities were established by the former Nuclear Safety Commission in Japan. The methods used for deriving the clearance levels for solid waste from nuclear reactor facilities are summarized in a review paper by Okoshi [Citation7]. He stated that analysing the lifecycle of the materials is necessary to determine its clearance levels. Although the quantity of recycled Pd will be significantly smaller than that of other materials such as iron or concrete blocks from reactor facilities and recycled Pd may have relatively limited applications, determining its clearance level is important. The first step for determining the clearance levels of 107Pd involves the collection of comprehensive data on the environmental behaviour of 107Pd and possible pathways of public exposure. In this paper, we have reviewed the lifecycle of Pd in Japan with special emphasis on possible public exposure pathways.
2. Overall supply and demand of Pd
The Pd demand tripled in the 1990s because of the increased use of Pd in automotive emission control catalysts due to the introduction of stricter standards and legislations on emissions from road vehicles [Citation1,Citation8–10]. The total demand of ∼100 t in 1990 increased to 300 t in 1999; it maintained a similar level thereafter. The worldwide total demand of Pd was 290 t in 2015. Palladium is mainly used as catalysts in automobile exhausts and chemical industries, dental prostheses, electrical devices, and jewellery. The total Pd demand in Japan was 88.5 t in 2015, but the actual domestic consumption (excluding export) was 64 t. Compared with 85 t in 2006, the domestic consumption has gradually decreased during this decade (). Palladium is an element that can be recovered and recycled relatively well. In 2015, 28% and 32% of the total supply of Pd was recycled in the world and Japan, respectively. The recovery rate of Pd from catalysts in the chemical industry and from automotive emission control catalysts in Japan is almost 100% and 60%, respectively.
Figure 1. Demand of Pd in Japan from 2011 to 2015. Data from reference [Citation1] were represented in a graph by the author.
![Figure 1. Demand of Pd in Japan from 2011 to 2015. Data from reference [Citation1] were represented in a graph by the author.](/cms/asset/5a3f9f98-9ce7-484a-8f54-2f04495b1a62/tnst_a_1435316_f0001_oc.jpg)
3. Major applications of Pd
3.1. Catalysts for automobile emission control and chemical reactions
shows that more than 60% of the total Pd resources in Japan are used as catalysts for automotive emission control and chemical reactions [Citation1]. With respect to automobile emission control catalysts (known as three-way catalyst), Pd is used in combination with platinum and rhodium. Although improvements of gasoline engines and catalysts might reduce the usage of Pd for this purpose, it is speculated that the total demand will not significantly change in the near future [Citation10]. During the production of automotive emission catalysts, Pd and other platinum-group metals are used in an acid solution. Ceramics or metal substrates are immersed in this acid solution and heated to keep these metals on the surface. Palladium is also an important industrial catalyst. It is essential for the chemical synthesis of vinyl acetate, terephthalic acid, and artificial fibres [Citation1,Citation11].
3.2. Dental appliances
Palladium is used in dentistry for both casts and fillings. The demand for this purpose is ∼5% of the global demand. The use of Pd in dental appliances is very common in Japan because Au–Ag–Pd alloys are standard materials for dental cast restorations and the therapeutic costs are covered by the National Health Insurance. In contrast, the usage of Pd for dental treatments has decreased in western countries because of the possible risk of metal allergies. Approximately 76% of the global Pd demand for dental appliances (15 t) was utilized in Japan in 2015. Considering the increasing concern about metal allergies caused by dental appliances used by the public and dentists, the demand for this purpose will not increase in future based on medical societies such as the Japanese Society of Metal-Free Dentistry.
3.3. Jewellery, personal ornaments, and investments
The Pd demand for jewellery and ornaments (rings, necklaces) is not very large compared with other usages; it decreased from 1.7 t in 2006 to 0.5 t in 2015 in Japan [Citation1]. Palladium is used as part of an alloy with gold to change the colour (white gold) and as an additional metal for platinum in jewellery, ornaments, and coinage. Due to the increase in Pd prices, a lower proportion of Pd is used; therefore, the total global demand is decreasing. There are no statistics on the amount of recycling, personal storage, and disposal.
3.4. Electrical equipment and others
Palladium is also used in electrical equipment as metal, alloy, or metallic paste. Its metals or alloys are used for electric devices such as electrodes, integrated circuits, and semiconductor memories [Citation12,Citation13]. Thin-film pastes are common materials used for capacitors, resistors, and conductors. Recently, metallic thin-film pastes have attracted a lot of attention with respect to hydrogen storage materials. There are no statistical data on the recycling rate of Pd used for electrical equipment. The use of Pd in anti-cancer drugs is being investigated. However, the amount utilized for this purpose is not large at present, in contrast to platinum, which is widely used for anti-cancer drugs such as Cisplatine [Citation14].
4. Levels of Pd in the environment and human exposure
4.1. Working environment
A high Pd concentration might exist in working environments, from refining or recycling to manufacturing of end products [Citation8]. The concentration of Pd in the air in a platinum and palladium refinery is reportedly in the range of 0.001–0.36 μg m−3. The highest level of soluble Pd was measured for the production of catalysts for automobiles (1.66 μg m−3). During the production of dental casts and crowns, Pd concentrations of 3.5 μg m−3 with local ventilation and 5.5 μg m−3 without dust control were measured in the breathing zone of dental technicians [Citation15]. These Pd concentrations are much higher than those in the environment accessible to the public, although they are high near busy roads, as mentioned in what follows.
4.2. Pd originating from automotive exhaust catalysts
Barbante et al. [Citation16] measured the concentration of platinum-group metals (Pt, Pd, Rh) in snow and ancient ice in Central Greenland. They found that the concentrations of these metals in recent snow from the 1990s were 40–120 times higher than that of 7000-year-old ice and that a large fraction of the recent increase might be due to the use in automobile catalytic converters. Considering the total amount of Pd used for this purpose, their conclusion might be reasonable. The amount of Pd released from automotive catalytic converters was reported to be 4–108 ng/km of Pd in the flesh converter of an ordinary passenger car with gasoline engine, although this concentration might be affected by the speed of driving, type and age of the catalyst, and engine type [Citation9,Citation17]. Most of the Pd (>85%) is released as particulate matter (PM) and exists as airborne PM in the atmosphere [Citation18–20]. The concentrations of Pd in PM10 (particles with an aerodynamic diameter <10 μm) were summarized previously; they range from 1.3–42.7 pg m−3 at busy roadsides downtown [Citation11].
To estimate the radiation doses of 107Pd in airborne Pd particles, information on particle size and solubility is necessary because the dose coefficient of the International Commission of Radiation Protection (ICRP) varies depending on these parameters [Citation20]. Rauch et al. [Citation21] reported that the average concentration of Pd in PM10 and PM2.5 is 4.9 and 1.5 pg m−3 at urban roadsides and 1.8 and 1.4 pg m−3 at rural roadsides, respectively. Zereini et al. [Citation22–24] measured the particle size distribution of PM using an 8-stage Andersen sampler and determined the Pd concentrations for each particle size range. Approximately 70% and 37% of the Pd in particle size fractions <10 microns were associated with particles <2.5 and <1 μm in diameter, respectively. Based on these studies, it is apparent that most of the Pd emitted from catalytic converters is distributed in the respiratory PM size fraction. For radiation dose estimation after inhalation, information on the solubility of the particles is essential. The dose coefficients vary depending on the particle solubility: slow (S), moderate (M), or fast (F) [Citation25]. At present, data on the solubility of Pd in road dust or automobile catalysts based on animal experiments are limited. Artelt et al. [Citation17] showed that 35% of platinum inhaled by cats as road dust was released from the dust in 90 days. By using an in vitro assay system with artificial lung fluid, Colombo et al. [Citation26,Citation27] determined a similar dissolution rate (36% for Pt and 88% for Rh). Based on these reports and data showing that metal Pd is much more soluble than Pt and Rh, Pd in road dust might be categorized as F- or M-class particle in the ICRP lung model.
4.3. Dental appliances
There are several reports on the dissolution of dental casting alloys containing Pd using in vitro experiments. The release rate of Pd ranges from 0.003–6 μg Pd cm−2 day−1 depending on the experimental conditions and alloys used, as reviewed by Kielhorn et al. [Citation8]. In vivo, the release rate might significantly vary depending on the dental conditions, total amount used, and personal habits (e.g. teeth brushing). The World Health Organization (WHO) estimated the uptake of Pd from dental appliances to be <1.5–15 μg person−1 day−1 [Citation12]. The absorption rate of dissolved Pd by the human gastrointestinal tract is not known. A low Pd absorption rate was observed in animal experiments [Citation28–30] and the ICRP recommended a gastrointestinal absorption rate (f1) of 0.03 in the alimentary model (Publ.100) [Citation31].
4.4. Other applications
The usage of Pd for jewellery, personal ornaments, coins, electrical parts, and devices plays an insignificant role for the direct intake of Pd by the human body. There is a possibility that Pd disintegrates from jewellery (e.g. rings, piercings) and is absorbed by the skin; however, the amount is assumed to be very small. Therefore, it is unlikely that internal radiation exposures occur. Palladium may be in close contact with the skin, but the external radiation dose is negligible because of the very low energy of beta rays emitted from 107Pd (31 keV). Environmental transfer after the disposal of Pd with waste is more important for human exposure. Palladium is distributed widely in the environment after disposal because Pd easily dissolves and becomes bioavailable compared with other platinum-group elements [Citation26,Citation27,Citation32,Citation33].
4.5. Water, soil, and sewage sludge
After release to the environment, Pd is transferred and distributed in ecosystems, like other environmental pollutants. Several measurements have been carried out on soil samples; a maximum of ∼300 ng/g Pd was observed. The concentrations were higher in samples collected in urban areas and near busy roads. The deposited Pd can be transferred to deeper soil. For example, in places with a Pd concentration of 193 ng/g in the topsoil layer, the concentration was 19 ng/g at 20–30 cm depth [Citation34]. Leopold et al. [Citation35] summarized data on Pd contents of road tunnel dust and sewage sludge ash from 1994 to 2007. The concentrations of Pd in road tunnel dust showed a ∼25-fold increase, that is, 13.5–21.8 and 311.4–516.2 ng/g in 1994 and 2007, respectively. In contrast, the concentration of Pd in urban sewage sludge ash decreased, 460 and 150 ng/g in 1996 and 2007, respectively. They concluded that this decrease is attributable to the decrease in the usage of Pd in the dental industry in Europe.
Although there are many reports on the concentration of platinum in water environments, that for Pd are very limited. Uchida et al. [Citation36] reported the concentrations of elements and ions and chemical characteristics, such as pH, in water of 45 major rivers in Japan. Based on their measurements, the concentration of Pd in the river is 0.14 ng/L on average and ranges from 0.02–0.83 ng/L. Values of 0.4 and 0.04 ng/L were reported for water of the Rhein River in Germany and Pacific Ocean, respectively [Citation37,Citation38].
4.6. Plant, animal, and human uptake
Although Pd is mainly released as metal or oxide from automobile catalysts, a significant fraction of it dissolves and becomes bioavailable after release into the environment. Recently, it was shown that inorganic anions enhance the bio-accessibility of released Pd [Citation39]. Transfer factors, defined as concentration ratios of an element in food plant vs. soil or culture medium, are important parameters for the estimation of human exposure (radiation dose) to radioactive nuclides in the soil. Based on the greenhouse experiment, the transfer factors of Pd are ∼0.1, significantly higher than that of platinum and rhodium [Citation40].
The Pd concentrations in biotic media, such as grass on roadsides, seaweeds, muscles of pigeons, freshwater isopods, and eels, vary from 0.2 to 800 μg kg−1. The Pd concentration is generally higher than that of other platinum elements (Pt and Rh), indicating the high bioavailability of this element. Although there are no reports on the daily uptake of Pd by the public in Japan, 0.03 and <2 μg person−1 day−1 were estimated for drinking water and food, respectively [Citation41].
5. Conclusion
The lifecycle of Pd is summarized in . In Japan, 64 t of Pd were consumed in 2015 in total, 35% of which was recycled. The major applications were catalysts for automobile exhausts and chemical industries, followed by electrical devices and dental prosthesis. The highest human exposure might occur at workplaces, where Pd-containing products are produced; air concentrations of up to 1.66 and 5.5 μg m−3 were observed in an automobile catalyst factory and a dental technician's workplace, respectively. For the public, the WHO reported a daily uptake of Pd from drinking water and food of 0.03 and <2 μg person−1, respectively; the uptake by people with dental prostheses containing Pd might reach up to 15 μg day−1. The Pd uptake by the general public via inhalation of PM from automobile exhaust catalysts is not very large, even in urban areas, although this industry contributes the most to the release of 107Pd into the environment.
The contamination with long half-life radionuclides of Pd is unavoidable when Pd is recovered from spent nuclear fuels of light-water cooling reactors and recycled. The results in this paper suggest that oral ingestion may be an important pathway to estimate the radiation dose for public. Palladium is used for a variety of applications; thus, it is necessary to evaluate the radiation doses for each of the applications described in this paper and to establish clearance levels.
Acknowledgments
This research was funded by ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Japan oil, gas and metals national corporation. [mineral resources, material flow 2015. 5. Platinum Group Metals (PMG)]. [ cited 2017 Jul 21]. Japanese. Available from: http://mric.jogmec.go.jp/public/report/2-15-11/05_201511_PGM.pdf.
- Fujita R. [Reduction and resource recycling of high-level radioactive wastes through nuclear transmutation, report of ImPACT program]. [ cited 2017 Jul 21]. Available from: http://www.jst.go.jp/ impact/en/program/08.html
- International Atomic Energy Agency. Implications of partitioning and transmutation in radioactive waste management. Technical Reports Series No.435; 2004.
- Nishihara K, Nakayama S, Morita Y, et al. Impact of partitioning and transmutation on LWR high-level waste disposal. J Nucl Sci Technol. 2008;45:84–97.
- Naito K, Matsui T, Tanaka Y. Recovery of noble metals from insoluble residue of spent fuel. J Nucl Sci Technol. 1986;23:540–549.
- Locke1 CR, Kobayashi T, Midorikawa K. Improved efficiency of selective photoionization of palladium isotopes via autoionizing Rydberg states. Appl Phys B. 2017;123:33. DOI:https://doi.org/10.1007/s00340-016-6623-5
- Okoshi M. Derivation methods on the clearance levels for nuclear reactors. Jpn J Health Physics. 1999;34:353–358. Japanese.
- Kielhorn J, Melber C, Keller D, et al. Palladium – a review of exposure and effects to human health. Int J Hyg Environ Health. 2005;205(6):417–432.
- Ravindra K, Bencs L, Van Grieken R. Platinum group elements in the environment and their health risk. Sci Total Environ. 2004;318(1–3):1–43.
- Zereini F, Alsenz H, Wiseman CL, et al. Platinum group elements (Pt, Pd, Rh) in airborne particulate matter in rural vs. urban areas of Germany: concentrations and spatial patterns of distribution. Sci Total Environ. 2012;416:261–268.
- Wiseman CL, Zereini F. Airborne particulate matter, platinum group elements and human health: a review of recent evidence. Sci Total Environ. 2009;407(8):2493–2500.
- World Health Organization. [Palladium. Environmental health criteria series 226, international program on chemical safety]. [cited 2017 Jul 21]. Available from: http://www.inchem.org/documents/ehc/ehc/ehc226.htm
- Johnson Matthey. [Precious metals management, the role of palladium]. [ cited 2017 Jul 21]. Available from: http://www.platinum.matthey.com/about-pgm/ applications/industrial/electronic-components
- Tanaka M, Kataoka H, Yano S, et al. Anti-cancer effects of newly developed chemotherapeutic agent, glycoconjugated palladium (II) complex, against cisplatin-resistant gastric cancer cells. BMC Cancer. 2013;13:237. DOI:org/https://doi.org/10.1186/1471-2407-13-237
- Purt R. Possible health danger from palladium-based fired alloys. quantitative determination of palladium and gallium in atmospheric zone of dental technicians. Quintessenz Zahntech. 1991;17(3):329–334.
- Barbante C, Veysseyre A, Ferrari C, et al. Greenland snow evidence of large scale atmospheric contamination for platinum, palladium, and rhodium. Environ Sci Technol. 2001;35(5):835–839.
- Artelt S, Creutzenberg O, Kock H, et al. Bioavailability of fine dispersed platinum as emitted from automotive catalytic converters: a model study. Sci Total Environ. 1999;228(2–3):219–242.
- Rauch S, Peucker-Ehrenbrink B, Molina LT, et al. Platinum group elements in airborne particles in Mexico City. Environ Sci Technol. 2006;40(24):7554–7560.
- Rauch S, Hemond HF, Peucker-Ehrenbrink B, et al. Platinum group element concentrations and osmium isotopic composition in urban airborne particles from Boston, Massachusetts. Environ Sci Technol. 2005;39(24):9464–9470.
- Wiseman CL, Hassan Pour Z, Zereini F. Platinum group element and cerium concentrations in roadside environments in Toronto, Canada. Chemosphere. 2016;145:61–67.
- Rauch S, Lu M, Morrison GM. Heterogeneity of platinum group metals in airborne particles. Environ Sci Technol. 2001;35(3):595–599.
- Zereini F, Wiseman C, Alt F, et al. Platinum and rhodium concentrations in airborne particulate matter in Germany from 1988 to 1998. Environ Sci Technol. 2001;35:1996–2000.
- Zereini F, Alsenz H, Wiseman C, et al. Platinum group elements (Pt, Pd, Rh) in airborne particulate matter in rural vs. urban areas of Germany: concentrations and spatial patterns of distribution. Sci Total Environ. 2012;416:261–218.
- Zereini F, Alt F, Messerschmidt J, et al. Concentration and distribution of heavy metals in urban airborne particulate matter in Frankfurt am Main, Germany. Environ Sci Technol. 2005;39:2983–2989.
- International Commission on Radiological Protection. 1990 Recommendations of the international commission on radiological protection. Publication 60. Oxford: Pregamon Press; 1991.
- Colombo C, Monhemius AJ, Plant JA. Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicol Environ Safety. 2008;71(3):722–730.
- Colombo C, Monhemius AJ, Plant JA. The estimation of the bioavailabilities of platinum, palladium and rhodium in vehicle exhaust catalysts and road dusts using a physiologically based extraction test. Sci Total Environ. 2008;389(1):46–51.
- Moore W, Hysell D, Hall L, et al. Preliminary studies on the toxicity and metabolism of palladium and platinum. Environ Health Perspect. 1975;10:63–71.
- Moore W Jr, Hysell D, Crocker W, et al. Biological fate of 103Pd in rats following different routes of exposure. Environ Res. 1974;8(2):234–240.
- Iavicoli I, Bocca B, Fontana L, et al. Distribution and elimination of palladium in rats after 90-day oral administration. Toxicol Ind Health. 2010;26(3):183–189.
- Inetrnational Commnission on Radiological Protection. Human alimentary tract model for radiological protection. Publication 100. Oxford: Pergamon Press; 2006.
- Moldovan M, Rauch S, Gómez M, et al. Bioaccumulation of palladium, platinum and rhodium from urban particulates and sediments by the freshwater isopod Asellus aquaticus. Water Res. 2001;35(17):4175–4183.
- Zimmermann S, Alt F, Messerschmidt J, et al. Biological availability of traffic-related platinum-group elements (palladium, platinum, and rhodium) and other metals to the zebra mussel (Dreissena polymorpha) in water containing road dust. Environ Toxicol Chem. 2002;21(12):2713–2718.
- Leopold K, Wörle K, Schindl R, et al. Determination of traffic-related palladium in tunnel dust and roadside soil. Sci Total Environ. 2017;583:169–175.
- Leopold K, Maier M, Weber S, et al. Long-term study of palladium in road tunnel dust and sewage sludge ash. Environ Pollut. 2008;156(2):341–347.
- Uchida S, Takeda H, Tagami K, et al. Elemental concentration in Japanese rivers 2002–2006. Chiba: National Institute of Radiological Sciences; 2007. Japanese.
- Lee DS. Palladium and nickel in northeast Pacific waters. Nature. 1983;305:47–48.
- Eller R, Alt F, Tölg G, et al. An efficient combined procedure for the extreme trace analysis of gold, platinum, palladium and rhodium with the aid of graphite furnace atomic-absorption spectrometry and total-reflection X-ray fluorescence analysis. Fresenius Z Anal Chem. 1989;334:723–739.
- Zereini F, Wiseman CL, Poprizki J, et al. Assessing the potential of inorganic anions (Cl-, NO3-, SO42- and PO43-) to increase the bioaccessibility of emitted palladium in the environment: experimental studies with soils and a Pd model substance. Environ Pollut. 2017;220(Pt B):1050–1058.
- Schäfer J, Hannker EJD, Stüben D. Uptake of traffic-related heavy metals and platinum group elements (PGE) by plants. Sci Total Environ. 1998;215:59–67.
- Rose M, Baxter M, Brereton N, et al. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(10):1380–1404.