?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effect of oxygen potential on the Fuel-Cladding Chemical Interaction (FCCI) of 15-15Ti cladding material was studied in this paper. In the test, the materials have been exposed to simulated fission products at 600◦C for 120 h under the oxygen potential in equilibrium with Mo/MoO2 and Cr/Cr2O3.The general corroded depth was roughly 30 µm. Furthermore, the results also show that corroded depth on the cladding surface increase with the increase of oxygen potential. No intergranular attacks were observed, which might be caused by the absence of the high-temperature gradient. A single layer of CrTe is assumed to be formed under low oxygen potential, whereas cesium and oxygen may play an important role under high oxygen potential. Moreover, FCCI may impose a great threat to the integrity of China designed 15-15Ti material.
1. Introduction
Currently, the designs of different kinds of advanced nuclear systems are investigated, including Lead-cooled Fast Reactor (LFR) and Accelerator Driven System (ADS). However, there are some open technical issues for the employment of these advanced nuclear systems. Among them, the choice of advanced materials, especially cladding material, is one of the crucial issues. In these advanced nuclear systems, the cladding materials will be exposed to high irradiation dose (100–200 dpa) and high temperature (480–600C), which will threaten the integrity of the cladding.
15-15Ti stainless steel is a derivative of type 316 steel, which has been widely used in the sodium-cooled fast reactor. Moreover, plenty of irradiation data have been accumulated [Citation1]. However, experimental data on the corrosion resistance of 15-15Ti in the reactor environment are insufficient.
In the reactor, when fission of U or Pu begins, fission products will be released immediately. Among these elements, Cs, Te, I are named volatile fission products because they are in a gas state in the reactor environment. Furthermore, they are expected to be the most aggressive in the reactors environment [Citation2,Citation3], which can easily cause Fuel-Cladding Chemical Interaction (FCCI). FCCI is the FP-accelerated oxidative attack of cladding that is frequently observed in fast reactor. It’s generally accepted that the high-temperature corrosion resistance of austenitic stainless steel is due to the thin protective layer of a chromium-rich oxide on the surface [Citation4]. In the process of FCCI, the corrosive fission products can destroy the integrity of the protective layer and penetrate into the cladding. Intergranular attacks induced by cesium and tellurium are considered to be the most crucial. Previous studies have shown that pure cesium cannot interact with the steel without the presence of oxygen [Citation5]. On the contrary, Te can penetrate into the steel even in the absence of oxygen [Citation6]. Furthermore, some studies [Citation4,Citation7,Citation8] have also suggested that there might be a competitive relationship between the formation of oxide and telluride, which means that the extent of Te attacks may be decreased by the increase of oxygen potential. Iron from the cladding materials has been observed within the irradiated fuel [Citation9,Citation10], which is attributed to the iodine transport of cladding constituents. According to the previous studies [Citation10,Citation11], metal diiodides can be formed due to the reaction between iodine and steel and they can diffuse into the fuel. Furthermore, radiation-induced decomposition of metal diiodides will release iodine for further reaction.
China has planned to build a sodium-cooled-fast-reactor CFR600 before 2023. Since 15-15Ti steel was chosen as a candidate cladding material for CFR600, China has designed its own 15-15Ti material, which is named as CN15-15Ti [Citation12,Citation13]. Compared with other types of 15-15Ti steel in the world, the elements such as Co, V were added in this type of steel. More specifications of CN15-15Ti will be explained in Material part. However, due to the high irradiation in the fast reactor, extensively works are devoted to the study of its irradiation resistance. The data about its corrosion resistance to simulated fission products are rare.
The purpose of this study is to investigate the corrosion phenomenon of CN15-15Ti in the simulated reactor environment and corrosion phenomena under different oxygen potential. The samples were immersed in the simulated fission products under different oxygen potential and heated at 600C for 120 h.
2. Material
The cold-worked 15-15Ti used in this project, which were provided by China Institute of Atomic Energy (CIAE), were designed for CFR600. The materials were annealed at about 1050C in the manufacture process. The average grain size of this kind of steel is about 100
. shows the composition of the evaluated steel.
Table 1. Chemical composition of evaluated alloys (in wt.%)
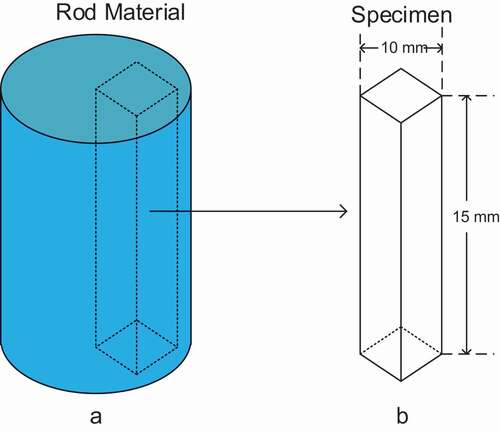
As shown in , rectangular-shaped specimens were cut out from the steel rod by wire cutting. Subsequently, their surfaces were polished to remove the oxidation layer, which was caused by cutting process. The dimensions of the specimens are also shown in .
3. Experimental
Pure cesium is in a liquid state at room temperature and very active. As a result, ,
were added as the simulated fission products in this test. Although this experiment is not a perfect simulation of the fuel-clad gap since the ratio of Te is higher, its results could show the role of the main fission products. The oxygen potential of Mo/
, Cr/
at 873 K are −425 kJ/mol and −598 kJ/mol according to thermodynamic calculation. In accordance to Ref [Citation14], the oxygen potential of irradiated fuel is suggested to be equilibrium condition between Mo/
and Cr/
.
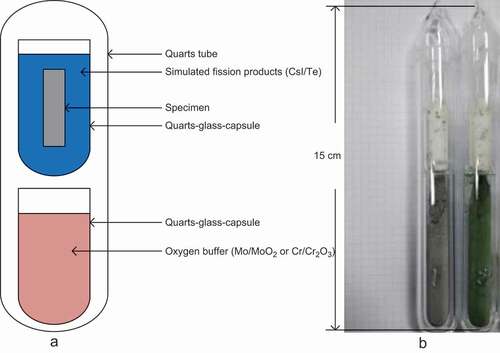
) exhibits the schematic diagram of the test equipment. Furthermore, the test capsules were shown in ). The oxygen buffer materials and simulated fission products were placed in two quartz capsules. After these quartz capsules were sealed in a larger quartz tube in the absence of oxygen, these tubes were placed in the electric furnace at 600C for 120 h.
After unmounted from the furnace, the additives were then removed. All of the sample surfaces were polished, about 45 with respect to the initial surface, by decreasing the abrasive particle size sequentially. Then the samples were observed and analyzed with SEM and EDS. An acceleration voltage of 15 kV was used in SEM, and an 80
X-Max Silicon Drift EDS Detectors from Oxford Instruments was used for elemental analysis. Although this technique is insensitive to the light elements such as carbon, nitrogen, or oxygen which is included in the final results, it’s fixed by using the atomic ratios of the interested elements.
4. Results
The experiment data and observation are summarized in . The detailed descriptions will be reported below.
Table 2. General results of the test
4.1. Mo/MoO3 oxygen buffer
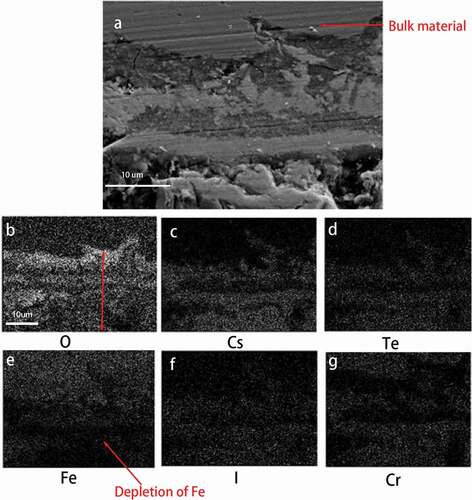
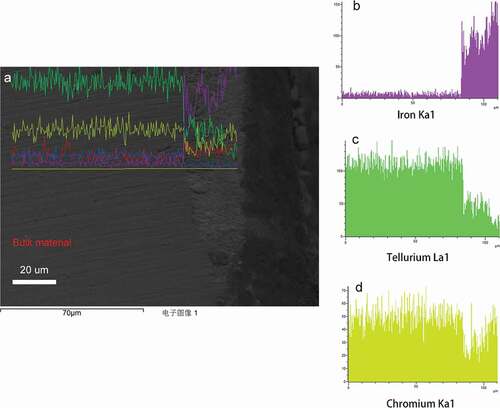
shows one of the element mapping images under higher oxygen potential in equilibrium with Mo/. In ), matrix attacks with a few layers, whose depth is roughly 30
, could be observed.
Moreover, show the element mapping images for each element, in which the white spot shows the presence of each element. In this microscopic image, corrosive elements penetrated into the bulk materials, which is placed on the middle part of the image. It can be easily observed from ) that the penetration of cesium is evident. Further more, ) and e also show the presence of oxidation and prominent iron depletion.
Element analysis has also been performed. ) shows the element analysis results of this area. In this site, although matrix attacks were prominent, local attacks were severer. Spectrum 1 in shows the penetration of fission products especially cesium and tellurium. Furthermore, spectrum 2 and 3 show the enrichment of Cr.
Table 3. EDS data of (in at.%)
4.2. Cr/Cr2O3 oxygen buffer
Unlike the results in the case of higher oxygen potential, the extent of corrosion is not so prominent in this case.
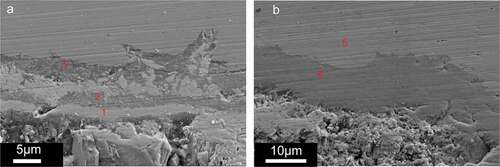
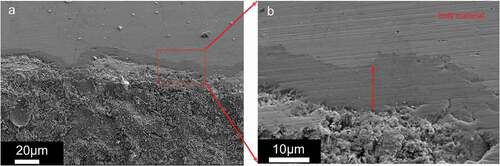
Line element analysis has been performed in this case. shows one of the corrosion sites. These line analysis images show the penetration of Te. Compared with the results in the case of higher oxygen buffer, the attack caused by cesium and iodine was hardly detected. Matrix attacks with layers, whose depth is roughly , were also observed.
In addition, one of the attack areas is shown in . The right image is the magnification of the left one. Layers of matrix attacks can be observed in this area. Furthermore, element analysis shows the presence of tellurium in this layer. Two points were chosen from the right image to be analysis, which is shown in ). The element analysis data of spectrum 4 in shows the Te/Cr ratio is almost 1.
5. Discussion
As illustrated in Result part, different kinds of penetration morphologies were observed in different cases. The corrosion mechanism and dominant reaction for each case will be discussed in this section.
5.1. Mo/MoO3 oxygen buffer
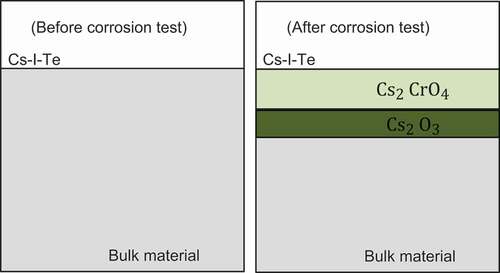
shows the cross section of the specimen before and after the test. Layers of Cs, Te are observed between Fe and Ni.
The corrosion depth is more than 30. Based on the thermodynamic calculation in Ref [Citation7], in the presence of Mo/
oxygen buffer, the following buffer reactions may happen:
The condense phase present may be ,
, element tellurium can also present. Although the oxygen buffer and fission products were placed in different tubes, the volatile fission products could migrate to react with Mo. Furthermore, matrix attacks were also observed, whereas the components of each layer are different. Cs and oxygen penetration are evident in . On the other hand, the attack of Te is not prominent compared to that of Cs and oxygen. The condense phrase present may be
,
, element Te can also present. The element analysis data in shows the presence of Cs, Te, O, and Cr, depletion of Fe is also evident. Several types of cesium chromate have been proposed in the previous studies [Citation15]:
, or
. In this case, the equation is suggested to be as follows based on the data:
Furthermore, is assumed to be formed in that layers. Below is the reaction equation [Citation3,Citation10]:
5.2. Cr/Cr2O3 oxygen buffer
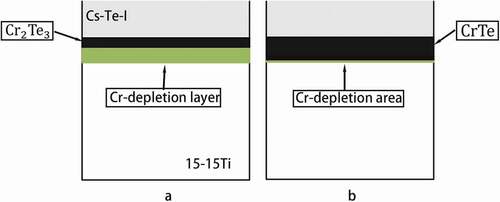
The absence of Cs attack in and might be caused by the lower oxygen potential [Citation6]. Furthermore, analysis data in shows the pattern of . The corrosion attacks are mainly caused by Te-steel reaction. It is assumed that the steel surface was converted to be a single layer of
. This result is in good agreement with that of Ref [Citation16], in which a single layer of
was formed in 20Cr-25Ni, Nb stabilized steel at 850
C in the absence of oxygen. The corrosion process, in this case, is suggested as following [Citation4,Citation16]:
was suggested to be formed at the beginning of Te penetration, which will induce the depletion of Cr underneath. The outer diffusion of Cr may continue until a single layer of
was formed. Additionally, these processes are graphically represented in .
5.3. The absence of intergranular attack
Intergranular attacked are often observed both in out-of-pile test and in-pile test [Citation9]. It is suggested that it was mainly caused by the penetration of Cs and Te. Whereas in this work, no trace of intergranular attacks was observed. It might be caused by the absence of the high-temperature gradient [Citation5]. Furthermore, the morphology of cladding attack depends heavily on the local concentration of the fission products [Citation6]. This factor may affect the results.
6. Conclusion
China designed 15-15Ti cladding material was tested with simulated fission products for 120 h. These two tests were performed under the oxygen potential in equilibrium with Mo/ and Cr/
. The test results show that attack depths increase while oxygen potential increases. No intergranular attacks have been detected which might be caused the presence of a high-temperature gradient. A single layer of
was suggested to have been formed under higher oxygen potential in equilibrium with
. Under higher oxygen potential,
and
are assumed to formed. Furthermore, FCCI can impose a threat to the integrity of China designed 15-15Ti cladding materials, plenty of R&D works still need to be done.
Acknowledgments
Present work was supported by Tsinghua University Initiative Scientific Research Program, number 20161080065. My thanks also extend to China Institute of Atomic Energy for kindly providing CN15-15Ti materials. This work was supported by the Tsinghua University Initiative Scientific Research Program [20161080065].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Nuclear Energy Agency. Handbook on lead-bismuth eutectic alloy and lead proper- ties, materials compatibility. Nucl Sci. 2015;571–611. https://www.oecd-nea.org/science/pubs/2015/7268-lead-bismuth-2015.pdf
- Antill J, Warburton J. Influence of tellurium on caesium-enhanced corrosion of stainless steel. J Nucl Mater. 1977;71:134–139.
- Sasaki K, Tanigaki T, Fujimura R, et al. The corrosion product of CsTe corrosive compound with 11Cr-Ferritic/Martensitic steel and 9Cr-Oxide dispersion strengthened steel. J Nucl Mater. 2015;460:107–113.
- Maiya P, Busch D. Grain-boundary penetration of type 316 stainless steel exposed to cesium or cesium and tellurium. Metall Trans A. 1975;6:409–415.
- Viswanathan R. Fuel clad chemical interactions in fast reactor MOX fuels. J Nucl Mater. 2014;444:101–111.
- Pulham R, Richards M. Chemical reactions between caesium, tellurium and oxygen with fast breeder reactor cladding alloys. J Nucl Mater. 1990;172:206–219.
- Ball R, Burns W, Henshaw J, et al. The chemical constitution of the fuel-clad gap in oxide fuel pins for nuclear reactors. J Nucl Mater. 1989;167:191–204.
- Arima T, Takaki M, Sato I, et al. Reaction of modified SUS316 with tellurium under low oxygen potentials. Corros Sci. 2003;45:1757–1766.
- Johnson C, Crouthamel C. Cladding interactions in mixed oxide irradiated fuels. J Nucl Mater. 1970;34:101–104.
- Reimann G. Development of an ultrafine grain size type-316 stainless-steel cladding. Nucl Technol. 1971;10:62–66.
- Corbett J, Clark R, Munday T. Solution of metals in their molten salts some manganese, chromium, uranium and antimony systems. J Inorg Nucl Chem. 1963;25:1287–1291.
- Xu Y, Wang J, Li J. The chemical interaction between domestic stainless steel cladding and fssion products. Out pile simulation test. Chin J Nucl Sci Eng. 1995;15(3):232–241.
- Xu Y, Li J, Wang J. Effects of oxygen potential on corrosion behaviour of stainless steel claddingtube used for FBR. Chin J Nucl Sci Eng. 1996;16(3):250–255.
- Kurata Y. Corrosion behavior of cold-worked austenitic stainless steels in liquid leadbismuth eutectic. J Nucl Mater. 2014;448:239–249.
- Bradbury M, Pickering S, Whitlow W. A proposed mechanism for internal cladding corrosion in LMFBR mixed oxide fuel pins. J Nucl Mater. 1978;78:272–280.
- Lobb R, Robins I. A study of the 20% Cr/25% Ni/Nb stabilised stainless steel-tellurium reaction. J Nucl Mater. 1976;62:50–62.